Introduction
Telomerase is a special type of RNA nuclear protease
that maintains the function of the telomere and has an important
role in the immortalization of cells, as well as the genesis and
progression of cancer (1,2). Telomerase is an RNA-dependent DNA
polymerase that synthesizes telomeric DNA sequences, which provide
tandem GT-rich repeats (TTAGGG) that compensate telomere shortening
and have an important role in cellular aging and carcinogenesis.
Human telomerase usually consists of three subunits: human
telomerase RNA (hTR), human telomerase associated protein 1 (TEP1)
and human telomerase reverse transcriptase (hTERT). The hTERT gene
is the most important regulator of telomerase activity (3).
Previous studies have demonstrated that telomerase
activity is absent from most normal human somatic cells, but
present in >90% of tumor cells and immortalized cells (4–6).
Numerous studies have shown that antisense gene therapy directed
against telomerase RNA or hTERT components may effectively inhibit
telomerase activity and induce apoptosis in gastric cancer,
malignant gliomas, colon cancer and ovarian cancer (7–10).
Previous studies have also demonstrated that the growth of tumor
cells may be inhibited by antisense oligodeoxynucleotides (ASODNs)
that are targeted to the hTERT gene in a wide variety of tumor
types (11,12). The positive rate of telomerase
expression has been found to be ≤90.48% in esophageal carcinoma
tissue, whereas telomerase is not expressed in normal esophageal
tissue and leiomyoma of the esophagus (13).
In the present study, a phosphorothioate antisense
oligodeoxynucleotide (PS-ASODN) against hTERT was used to treat the
human Eca-109 esophageal cancer cell line. The inhibitory effect
and the mechanism of the PS-ASODN were investigated in the
esophageal cancer cells in order to explore novel strategies for
esophageal cancer gene therapy.
Materials and methods
Materials
Human esophageal cancer cells (Eca-109) were
obtained from the Cancer Institute and Hospital (Chinese Academy of
Medical Sciences, Beijing, China). RPMI-1640 medium was purchased
from Gibco-BRL (Gaithersburg, MD, USA) and MTT was obtained from
Sigma (St. Louis, MO, USA). Based on the sequencing of hTERT gene
cDNA, an antisense sequence was designed that was complementary to
the original code sequence and was 15 base pairs in length
(5′-GGAGCGCGCGGCATC-3′). The original code sequence is absent from
all known human genes other than hTERT. In addition, a control
N-ASODN was designed (5′-ACCTGGCACCGGCGG-3′). The hTERT-targeted
antisense oligodexynucleotide (PS-ASODN) and N-ASODN (all
phosphorothioate modified) were provided by Shanghai Biotechnology
Corporation (Shanghai, China). Liposomes (Lipofectin) were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
The semi-quantitative telomerase detection kit was obtained from
the Department of Genetics of Shandong University (Jinan, China),
the microplate reader model 550 was purchased from Bio-Rad
(Hercules, CA, USA) and the polymerase chain reaction (PCR) thermal
cycler was obtained from PerkinElmer (Waltham, MA, USA). The image
scanning and analysis system was obtained from AlphaInnotech
Corporation (San Leandro, CA, USA).
Cell culture and transfection
Eca-109 cells were cultured in RPMI-1640 medium
(5×104 cells/ml, 37°C, 5% CO2 and under
saturated humidity). The medium was changed every other day. Cells
in the logarithmic growth phase were used for transfection.
MTT colorimetric assay
Eca-109 cells were seeded in 96-well plates with 100
μl cell-culture medium in each well at a cell concentration of
5×104/ml. Cells were cultured to the logarithmic growth
phase and PS-ASODN was then added to the experimental group with
final concentrations of 1, 2, 3, 4 and 5 μmol/l, while N-ASODN was
added to the control group at a concentration of 5 μmol/l.
RPMI-1640 cell culture medium was added to the blank control group.
The cells were then cultured at 37°C, 5% CO2 and
saturated humidity. Cells were cultured for various times (1, 5, 10
and 15 days). The cell survival rate was measured using the MTT
colorimetric assay.
