Introduction
Statistical data show that ~1 million patients with
cardiac arrest (CA) are recorded annually in the United States and
Europe (1). However, treatment of
CA with cardiopulmonary resuscitation (CPR) often leads to an
unsatisfactory outcome. For example, 30–40% of patients that have
undergone CPR achieve a return of spontaneous circulation (ROSC),
10–30% are completely healed and discharged from hospital, while
the remaining patients eventually succumb to the illness. In
addition, patients that have been successfully resuscitated can
often experience permanent neurological complications, including
cognitive impairment and motor deficit (2,3).
Therefore, providing an effective regimen for the treatment and
prevention of neurological injury following CPR is important.
Organ injury following successful CPR from CA is
considered to be associated with systemic inflammatory responses;
levels of various cytokines and lipopolysaccharide (LPS) have been
shown to be markedly elevated in patients that have been
successfully resuscitated from CA (4,5). LPS
can be identified by the cell-surface Toll-like receptor 4 (TLR4),
which induces inflammatory responses and promotes the production of
a number of inflammatory cytokines, resulting in a sepsis-like
syndrome. The dynamic changes in the levels of inflammatory
cytokines and LPS have been shown to be closely associated with
prognosis in patients that have undergone CPR (6). A previous study demonstrated that
neurological injury following CPR is associated with the activation
of cerebral inflammatory responses, with the TLR4 signaling pathway
playing a contributory role in the occurrence of neurological
injury (7). Under cerebral
ischemia and anoxia, the activated TLR4 promotes the expression of
nuclear factor (NF)-κB and induces the production of cytokines,
such as tumor necrosis factor (TNF)-α and interleukin (IL)-6,
thereby causing inflammation and neurological injury (7). Therefore, it is hypothesized that the
TLR4 signaling pathway may be inhibited by hypoxic preconditioning
to attenuate inflammatory responses and neurological injury; thus,
protecting brain function. Notably, a significantly reduced area of
cerebral infarction and an alleviation of inflammatory responses in
the brain have been observed in TLR4 knockout mice following
cerebral ischemia (8). However,
attenuation of brain injury following CA by hypoxic preconditioning
remains difficult in clinical settings. Therefore, postconditioning
with an effective medicine is of vital importance.
Ulinastatin (UTI), a urinary trypsin inhibitor
extracted and purified from human urine, has been shown to possess
anti-inflammatory properties, suppressing the infiltration of
neutrophils and the release of elastinase and chemokines (9). In addition, UTI protects
mitochondrial function by reducing the calcium overload in injured
cells and exhibits protective effects against ischemia-reperfusion
(I/R) injury in the heart, lung, liver and kidney (9). Furthermore, previous studies have
demonstrated that UTI can alleviate LPS-induced lung and kidney
injury by inhibiting inflammatory responses in these organs
(10,11). Thus, we hypothesized that UTI may
protect against cerebral I/R injury following CPR and inhibit
TLR4-induced inflammatory responses in the brain following
resuscitation. In the present study, a rat model of CPR following
asphyxial CA was established, in which the effects of UTI on the
TLR4 signaling pathway were evaluated, as well as the protective
mechanisms against cerebral I/R injury in rats subjected to CPR
following CA.
The effects of UTI were investigated with regard to
the mortality rate, TLR4 mRNA expression, NF-κB activity and the
levels of TNF-α and IL-6. The aim of the present study was to
determine whether UTI improved neurological function; thus, may be
used in combination with CPR for the treatment of brain injury
following CA.
Materials and methods
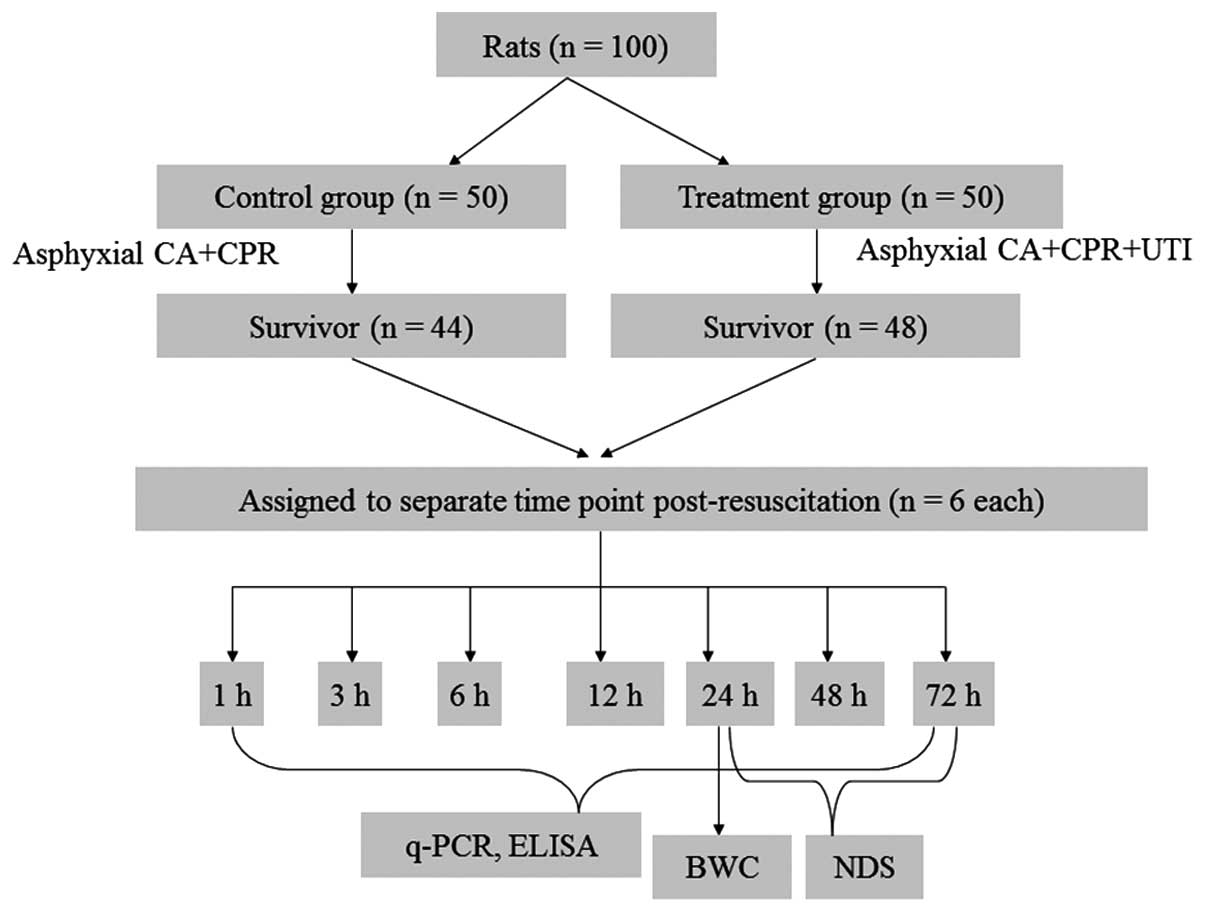
Animal treatments
The study was approved by the Experimental Animal
Protection and Ethics Committee of the Second Artillery General
Hospital (Beijing, China). In total, 100 male Wistar rats (age, 2
months; weight, 250–350 g) were randomly assigned to control and
treatment groups (n=50). After 4 min of asphyxial CA, all the rats
were immediately subjected to CPR. The treatment group animals were
administered 15 mg/kg UTI (Techpool Bio-Pharma, Guangdong, China)
at the onset of resuscitation (Fig.
