Introduction
Colorectal cancer (CRC) is one of the most common
malignancies worldwide. In the USA, despite a small decrease in the
incidence and mortality rates during the past two decades, CRC
remains the second most common type of cancer (1). Due to distant invasion and migration,
the five-year survival rate of colon cancer patients is low
(2). In China, the incidence rate
of CRC was initially low; however, due to changes in lifestyle and
nutritional habits, the incidence rate of CRC has increased rapidly
since the 1980s (3,4). CRC now ranks as the fifth leading
cause of cancer-associated mortality in China (5). Thus, several alternative therapeutic
strategies are being actively pursued, including immunotherapy.
According to whether the reagent has
antigen-specificity to adjust to the immunity, reagents are divided
into specific and non-specific immunotherapy for cancers, of which
the former is more promising due to targeting. The first step of
specific immunotherapy is to identify rational antigen targets.
Among the tumor antigens identified to date, the cancer-testis (CT)
antigen has been recognized as one of the most potent
tumor-associated antigens (TAAs). CT antigens are expressed in
tumors, as well as in germ cells, but not in normal tissues.
Furthermore, since the testis is an immune-privileged organ
(6), cancer vaccination of CT
antigens is not hypothesized to cause damage to normal tissues via
autoimmune responses. NY-ESO-1 is a CT antigen that induces strong
cellular and humoral immune responses (7,8).
Therefore, NY-ESO-1 represents an ideal target for
immunotherapeutic applications. Theoretically, patients who suffer
from CRC and exhibit NY-ESO-1 expression can profit from
NY-ESO-1-based immunotherapeutic strategies, as demonstrated in a
previous study on vaccines targeted against NY-ESO-1 (9).
The most direct method to measure NY-ESO-1 is from
tumor tissues. However, this is difficult to perform in clinical
practice due to the limitations of available fresh tumor specimens.
Therefore, an alternative method is required, such as testing serum
samples rather than tissue samples. NY-ESO-1 has been reported to
elicit humoral and cellular immune responses in patients with
NY-ESO-1-positive cancers, and spontaneous serum antibodies (Abs)
induced by humoral immunity against NY-ESO-1 can be detected in
40–50% of patients with NY-ESO-1-positive tumors (8,10).
Furthermore, several studies have hypothesized that Ab titers
against NY-ESO-1 correlate with advanced stages of antigen-positive
tumors, including melanoma, transitional cell carcinoma and
prostate cancer (8,11–13),
indicating the later the stage, the higher the whole expression
rate. Therefore, in the present study, only advanced-stage patients
(stages III and IV) were selected for the analysis of serum Abs
against NY-ESO-1 by ELISA. The aim of the present study was to
identify patients with strong NY-ESO-1 immunogenicity to aid the
selection of suitable patients for NY-ESO-1-specific immunotherapy.
However, this selection process may have missed a few patients at
an early stage. In addition, the dynamic expression of
anti-NY-ESO-1 Abs was analyzed in 59 patients to further
investigate the association with clinical status and the possible
influencing factors.
Materials and methods
Patients and sera
In total, 155 patients were pathologically diagnosed
with advanced-stage CRC (TNM stage III/IV) and hospitalized in the
Department of Medical Oncology or Department of Multimodality
Therapy of Oncology in the Chinese PLA General Hospital (Beijing,
China) between July 2012 and December 2012. Serum samples were
collected at the time of admission. There were 59 patients whose
serum samples were randomly collected more than twice at different
hospitalization episodes. Thus, there were 239 serum samples in
total. Pathological grading was characterized according to criteria
from the World Health Organization (14); tumors were classified as G1, G2 and
G3 for well, moderately and poorly differentiated tumors,
respectively. The study was approved by the Ethics Committee of the
Chinese PLA General Hospital and written informed consent was
obtained from the patients.