The absorbance (A value) was measured at a
wavelength of 570 nm by a microplate reader, with the A value at a
wavelength of 620 nm used as a reference, and the inhibition rate
of the cells was calculated as follows: Inhibition rate (%) = A
value (control group) - A value (test group)/A value (control
group) - A value (blank group) ×100.
Morphological observation
Eca-109 cells were treated with PS-ASODN (1–5
μmol/l) and N-ASODN (5 μmol/l) under culture conditions and the
growth status and morphologic changes of the cells were then
observed using an inverted phase-contrast microscope (Nikon
Corporation, Tokyo, Japan).
Giemsa staining
Initially, 100 μl of conventional trypsin digestion
cells were extracted onto a slide glass and dried up naturally.
Next, Giemsa solution was added diluted by phosphate buffer
solution and stored at room temperature for 3–5 min; The rear side
of the glass was rinsed and after natural drying, the glass was
sealed with resin and covered with coverslips. Finally, it was
observed using an optical microscope (TMS phase contrast
microscope; Nikon Corporation).
Telomeric repeat amplification protocol
(TRAP)-silver staining
Cells were collected following treatment under
various conditions, and the number of living cells was counted
using Trypan blue staining. Approximately 1×104 cells
were obtained and washed using phosphate-buffered saline (PBS). The
cells were centrifuged and the supernatant was discarded. CHAPS
lysis buffer (10 μl) was then added and telomerase was extracted
for the TRAP reaction. The procedure was as follows: the
supernatant (1 μl) was added to a reaction liquid containing the
forward primer (25 μl) and the reaction mixture was incubated at
30°C for 30 min in order to complete the process of telomere
extension mediated by telomerase. Primers (1 μl), Taq enzyme (1 μl)
and proline (20 μl) were then added. A PCR reaction was then
performed under the following conditions: 94°C for 30 sec, 55°C for
30 sec and 72°C for 30 sec for a total of 30 cycles. The forward
primer (TS) sequence was 5′-GGAGCGCGCGGCATC-3′, and the reverse
primer (CX) sequence was 5′-ACCTGGCACCGGCGG-3′. Following PAGE gel
casting, electrophoresis buffer (1X Tris-borate-EDTA) was placed in
the electrophoresis tank. The TRAP amplification reaction products
and loading buffer (containing bromophenol blue and xylenocyanol)
were combined and electrophoresed at 200 V until the bromophenol
blue reached the bottom of the gel and xylenocyanol was ~2 cm from
the bottom. The gel was removed and subjected to dyeing for 10 min
with 0.2% silver nitrite, fixing with alcohol for 5 min and rinsing
with double-distilled water. Images were then captured for data
analysis. The strength of the telomerase activity was indicated by
the number of 6-bp spaced bands and the gray strength of the bands.
The results from the electrophoresis were scanned and stored using
SmartView (Oracle, Redwood City, CA, United States), a biological
electrophoresis image analysis system. The telomerase activity of
the control group was taken as 100, and the relative telomerase
activity was calculated for each of the other groups. The
associations of the concentration and treatment time of the
PS-ASODN with the telomerase activity of the Eca-109 cells were
then analyzed.