1).
Establishment of a rat model of CPR
following asphyxial CA
Asphyxial CA was induced as previously described
(12–16). Rats in the two groups were
anesthetized with 30 mg/kg chloral hydrate, orally intubated and
mechanically ventilated using a small-animal ventilator. The tidal
volume, respiratory rate, inspiratory/expiratory ratio (I/E) and
the fraction of inspired oxygen were set at 8 ml/kg, 40
breaths/min, 1/2 and 1.0, respectively. Next, the rats were
intravenously injected with 2 mg/kg vecuronium bromide to prevent
spontaneous respiration. The rectal temperature was maintained at
37.5±0.3°C with a heating lamp during the induction of CA. The
femoral vein and artery were cannulated in order to provide fluid
and medications and to monitor the arterial pressure, heart rate
(HR) and arterial blood gas.
Baseline physiological variables were recorded
continuously for 10 min following surgery. CA was defined as a
reduction in the mean arterial pressure (MAP) to a value of <10
mmHg after ventilation had been discontinued for ~3 min. After 1
min of CA, CPR was performed with continuous ventilation and
external cardiac compressions at a rate of 200/min, along with an
intravenous injection of 0.01 mg/kg epinephrine and 1 mmol/kg
sodium bicarbonate. Successful CPR was defined as an achievement of
ROSC, indicated by a rise in the MAP to a value of >50 mmHg. CPR
was considered to have failed if no sign of ROSC was observed
during the 5 min post-resuscitation.
Following resuscitation, the rats were mechanically
ventilated with an increased I/E ratio of 1/1.5 and treated with a
continuous infusion of 8 ml/h lactated Ringer’s solution until the
restoration of adequate spontaneous respiration. The rats were then
weaned from the ventilator, extubated and observed for 10 min. This
was followed by arteriovenous ligation and skin sutures if there
were no abnormalities observed in the circulation and respiration.
Following restoration in an oxygen tank for 30 min, the rats were
finally returned to their respective cages with access to food and
water ad libitum.
Determination of the mortality rate
The mortality rate in the two groups was recorded at
24 h post-resuscitation.
Assessment of the circulatory
function
MAP and HR were determined for all the animals
during the first 60 min post-resuscitation. The time to CA (TCA)
and time to ROSC (TROSC) were also recorded. The TCA was defined as
the time from the initiation of ventilation to the onset of CA,
while the TROSC was defined as the time from the start of
resuscitation to ROSC.
Sample collection
At 1, 3, 6, 12, 24, 48 and 72 h post-resuscitation,
all the rats were decapitated, and their brains quickly removed and
placed on ice for dissection. Following washing in ice-cold normal
saline, the surface water on the organs was gently blotted off with
filter paper. Left hemisphere brain tissues were stored in liquid
nitrogen, until required for the analysis of TLR4 mRNA expression
and NF-κB, TNF-α and IL-6 protein levels.
Determination of brain water content
(BWC)
A small sample of brain tissue (~50 mg) from the
right hemisphere was extracted at 24 h post-resuscitation, as
described previously. The brain tissues were immediately weighed on
an electronic analytical balance, dried at 105°C for 24 h and
weighed again. The BWC was calculated as follows: (wet weight - dry
weight)/wet weight × 100%.
Neurological assessment
Neurological function was evaluated in the rats at
24, 48 and 72 h post-resuscitation using a neurological deficit
scale (NDS) scoring system (score range, 0–100; score 0, normal;
score 100, brain dead; Table I)
(13,14).
 | Table IModified NDS scores. |
Table I
Modified NDS scores.