Statistical analyses
All data were analyzed using SPSS software, version
11.0 (SPSS, Inc., Chicago, IL, USA). Variance analysis, t-tests and
linear correlation were used for the analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of PS-ASODN on Eca-109 cell
growth
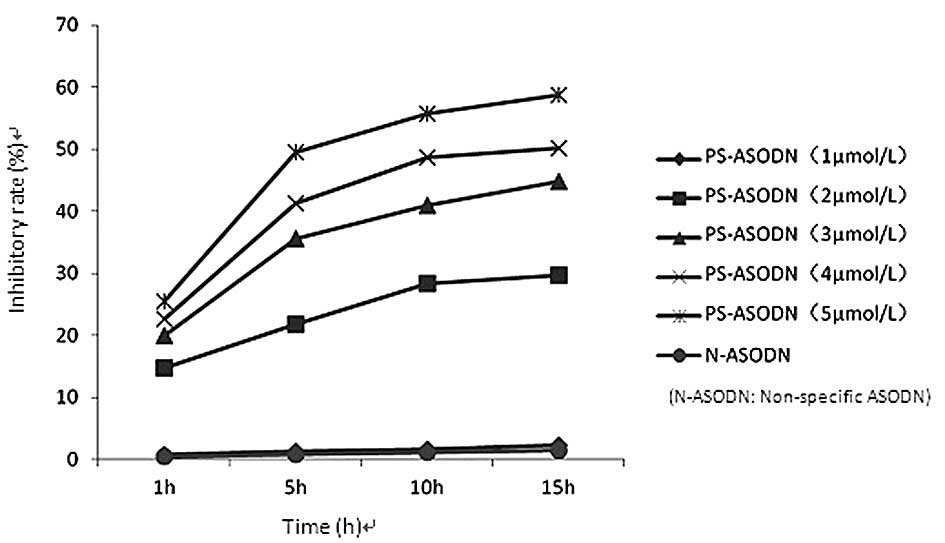
The proliferation of Eca-109 cells was found to be
inhibited following treatment with PS-ASODN (1–5 μmol/l). The
difference in the inhibition rate between the PS-ASODN group and
the blank control group was statistically significant (P<0.05)
when the concentration of PS-ASODN was ≥2 μmol/l, whereas no
significant difference was observed between the N-ASODN group and
blank control group (Table I,
Fig. 1). The inhibition rate
increased with time and as the concentration of PS-ASODN increased,
which indicates that PS-ASODN inhibited the growth of Eca-109 cells
in a concentration-, time- and sequence-specific manner.
 | Table IEffect of a hTERT-targeted PS-ASODN on
Eca-109 cell growth (inhibition ratio, %). |
Table I
Effect of a hTERT-targeted PS-ASODN on
Eca-109 cell growth (inhibition ratio, %).
| Group | Concentration
(μmol/l) | Time (days) |
|---|
|
|---|
| 1 | 5 | 10 | 15 |
|---|
| B | 5 | 0.3 | 0.7 | 1.0 | 1.3 |
| C (N-ASODN) | 5 | 0.4 | 0.8 | 1.1 | 1.4 |