| Parameter | Points | Maximum points |
|---|
| Arousal | | 19 |
| Alerting
(normal/stuporous/comatose) | 10/5/0 | |
| Eye opening (open
spontaneously/open to pain/absent) | 3/1/0 | |
| Spontaneous
respiration (normal/abnormal/absent) | 6/0/0 | |
| Brainstem
function | | 21 |
| Olfaction
(present/weak/absent) | 3/1/0 | |
| Vision
(present/weak/absent) | 3/1/0 | |
| Papillary light
reflex (present/weak/absent) | 3/1/0 | |
| Corneal reflex
(present/weak/absent) | 3/1/0 | |
| Startle reflex
(present/weak/absent) | 3/1/0 | |
| Whisker stimulation
(present/weak/absent) | 3/1/0 | |
| Swallowing
(present/weak/absent) | 3/1/0 | |
| Motor assessment
(each side tested and scored separately) | | 6 |
| Strength
(normal/weak movement/no movement) | 3/1/0 | |
| Sensory assessment
(each side tested and scored separately) | | 6 |
| Pain (brisk
withdrawal/weak withdrawal/no movement) | 3/1/0 | |
| Motor behavior | | 6 |
| Gait coordination
(normal/abnormal/absent) | 3/1/0 | |
| Balance during
walking (normal/abnormal/absent) | 3/1/0 | |
| Behavior | | 12 |
| Righting reflex
(normal/abnormal/absent) | 3/1/0 | |
| Negative geotaxis
(normal/abnormal/absent) | 3/1/0 | |
| Visual placing
(normal/abnormal/absent) | 3/1/0 | |
| Turning alley
(normal/abnormal/absent) | 3/1/0 | |
| Seizures (no
seizure/focal seizure/generalized seizure) | 10/5/0 | 10 |
| Feeding
(normal/abnormal/absent) | 10/5/0 | 10 |
| Grooming
(normal/abnormal/absent) | 10/5/0 | 10 |
Isolation of total RNA and cDNA
synthesis
Total RNA was extracted from the brain tissue
specimens of rats using a TriPure isolation reagent (Roche
Diagnostics, Mannheim, Germany), according to the manufacturer’s
instructions. Briefly, fresh or frozen tissues (~100 mg) were
homogenized in 1 ml TriPure reagent and submitted to centrifugation
at 21,578 × g for 10 min at 4°C. Following the addition of 0.2 ml
chloroform to the supernatants, the mixture was incubated at room
temperature for 20 min with occasional stirring. The samples were
then submitted to centrifugation again, as aforementioned. The
resulting supernatants were mixed with 0.5 ml isopropanol,
incubated for 5–10 min and centrifuged as aforementioned. The
pellet containing total RNA was washed with 1 ml ethanol (75%) and
centrifuged at 8,429 × g for 5 min at 4°C. The RNA pellet was air
dried, dissolved in 3 ml diethylpyrocarbonate-treated water and the
optical density (OD) values were measured at 260 and 280 nm on a
Lambda 20 UV/Vis spectrophotometer (Perkin-Elmer, Norwalk, CT,
USA). The RNA concentration was calculated as follows: RNA
concentration (μg/ml) = OD260 × dilution factor ×
40/1,000. RNA purity was assessed by the
OD260/OD280 ratio.
Detection of TLR4 mRNA expression
levels
TLR4 gene expression was evaluated using
quantitative polymerase chain reaction (qPCR). Plasmids of TLR4 and
the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), were obtained at 3.67 and 2.67×1010 copies/μl,
respectively, and diluted with sterile water to final
concentrations of 1×106, 1×105,
1×104 and 1×103 copies/μl. The qPCR mixture
(25 μl) contained 1X PCR buffer, 4 mM MgCl2, 0.4 μM each
primer, 0.4 μM TaqMan probe, 0.5 units AmpErase Uracil
N-Glycosylase, 0.5 units AmpliTaq Gold DNA polymerase, 50 ng
cDNA, double distilled water, 0.2 mM dUTP and 0.1 mM dATP, dGTP and
dCTP.
qPCR was conducted using an ABI Prism 7500 SDS
RT-PCR system (Applied Biosystems, Foster City, CA, USA). The
reaction conditions for the amplification of TLR4 and GAPDH were 40
cycles of 50°C for 2 min, 95°C for 2 min, 95°C for 15 sec and 60°C
for 60 sec. The amplified products for TLR4 and GAPDH were 122 and
138 bp in size, respectively. The primers and fluorescent probes
used for qPCR were as follows: TLR4 forward,
5′-TGAGAAACGAGCTGGTAAAGAATT-3′, reverse,
5′-GTGGAAGCCTTCCTGGATGATG-3′; and probe,
5′-AGTGCCCCGCTTTCAGCTTTGCCT-3′; GAPDH forward,
5′-CATGACCTTCCGTGTTCCTACC-3′, reverse, 5′-TAGCCCAGGATGCCCTTCAG-3′
and probe, 5′-CCTCAGACGCCTGCTTCACCACCT-3′.
The threshold level was set at 10-times the standard
deviation (SD) of the baseline fluorescence measured during PCR
cycles 3–15. The threshold cycle (Ct) was defined as the cycle
number at which fluorescence passed the fixed threshold, normalized
against GAPDH. Standard curves of the Ct values (serial dilutions
of the control plasmids plotted against the logarithm of the copy
numbers) were constructed for TLR4 and GAPDH. The correlation
coefficients of the standard curves for TLR4 and GAPDH were 0.986
and 0.971, respectively. The absolute copy numbers of TLR4 mRNA in
each unknown sample were then calculated based on the Ct values.
The GAPDH mRNA copy number in each sample was used to normalize
TLR4 mRNA expression levels.
Extraction of nucleoproteins from the
brain tissues
Nucleoproteins were extracted from the brain tissues
using a nucleoprotein extraction kit (Active Motif, Carlsbad, CA,
USA). Briefly, ~200 mg tissue samples were homogenized in 2 ml
nuclear extraction buffer (NEB) A. The homogenates were then
centrifuged at 599 × g for 30 sec, and the supernatants containing
the complete nuclei were incubated on ice for 5 min. Following
centrifugation at 3,746 × g for 10 min at 4°C, the nuclear
precipitates (pellet) were resuspended in 150 μl NEB B and
incubated for 30 min on ice. A final centrifugation at 29,371 × g
for 1 min at 4°C yielded nucleoproteins in the supernatants, which
were stored at −70°C until required for the assessment of protein
concentrations using the Coomassie Brilliant Blue G-250 method
(8).
Detection of NF-κB activity
NF-κB activity levels in the nuclear extracts were
detected by a specific high-sensitive enzyme-linked immunosorbent
assay (ELISA), using a TransAM NF-κB detection kit (Active Motif),
according to the manufacturer’s instructions. Antibodies were used
at a 1:1,000 dilution in antibody binding buffer and incubations
were performed at room temperature for 1 h. The reactions were
visualized using 100 μl tetramethylbenzidine (TMB) chromogenic
substrate for 10 min in the dark, and were stopped following the
addition of 100 μl stop solution. Absorbance was measured
immediately at 450 nm on a microplate reader.
Detection of TNF-α and IL-6 levels
TNF-α and IL-6 levels were assessed using specific
ELISA kits (Sigma-Aldrich, St. Louis, MO, USA). Briefly, samples or
standards (2,000, 1,000, 500, 250 and 125 pg/ml) were added to the
wells. Following incubation for 1 h at 37°C, and four washes in 350
μl washing solution, the plates were incubated in the presence of
100 μl biotin antibodies for 1 h at 37°C. Next, the plates were
washed and incubated with 100 μl enzyme binding buffer for 30 min
at 37°C. The reactions were visualized using 100 μl TMB chromogenic
substrate for 15–20 min in the dark, and stopped following the
addition of 100 μl stop solution. Absorbance was measured within 15
min at 450 nm on a microplate reader. Based on the measured OD
values, the protein levels of TNF-α and IL-6 were determined using
the corresponding standard curves.
Statistical analysis
Statistical analyses were performed using SPSS
statistical software version 12.0 (SPSS, Inc., Chicago, IL, USA).
All the data are expressed as the mean ± SD. The Student’s t-test
was used to compare differences between the two groups, where
P<0.05 was considered to indicate a statistically significant
difference, and P<0.01 indicated a higher degree of statistical
significance.
Results
Baseline characteristics
No statistically significant differences were
observed in the baseline characteristics, including the body
weight, HR, MAP and NDS, between the two groups (data not
shown).
Analysis of the mortality rate, TCA,
TROSC and BWC
As shown in Table
II, at 24 h post-resuscitation, six and two mortalities were
recorded in the control and treatment groups, respectively; a
statistically significant difference was observed in the mortality
rates (P<0.01). TROSC values were significantly higher in the
control animals as compared with the treatment group (99.3±16.6 vs.
73.9±22.4 sec; P<0.01). In addition, the BWC was markedly
reduced following treatment with UTI when compared with the control
rats (77.33±4.55 vs. 84.06±1.83; P<0.01). However, no
statistically significant difference in the TCA was observed
between the control and treatment groups (191.1±20.8 vs. 190.3±19.6
sec; P>0.05).
 | Table IIComparison of mortality rates, TCA,
TROSC, BWC and NDS scores between the control and treatment
groups. |
Table II
Comparison of mortality rates, TCA,
TROSC, BWC and NDS scores between the control and treatment
groups.
| Variable | Treatment
group | Control group |
|---|
| Mortality rate,
(n=50) | 2/50 (4)a | 6/50 (12) |
| TCA, sec
(n=50) | 190.3±19.6 | 191.1±20.8 |
| TROSC, sec
(n=42) | 73.9±22.4a | 99.3±16.6 |
| BWC (n=6) | 77.33±4.5 a | 84.06±1.83 |
| NDS (n=6) |
| 24 h | 69.44±6.3a | 54.38±6.8 |
| 48 h | 73.77±5.69a,b | 63.44±6.48b |
| 72 h | 88.55±5.66a,c | 70.44±8.62c |
Evaluation of neurological function using
the NDS scores
Neurological data are summarized in Table II. The NDS scores were similar at
the baseline in the two groups of rats. At 24, 48 and 72 h
post-resuscitation, the NDS scores were significantly higher in the
treatment group when compared with control animals (P<0.01). In
addition, a statistically significant increase was observed in the
NDS scores at 48 h post-resuscitation when compared with the values
obtained at 24 h for each group (P<0.05); the trend continued to
72 h.
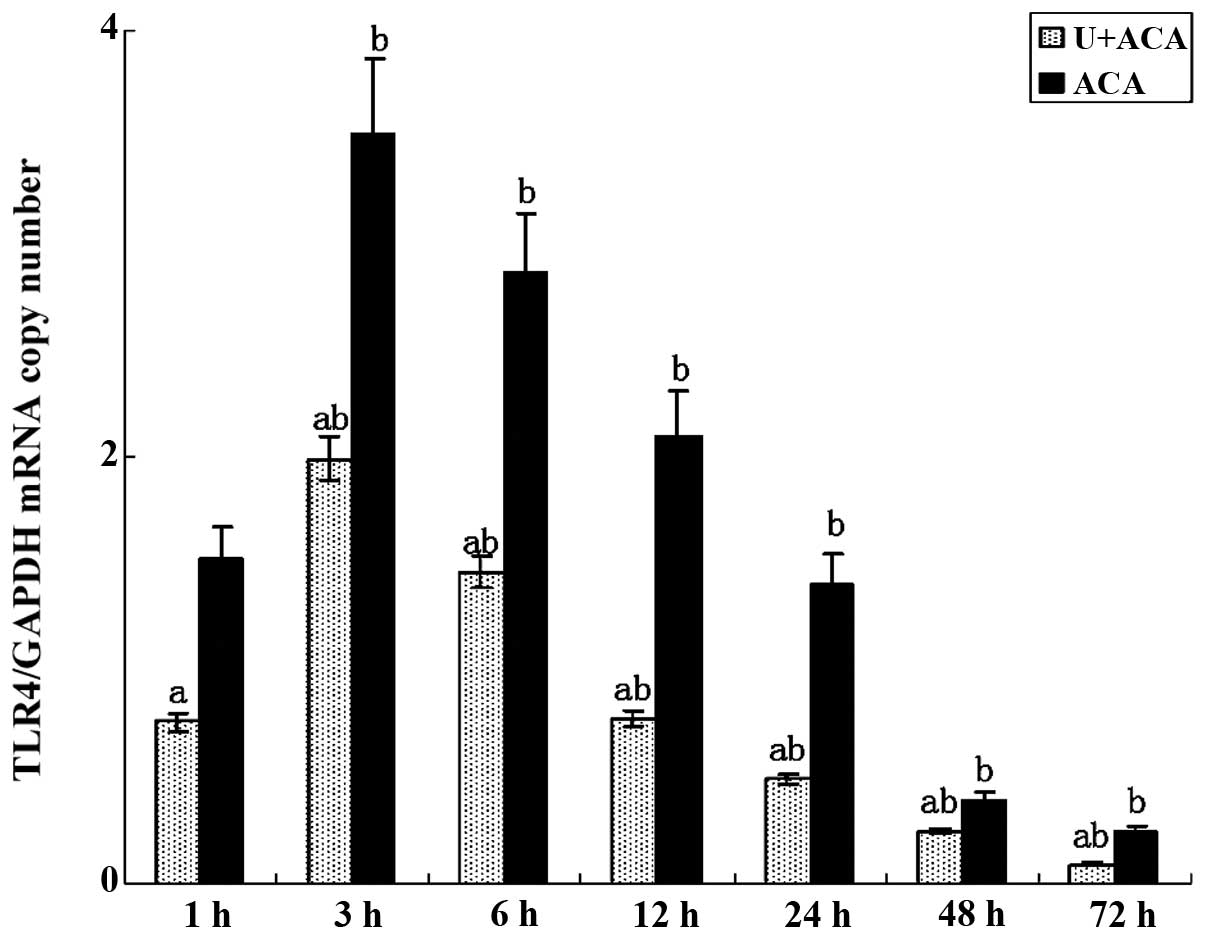
Effect of UTI on TLR4 gene expression
levels
According to the qPCR results (Fig. 2), TLR4 mRNA expression was detected
in the brain tissues collected from all the animals at 1 h after
resuscitation. Peak expression was observed between 3 and 6 h,
prior to markedly decreasing after 12 h, although expression
remained detectable at 72 h. Notably, TLR4 gene expression was
significantly reduced in the treatment group as compared with the
control rats at each time point (P<0.05).