| T1 (PS-ASODN) | 1 | 0.8 | 1.2 | 1.6 | 2.2 |
| T2 (PS-ASODN) | 2 | 14.7a | 21.7a | 28.3a | 29.6a |
| T3 (PS-ASODN) | 3 | 20.0a | 35.6a | 40.9a | 44.8a |
| T4 (PS-ASODN) | 4 | 22.7a | 41.3a | 48.7a | 50.2a |
| T5 (PS-ASODN) | 5 | 25.4a | 49.5a | 55.8a | 58.8a |
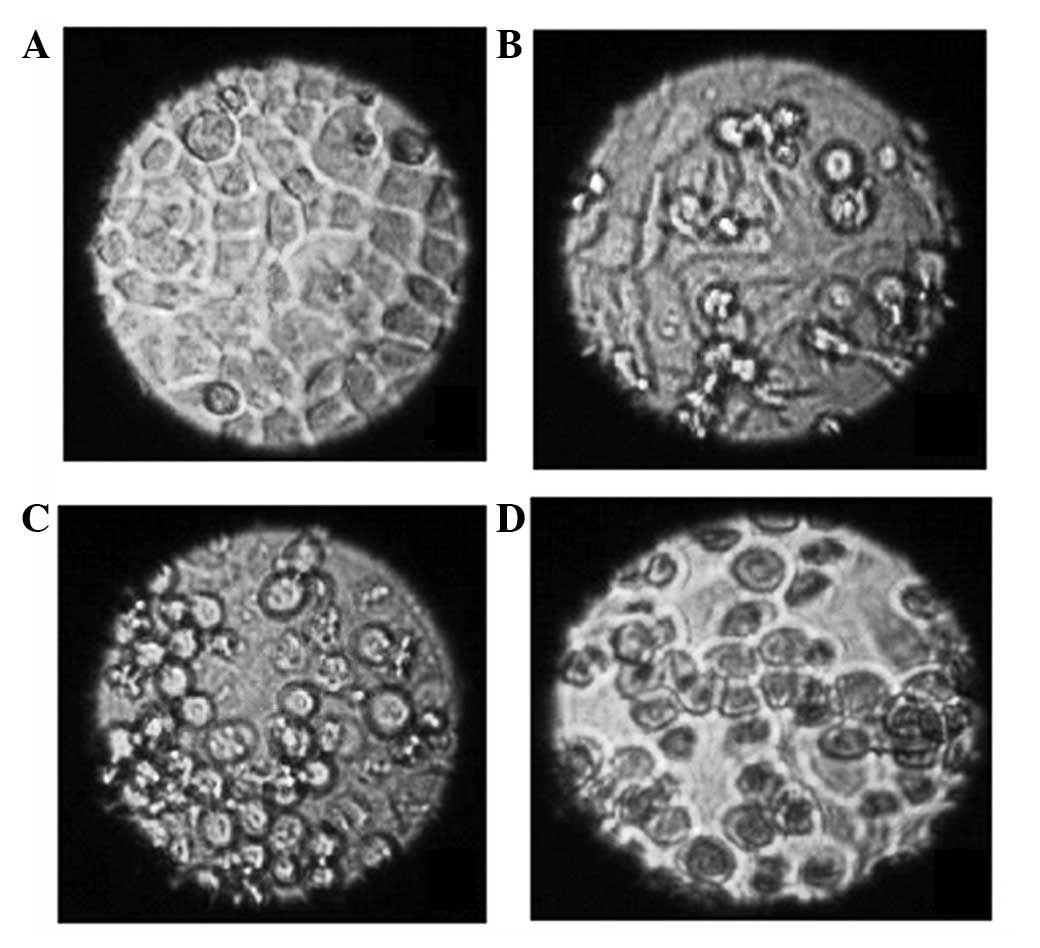
Morphological changes of the cells
Living cells were observed using an inverted
microscope and it was found that cells treated with N-ASODN and
cells in the blank control group were all epithelial and adhered to
the well, the cell outlines were clear, the cells were close
together and proliferation was rapid (Fig. 2A). There was no significant
difference between the treatment group (2 μmol/l PS-ASODN, 24 h)
and control group in morphology; however, cell growth was slower in
the PS-ASODN group (Fig. 2B).
After 10 days, the cells treated with PS-ASODN gradually became
round and began to float in the medium and the number of floating
cells gradually increased with time. Furthermore, certain cells
were reduced in size, chromatin condensation was observed, the
density increased and an increased number of granules was observed
in the cells (Fig. 2C). These
morphological changes were more marked when the concentration of
PS-ASODN was increased to 5 μmol/l, and as the time of treatment
was extended (Fig. 2D).