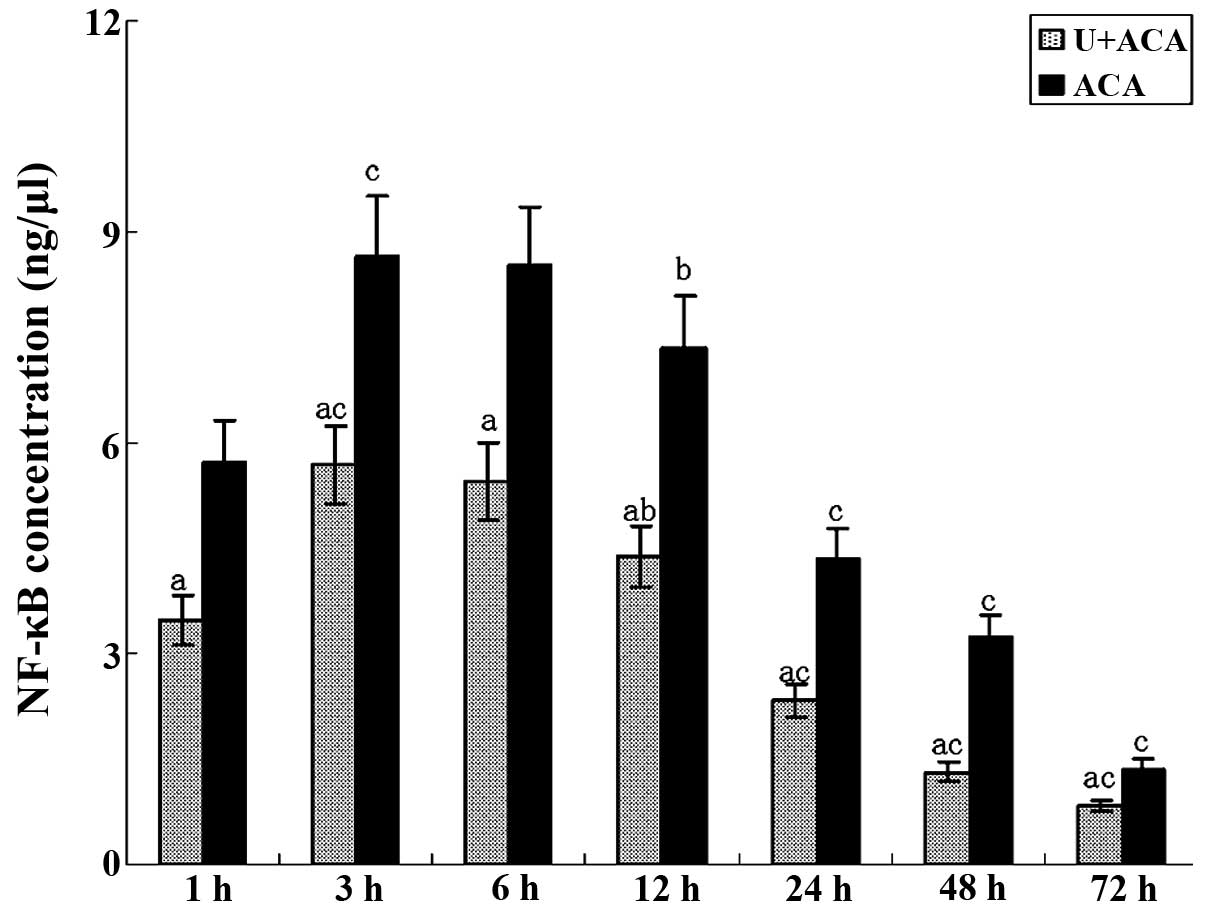
Evaluation of NF-κB activity levels
As shown in Fig. 3,
NF-κB activity was detected at 1 h post-resuscitation in the two
groups. The values increased and peaked between 3 and 6 h. At 24 h,
NF-κB activity was significantly reduced and continued to decrease
further, although a degree of activity was detected at 72 h.
Notably, a significant reduction in the NF-κB activity levels was
observed in the treatment group at each time point when compared
with the control animals (P<0.05). NF-κB activity levels were
consistent with the trends observed for TLR4 mRNA expression.