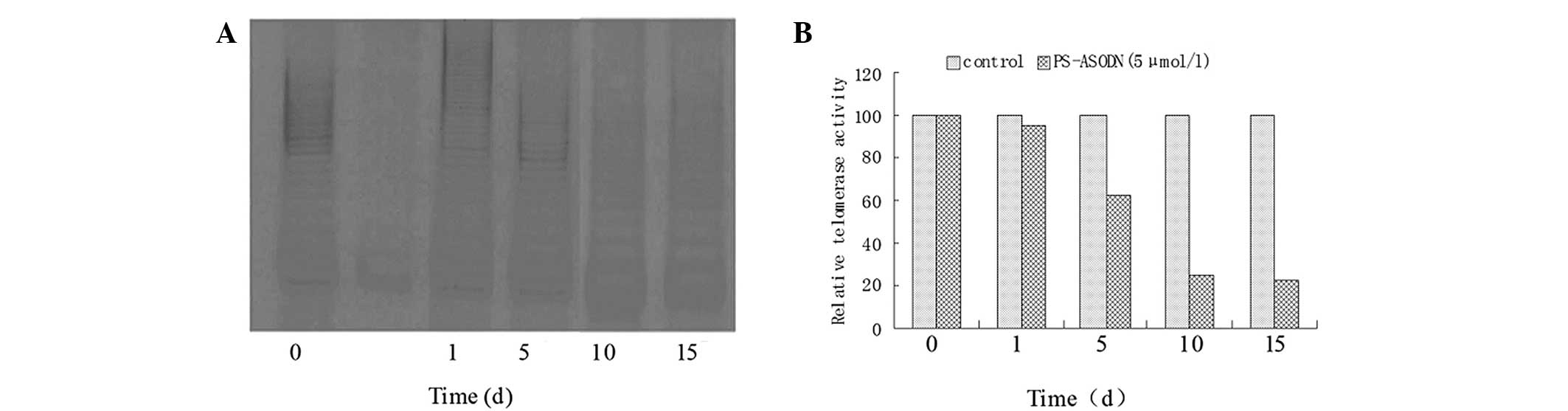
Time-dependent effect of telomerase
regulation by PS-ASODN in Eca-109 cells
The telomerase activity decreased slightly after 24
h of treatment with 5 μmol/l PS-ASODN and the relative telomerase
activity was 95 (vs. 100 at 0 h). After 5 days, the telomerase
activity decreased sharply and the relative telomerase activity was
63 (37% decrease), which was significantly lower compared with the
control group (P<0.05). After 10 and 15 days, the relative
telomerase activity was 25 and 22, respectively (Fig. 3). By contrast, no reduction in the
telomerase activity was observed in cells following treatment with
5 μmol/l N-ASODN (data not shown).
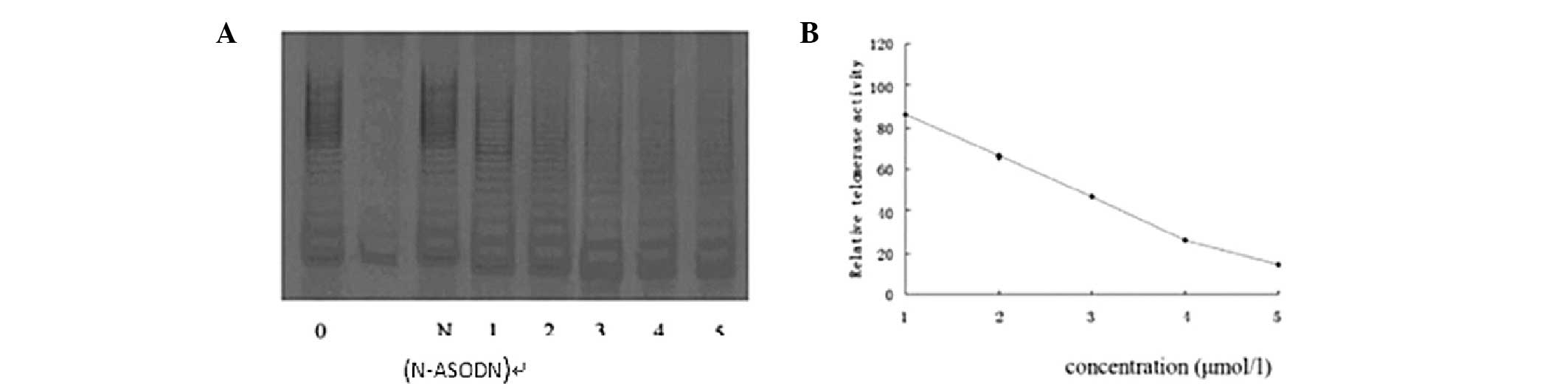
Dose-effect association of telomerase
regulation by PS-ASODN in Eca-109 cells
After 10 days, the relative telomerase activity was
86, 66, 47, 26 and 14, respectively, when Eca-109 cells were
treated with 1, 2, 3, 4 and 5 μmol/l PS-ASODN. The telomerase
activity declined by 14, 34, 53, 74 and 86% accordingly. This
indicates that as the concentration of PS-ASODN increased, the
inhibitory effect on Eca-109 cells increased, which indicates that
PS-ASODN acts in a concentration-dependent manner (Fig. 4).
Discussion
High telomerase activity has been observed in
malignant tumors; however, in the majority of normal tissues (with
the exception of germ cells, hematopoietic cells and certain stem
cells that have proliferation potential) and benign tumors,
telomerase activity has been found to be low (14). The activation of telomerase is
thought to be a final shared pathway for cell malignant
transformation or immortalization; therefore, telomerase is
considered to be an ideal target for gene therapy. Telomerase
consists of 3 subunits, hTR, hTERT and TEP1. hTERT is a single copy
gene that has recently been cloned and whose gene group contains a
unique, highly conserved sequence. Previous studies have
demonstrated that hTERT mRNA is only expressed in malignant tumors,
tumor cells or certain highly potential auto-regenerated tissues
(for example, the endometrium), and the expression of hTERT mRNA
has been found to be correlated with telomerase activity. hTERT has
been demonstrated to be specifically expressed in cancers and it
has been shown to regulate the rate of telomerase activity
(15). ASODNs have gained an
increasing amount of attention recently due to their high
specificity and targeting and low toxicity, and may potentially be
used for gene therapy. ASODN gene therapy is based on the
Watson-Crick principle of complementary base pairing. Artificial or
biologically compounded DNA or a section of DNA is synthesized
comprising a short chain of ~15–27 nucleotides that is chemically
treated and complementary to the target sequence. The ASODN then
targets the gene or mRNA, forming a double chain structure that
inhibits transfixion or translation, thereby inhibiting the
production of the protein encoded by the gene. The mechanism of
ASODN treatment is as follows: i) Combination with the target DNA
sequence and establishment of a tri-chain structure (U-type
complementation), prohibiting the replication or transfixion of the
target gene; ii) combination with the particular sequence of mRNA
to form a double chain structure that prevents the ribosome from
binding to the target mRNA, resulting in the inhibition of the
expression of mRNA or activation of RNase H, which degrades the
target mRNA by degrading the unusual double chain structure of the
hetero-molecules; iii) complementing to the end coding sequence or
the bottom sequence of mRNA5’ to inhibit the translation. It is
essential to ensure that the ASODN is stabilized prior to its use
for inhibition of the target gene. In order to do this, chemically
treated oligodeoxynucleotides are introduced into the ASODN,
including modifications to the phosphate backbone and pentose units
(primarily at the 2-hydroxy group of ribose). Among these
chemically treated derivatives, PS-ASODNs have a greatly increased
resistance to degradation of the oligodeoxynucleotide by nuclease.
In addition, PS-ASODNs have a good aqueous solubility, are stable
and may be rapidly prepared in large quantities, and so can meet
the clinical need (16,17).
A number of previous studies have used hTR as target
and used ASODNs to affect the cell cultures of ovarian cancer and
prostate cancer in vitro, and demonstrated that ASODNs
inhibit telomerase activity and induce cell apoptosis (18,19).
However, whether antisense hTR decreases the activity of telomerase
and inhibits tumor growth in all types of cancer (for example,
esophageal cancer) requires further investigation. The telomerase
activity is high in the Eca-109 esophageal carcinoma cell line;
however, studies concerning the effects of telomerase-targeting
ASODNs in esophageal carcinoma cell lines are rare. In a previous
study, we designed a PS-ASODN against hTR and applied it to Eca-109
cell cultures, and the results demonstrated that the PS-ASODN
inhibited the activity of telomerase and inhibited tumor growth
(20). However, hTR is widely
expressed in normal tissues; therefore, adverse reactions may occur
in normal cells and tissues if hTR is used as a target for
antisense therapy. Previous studies have demonstrated that hTERT is
the catalytic subunit of telomerase and also regulates telomerase
activity. In addition, the expression of hTERT in cells and tissues
has been found to be associated with telomerase activity (15,21,22).