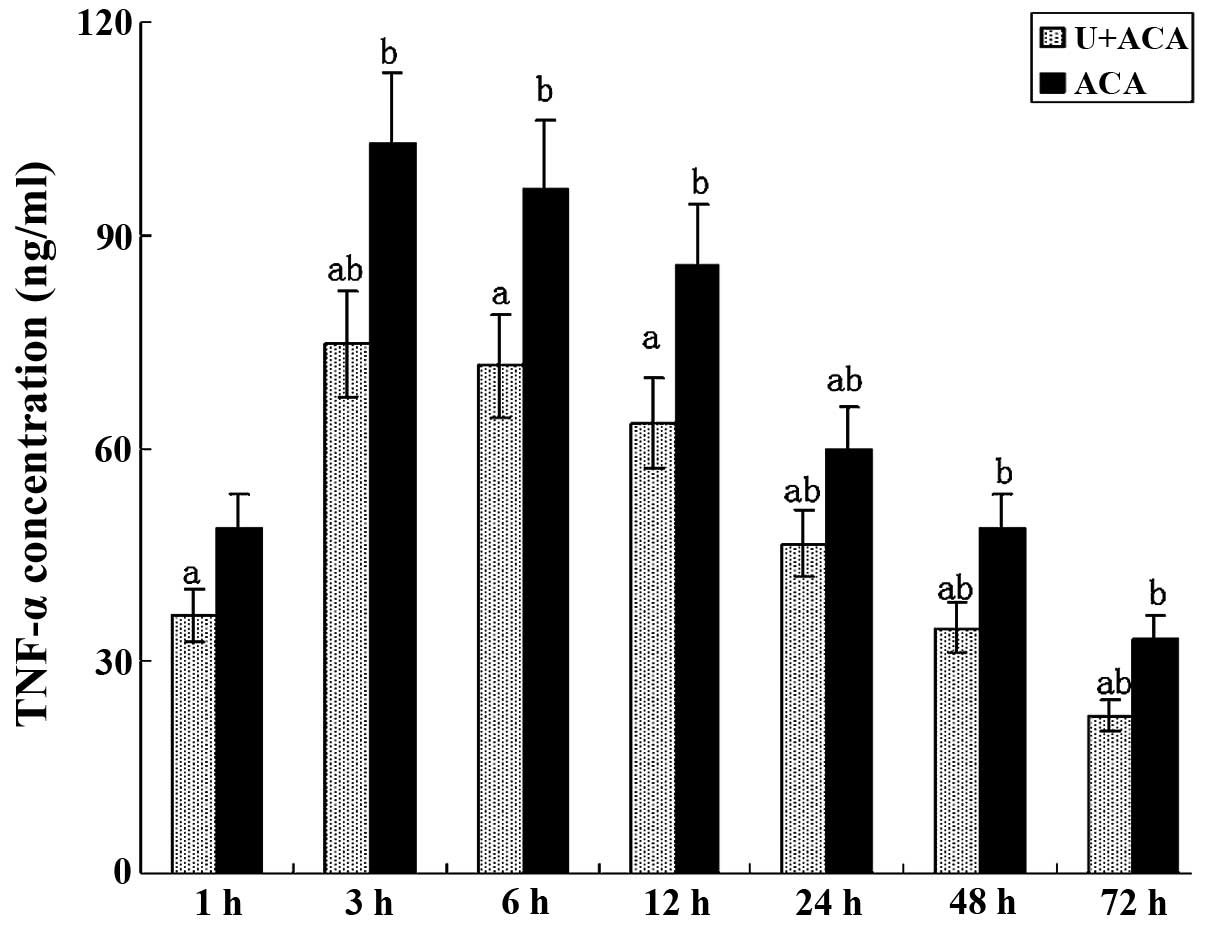
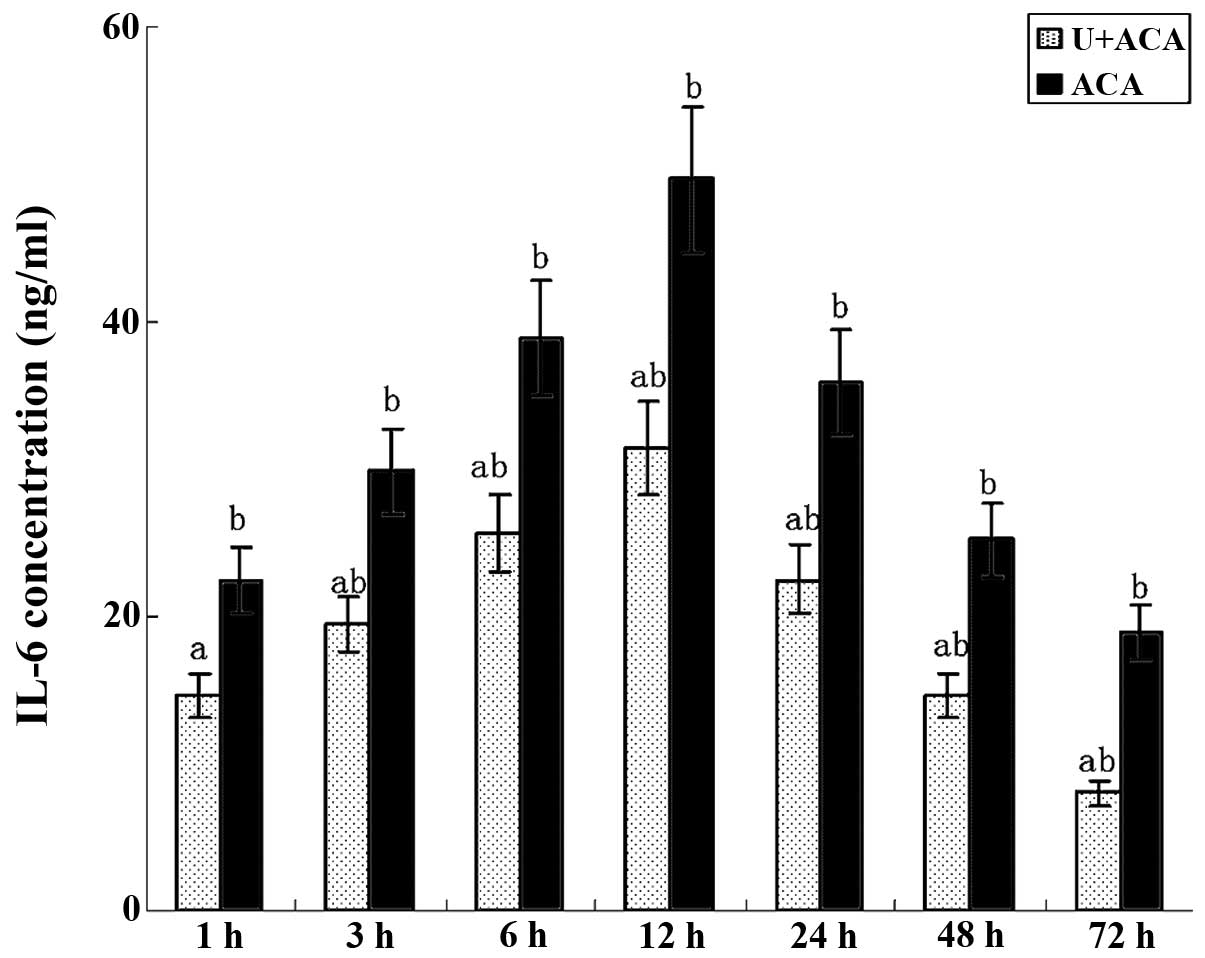
Effect of UTI on TNF-α and IL-6
production
As shown in Figs. 4
and 5, the ELISA results revealed
changes in the cytokine levels similar to those observed for TLR4
gene expression and NF-κB activity. TNF-α and IL-6 were readily
secreted in the brain cells at 1 h post-resuscitation. TNF-α levels
peaked at 3 h and remained high prior to a significant decrease
observed at 24 h (Fig. 4). IL-6
levels reached the highest value at 12 h and gradually decreased
thereafter (Fig. 5). Minimal
production of TNF-α and IL-6 was observed at 72 h
post-resuscitation. Notably, significantly lower levels of TNF-α
and IL-6 were observed in the UTI-treated animals at each time
point when compared with the control group (P<0.05).