Therefore, this suggests that hTERT may be a more suitable target
than hTR for antisense therapy.
Shammas et al (16) demonstrated that GRN163L, an
antisense oligonucleotide targeting telomerase RNA, inhibited
telomerase activity in bone marrow cells and induced cell death.
Telomerase activity has been found to be associated with cell
proliferation; therefore, the inhibition of telomerase activity may
inhibit cell proliferation (23).
Another study demonstrated that an antisense oligonucleotide
specifically targeted against hTERT in human prostate cancer cells
reduced telomerase activity, decreased proliferation and,
eventually, induced cell death, indicating that hTERT is directly
associated with the growth and proliferation of tumor cells
(11). The interference of RNA
with the expression of hTERT in cancer cells may effectively
inhibit telomerase activity and strengthen the sensitivity of the
cancer cells to ionizing radiation and chemotherapy (24).
In the present study, an 15-base ASODN was
synthesized, which was then liposome-encapsulated and used to
target hTERT in Eca-109 cells following phosphorothioate
modification. The PS-ASODN was demonstrated to have an inhibitory
effect on cell growth in a concentration- and time-dependent
manner. However, N-ASODN had no inhibitory effect on the growth of
Eca-109 cells, indicting that the inhibitory effect of PS-ASODN on
cell growth was sequence specific. The results from the TRAP-silver
staining assay used to detect the telomerase activity demonstrated
that the telomerase activity was negatively correlated with
concentration and time, and the inhibitory effect was dose- and
time-dependent. Treatment with the ASODN caused the cells to decay
and induced morphological changes. Using an inverted phase-contrast
microscope, cells of the N-ASODN and blank groups grew well, close
to the well wall and close together. The outlines of the cells were
clear and spindle shapes were observed. Particles within the
cytoplasm were rare and the nucleus was visible, which indicated
that the cells were growing rapidly. However, in the PS-ASODN
group, a number of cells were round and floating, the cells were
loosely associated with vague outlines, an increasing number of
particles was observed and proliferation was slow. Following Giemsa
staining, the N-ASODN and blank group cells looked full, with
evenly stained, clear nuclei. However, in the antisense group, the
cytoplasms were condensed and rounded, and the nuclei were stained
unevenly and new-moon-shaped or bulky, and contained large amounts
of decayed materials.
The results from the present study demonstrate that
the ASODN targeted against hTERT induced the death of esophageal
neoplasm cells, and inhibited the activity of telomerase, resulting
in the inhibition of cancer cell growth. The results indicate that
this ASODN effectively inhibits the growth of cancer cells and may
provide an experimental foundation for an anti-telomerase tumor
therapy. The ASODN was designed in accordance with the target-gene
sequences and base pairing; therefore, it only acted on the target
gene and had no effect on other genes within the cells. There are
numerous advantages to ASODNs, including high pertinence, high
specificity and small size. ASODNs enter the nucleus completely and
have strong anti-ribozyme activity when modified by
phosphorothioates. Therefore, this technology may have broad
applications.
References
|
1
|
Nittis T, Guittat L and Stewart SA:
Alternative lengthening of telomeres (ALT) and chromatin: is there
a connection? Biochimie. 90:5–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rao YK, Kao TY, Wu MF, Ko JL and Tzeng YM:
Identification of small molecule inhibitors of telomerase activity
through transcriptional regulation of hTERT and calcium induction
pathway in human lung adenocarcinoma A549 cells. Bioorg Med Chem.
18:6987–6994. 2010. View Article : Google Scholar
|
|
3
|
Hiyama E and Hiyama K: Telomerase as tumor
marker. Cancer Lett. 194:221–233. 2003. View Article : Google Scholar
|
|
4
|
Zhang RG, Wang XW, Yuan JH, et al: Human
hepatoma cell telomerase activity inhibition and cell cycle
modulation by its RNA component antisense
oligodeoxyribonucleotides. Acta Pharmacol Sin. 21:742–746.
2000.
|
|
5
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellon M and Nicot C: Regulation of
telomerase and telomeres: human tumor viruses take control. J Natl
Cancer Inst. 100:98–108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naka K, Yokozaki H, Yasui W, Tahara H and
Tahara E and Tahara E: Effect of antisense human telomerase RNA
transfection on the growth of human gastric cancer cell lines.
Biochem Biophys Res Commun. 255:753–758. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mukai S, Kondo Y, Koga S, Komata T, Barna
BP and Kondo S: 2–5A antisense telomerase RNA therapy for
intracranial malignant gliomas. Cancer Res. 60:4461–4467. 2000.
|
|
9
|
Jiang YA, Luo HS, Fan LF, Jiang CQ and
Chen WJ: Effect of antisense oligodeoxynucleotide of telomerase RNA
on telomerase activity and cell apoptosis in human colon cancer.
World J Gastroenterol. 10:443–445. 2004.PubMed/NCBI
|
|
10
|
Yuan Z and Mei HD: Inhibition of
telomerase activity with hTERT antisense increases the effect of
CDDP-induced apoptosis in myeloid leukemia. Hematol J. 3:201–205.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folini M, Brambilla C, Villa R, et al:
Antisense oligonucleotide-mediated inhibition of hTERT, but not
hTERC, induces rapid cell growth decline and apoptosis in the
absence of telomere shortening in human prostate cancer cells. Eur
J Cancer. 41:624–634. 2005. View Article : Google Scholar
|
|
12
|
Kraemer K, Fuessel S, Schmidt U, et al:
Antisense-mediated hTERT inhibition specifically reduces the growth
of human bladder cancer cells. Clin Cancer Res. 9:3794–3800.
2003.
|
|
13
|
Yu HP, Xu SQ, Lu WH, et al: Telomerase
activity and expression of telomerase genes in squamous dysplasia
and squamous cell carcinoma of the esophagus. J Surg Oncol.
86:99–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sekaran VG, Soares J and Jarstfer MB:
Structures of telomerase subunits provide functional insights.
Biochim Biophys Acta. 1804:1190–1201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shammas MA, Koley H, Bertheau RC, et al:
Telomerase inhibitor GRN163L inhibits myeloma cell growth in vitro
and in vivo. Leukemia. 22:1410–1418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Koeneman KS and Corey DR:
Consequences of telomerase inhibition and combination treatments
for the proliferation of cancer cells. Cancer Res. 63:5917–5925.
2003.PubMed/NCBI
|
|
18
|
Kushner DM, Paranjape JM, Bandyopadhyay B,
et al: 2–5A antisense directed against telomerase RNA produces
apoptosis in ovarian cancer cells. Gynecol Oncol. 76:183–192.
2000.
|
|
19
|
Kondo Y, Koga S, Komata T and Kondo S:
Treatment of prostate cancer in vitro and in vivo with
2–5A-anti-telomerase RNA component. Oncogene. 19:2205–2211.
2000.
|
|
20
|
Fan Xang-kui, Yan Rui-Hua and Li Yi:
Antisense oligodeoxynucleotides of human telomerase inhibits
esophagus carcinoma cell Eca-109 proliferation. China Oncology.
15:88–89. 2005.
|
|
21
|
Donate LE and Blasco MA: Telomeres in
cancer and ageing. Philos Trans R Soc Lond B Biol Sci. 366:76–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zvereva MI, Shcherbakova DM and Dontsova
OA: Telomerase: structure, functions, and activity regulation.
Biochemistry (Mosc). 75:1563–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura M, Masutomi K, Kyo S, et al:
Efficient inhibition of human telomerase reverse transcriptase
expression by RNA interference sensitizes cancer cells to ionizing
radiation and chemotherapy. Hum Gene Ther. 16:859–868. 2005.
View Article : Google Scholar
|