Discussion
Patients that are successfully resuscitated from CA
often experience neurological complications, including cognitive
impairment and motor deficit, which potentially incapacitate the
individuals and may result in mortality (2,3).
Thus, providing an effective regimen for neurological injury
attenuation following CPR is important. The TLR4 inflammatory
signaling pathway has previously been reported to play a critical
role in brain injury following CPR, since brain injury has been
shown to be alleviated by inhibiting the TLR4 signaling pathway via
hypoxic preconditioning (7).
Therefore, hypoxia-based strategies have been widely used to
investigate the mechanisms involved in the pathogenesis of cerebral
ischemic injury. However, since the onset of CA is unpredictable,
hypoxic preconditioning is not applicable for patients experiencing
CA. UTI, a urinary trypsin inhibitor, has been shown to possess
anti-inflammatory properties and attenuate LPS-induced acute lung
injury (10), inhibit systemic
inflammatory responses resulting from pulmonary I/R (11) and alleviate pulmonary I/R injury
via the inhibition of TNF-α expression in rats (17). In addition, previous studies have
demonstrated that UTI suppresses the elevation of IL-6 and IL-8
levels in patients undergoing coronary artery bypass grafting under
extracorporeal circulation (18),
alleviates forebrain I/R injury by inhibiting the production of
superoxide radicals and intercellular adhesion molecule-1 (19) and improves oleic acid-induced acute
lung injury by reducing TNF-α levels and inducing leukocyte
activation (20). In addition, UTI
has been proposed as a therapeutic option for endotoxin-associated
inflammatory disorders, including acute lung and liver injuries
(21), due to its
anti-inflammatory properties (22–24).
However, whether UTI protects against brain injury by inhibiting
the inflammatory responses following CA is yet to be elucidated.
Therefore, a prospective rat study was conducted to evaluate the
role of UTI. Compared with the control group, the mortality rate
was significantly reduced following UTI treatment at 24 h after
resuscitation from CA. In addition, the NDS scores were markedly
improved in the treatment group when compared with the control
animals at 24, 48 and 72 h post-resuscitation.
TLR4 is a pattern recognition receptor that
predominantly recognizes endotoxins. A previous study demonstrated
that TLR4 can bind to various exogenous ligands, including heat
shock proteins, protein fragments from the extracellular matrix,
hyaluronan, heparitin sulfate, vascular fibrin monomer-fibrinogen
complex and phylaxin and elastase released from immune cells
(25). The suppression of
exogenous substance release by UTI may contribute to the inhibitory
effects on TLR4 expression. However, further studies are required
to clarify these possible mechanisms.
Once TLR4 is activated, the interaction of the TLR4
intracellular domains with the adapter protein, MyD88, promotes the
expression of IL-1 receptor-associated kinase and TNF
receptor-associated factor 6, which then upregulate NF-κB.
Activated NF-κB further stimulates the expression of various
inflammatory cytokines, including TNF-α, IL-1 and IL-6, resulting
in inflammation (26). TNF-α and
IL-6 are the most important cytokines regulated by NF-κB and are
considered to play a central role in the development of
inflammatory diseases. TNF-α levels have been shown to positively
correlate with mortality rates, with high levels inducing
hypotension, tissue injury and consequently mortality in animals
(27). In addition, IL-6 plasma
levels have been demonstrated to be closely associated with
survival and mortality (28).
Based on these observations, the present study was designed to
detect the changes in the levels of NF-κB, TNF-α and IL-6 in a rat
model of CPR following asphyxial CA. Significant elevations in the
levels of TLR4 gene expression and NF-κB activity were observed, as
well as markedly increased levels of TNF-α and IL-6 in the brains
tissues of rats resuscitated from CA at each time point. These
observations indicate that UTI treatment at the onset of CPR
significantly suppresses the TLR4/NF-κB signaling cascade following
resuscitation, thereby alleviating the inflammatory responses in
the brain. The overt reduction in the mortality rate and the
improvement in the NDS scores were likely to have resulted from UTI
exerting anti-inflammatory properties in the brain tissues.
In conclusion, UTI was found to alleviate brain
injury following CPR by inhibiting the TLR4 signaling pathway and
reducing the release of inflammatory cytokines; thus, exerting
anti-inflammatory effects.
Acknowledgements
This study was supported by Beijing Municipal
Natural Science Foundation (No. 7142169).
References
|
1
|
Cobb LA, Fahrenbruch CE, Olsufka M and
Copass MK: Changing incidence of out-of-hospital ventricular
fibrillation, 1980–2000. JAMA. 288:3008–3013. 2002.PubMed/NCBI
|
|
2
|
de Vreede-Swagemakers JJ, Gorgels AP,
Dubois-Arbouw WI, et al: Out-of-hospital cardiac arrest in the
1990’s: a population-based study in the Maastricht area on
incidence, characteristics and survival. J Am Coll Cardiol.
30:1500–1505. 1997.
|
|
3
|
Peters R and Boyde M: Improving survival
after in-hospital cardiac arrest: the Australian experience. Am J
Crit Care. 16:240–247. 2007.PubMed/NCBI
|
|
4
|
Adrie C, Adib-Conquy M, Laurent I, et al:
Successful cardiopulmonary resuscitation after cardiac arrest as a
‘sepsis-like’ syndrome. Circulation. 106:562–568. 2002.
|
|
5
|
Neumar RW, Nolan JP, Adrie C, et al:
Post-cardiac arrest syndrome: epidemiology, pathophysiology,
treatment, and prognostication. A consensus statement from the
International Liaison Committee on Resuscitation (American Heart
Association, Australian and New Zealand Council on Resuscitation,
European Resuscitation Council, Heart and Stroke Foundation of
Canada, InterAmerican Heart Foundation, Resuscitation Council of
Asia, and the Resuscitation Council of Southern Africa); the
American Heart Association Emergency Cardiovascular Care Committee;
the Council on Cardiovascular Surgery and Anesthesia; the Council
on Cardiopulmonary, Perioperative, and Critical Care; the Council
on Clinical Cardiology; and the Stroke Council. Circulation.
118:2452–2483. 2008.
|
|
6
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar
|
|
7
|
Li YW, Jin HL, Wang BG, et al: Toll-like
receptor 4 signal pathway may be involved in cerebral ischemic
tolerance induced by hypoxic preconditioning: experiment with rats.
Zhonghua Yi Xue Za Zhi. 87:2458–2462. 2007.(In Chinese).
|
|
8
|
Caso JR, Pradillo JM, Hurtado O, et al:
Toll-like receptor 4 is involved in brain damage and inflammation
after experimental stroke. Circulation. 115:1599–1608. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu L, Ren B, Li M, et al: Ulinastatin
suppresses systemic inflammatory response following lung
ischemia-reperfusion injury in rats. Transplant Proc. 40:1310–1311.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bae HB, Jeong CW, Li M, et al: Effects of
urinary trypsin inhibitor on lipopolysaccharide-induced acute lung
injury in rabbits. Inflammation. 35:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ueki M, Taie S, Chujo K, et al: Urinary
trypsin inhibitor reduces inflammatory response in kidney induced
by lipopolysaccharide. J Biosci Bioeng. 104:315–320. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao F, Pardue S, Arnold T, et al: Effect
of ifenprodil, a polyamine site NMDA receptor antagonist, on brain
edema formation following asphyxial cardiac arrest in rats.
Resuscitation. 61:209–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geocadin RG, Malhotra AD, Tong S, et al:
Effect of acute hypoxic preconditioning on qEEG and functional
recovery after cardiac arrest in rats. Brain Res. 1064:146–154.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geocadin RG, Ghodadra R, Kimura T, et al:
A novel quantitative EEG injury measure of global cerebral
ischemia. Clin Neurophysiol. 111:1779–1787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCaul CL, McNamara PJ, Engelberts D, et
al: Epinephrine increases mortality after brief asphyxial cardiac
arrest in an in vivo rat model. Anesth Analg. 102:542–548. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fink EL, Alexander H, Marco CD, et al:
Experimental model of pediatric asphyxial cardiopulmonary arrest in
rats. Pediatr Crit Care Med. 5:139–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren B, Wu H, Zhu J, et al: Ulinastatin
attenuates lung ischemia-reperfusion injury in rats by inhibiting
tumor necrosis factor alpha. Transplant Proc. 38:2777–2779. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakanishi K, Takeda S, Sakamoto A and
Kitamura A: Effects of ulinastatin treatment on the cardiopulmonary
bypass-induced hemodynamic instability and pulmonary dysfunction.
Crit Care Med. 34:1351–1357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koga Y, Fujita M, Tsuruta R, et al:
Urinary trypsin inhibitor suppresses excessive superoxide anion
radical generation in blood, oxidative stress, early inflammation,
and endothelial injury in forebrain ischemia/reperfusion rats.
Neurol Res. 32:925–932. 2010. View Article : Google Scholar
|
|
20
|
Ito K, Mizutani A, Kira S, et al: Effect
of Ulinastatin, a human urinary trypsin inhibitor, on the oleic
acid-induced acute lung injury in rats via the inhibition of
activated leukocytes. Injury. 36:387–394. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inoue K and Takano H: Urinary trypsin
inhibitor as a therapeutic option for endotoxin-related
inflammatory disorders. Expert Opin Investig Drugs. 19:513–520.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka R, Fujita M, Tsuruta R, et al:
Urinary trypsin inhibitor suppresses excessive generation of
superoxide anion radical, systemic inflammation, oxidative stress,
and endothelial injury in endotoxemic rats. Inflamm Res.
59:597–606. 2010. View Article : Google Scholar
|
|
23
|
Wang X, Xue Q, Yan F, et al: Ulinastatin
as a neuroprotective and anti-inflammatory agent in infant piglets
model undergoing surgery on hypothermic low-flow cardiopulmonary
bypass. Paediatr Anaesth. 23:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu CE, Zhang MY, Zou CW and Gou L:
Evaluation of the pharmacological function of ulinastatin in
experimental animals. Molecules. 17:9070–9080. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karikó K, Weissman D and Welsh FA:
Inhibition of Toll-like receptor and cytokine signaling - a
unifying theme in ischemic tolerance. J Cereb Blood Flow Metab.
24:1288–1304. 2004.PubMed/NCBI
|
|
26
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raeburn CD, Sheppard F, Barsness KA, et
al: Cytokines for surgeons. Am J Surg. 183:268–273. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song M and Kellum JA: Interleukin-6. Crit
Care Med. 33(12 Suppl): S463–S465. 2005. View Article : Google Scholar : PubMed/NCBI
|