ELISA
Anti-NY-ESO-1 Abs were detected by ELISA. Briefly, 1
μg/ml NY-ESO-1 purified protein (Pharos Vaccine, Inc., Seoul,
Korea) or 3 μg/ml bovine serum albumin (background control;
10099141; Gibco Life Technologies, Grand Island, NY, USA) in
coating buffer [15 mM Na2CO3, 30 mM
NaHCO3 (pH 9.6)] was absorbed to flat-bottom 96-well
plates (50 μl/well; eBioscience, San Diego, CA, USA) at 4°C
overnight. Following blocking with 5% fetal bovine serum in
phosphate-buffered saline with Tween 20 (p9416; Sigma-Aldrich; St.
Louis, MO, USA) and washing, the plates were incubated for 2 h with
1:25, 1:125 and 1:625 dilutions of patient sera.
Peroxidase-conjugated rabbit anti-human IgG (whole molecule;
Sigma-Aldrich) was used as a secondary Ab and the reaction was
allowed to proceed for 30 min. The plates were incubated with the
substrate, 3,3′,5,5′-tetramethylbenzidine (860336; Sigma Aldrich),
for 5–10 min and analyzed using an ELISA reader (Bio-Rad
Laboratories, Hercules, CA, USA).
A strong positive reaction was defined as the
optical density (OD) values exceeding the corresponding cutoff
value in all the diluted serum titers (1:25, 1:125 and 1:625),
while a weak positive reaction was classified as the OD values
exceeding the cutoff value in two of the diluted titers. The
remaining situations were defined as a negative reaction. All the
experiments were performed in triplicate at different times. The
cutoff value was equal to the mean OD value of the sera from the
normal donors (n=10) plus three times the standard deviation. At
present, there is not an ELISA kit that can be used to obtain the
standard curve of NY-ESO-1; thus, this method was used to determine
the reaction. Sera samples from ten normal donors served as the
negative control, while a serum sample from a patient with
non-small cell lung cancer (NSCLC) served as the positive control,
which had been verified as NY-ESO-1 strong positive in preliminary
experiments.
Statistical analysis
Statistical analysis was performed with SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA), using the χ2
test. P<0.05 (two-tailed) was considered to indicate a
statistically significant difference.
Results
Baseline clinical characteristics of the
patients with CRC
In total, 93 of the 155 patients with CRC were male,
while 62 were female, with a mean age of 55 years (range, 18–83
years). The majority of the patients were pathologically confirmed
with a diagnosis of colorectal adenocarcinoma, while there were a
few cases of adenocarcinoma with mucinous adenocarcinoma and/or a
signet ring cell carcinoma component and two cases of
neuroendocrine carcinoma. All the patients had advanced-stage CRC,
including 32 patients with stage III, 121 individuals with stage IV
and two patients who were unable to be exactly grouped to stage III
or stage IV. There were 83 patients whose malignancies were located
in the colon, while the other 72 tumors were located in the rectum.
The most frequent site of metastases was the liver, followed by the
lungs and lymph nodes (Table
I).
 | Table IBaseline clinical characteristics of
the patients with colorectal cancer (n=155). |
Table I
Baseline clinical characteristics of
the patients with colorectal cancer (n=155).
| Characteristic | Patients, n (%) |
|---|
| Gender |
| Male | 93 (60.0) |
| Female | 62 (40.0) |
| Pathology |
| Unknown | 3 (1.9) |
| Adenocarcinoma | 123 (79.4) |
| Mucinous
adenocarcinoma and/or signet ring cell carcinoma | 7 (4.5) |
| Adenocarcinoma with
mucinous adenocarcinoma and/or signet ring cell carcinoma
component | 20 (12.9) |
| Neuroendocrine
carcinoma | 2 (1.3) |
| Location |
| Colon | 83 (53.5) |
| Rectum | 72 (46.5) |
| Surgical history |
| None | 28 (18.0) |
| Palliative | 26 (16.8) |
| Radical | 101 (65.2) |
| Stage |
| Unknown | 2 (1.3) |
| III | 32 (20.6) |
| IV | 121 (78.1) |
| Sites of metastases
(stage IV) |
| Lung | 56 (36.1) |
| Liver | 79 (50.9) |
| Bone | 18 (11.6) |
| Lymph nodes | 34 (21.9) |
| Others (adrenal
gland/ovarium/peritoneal) | 43 (27.7) |
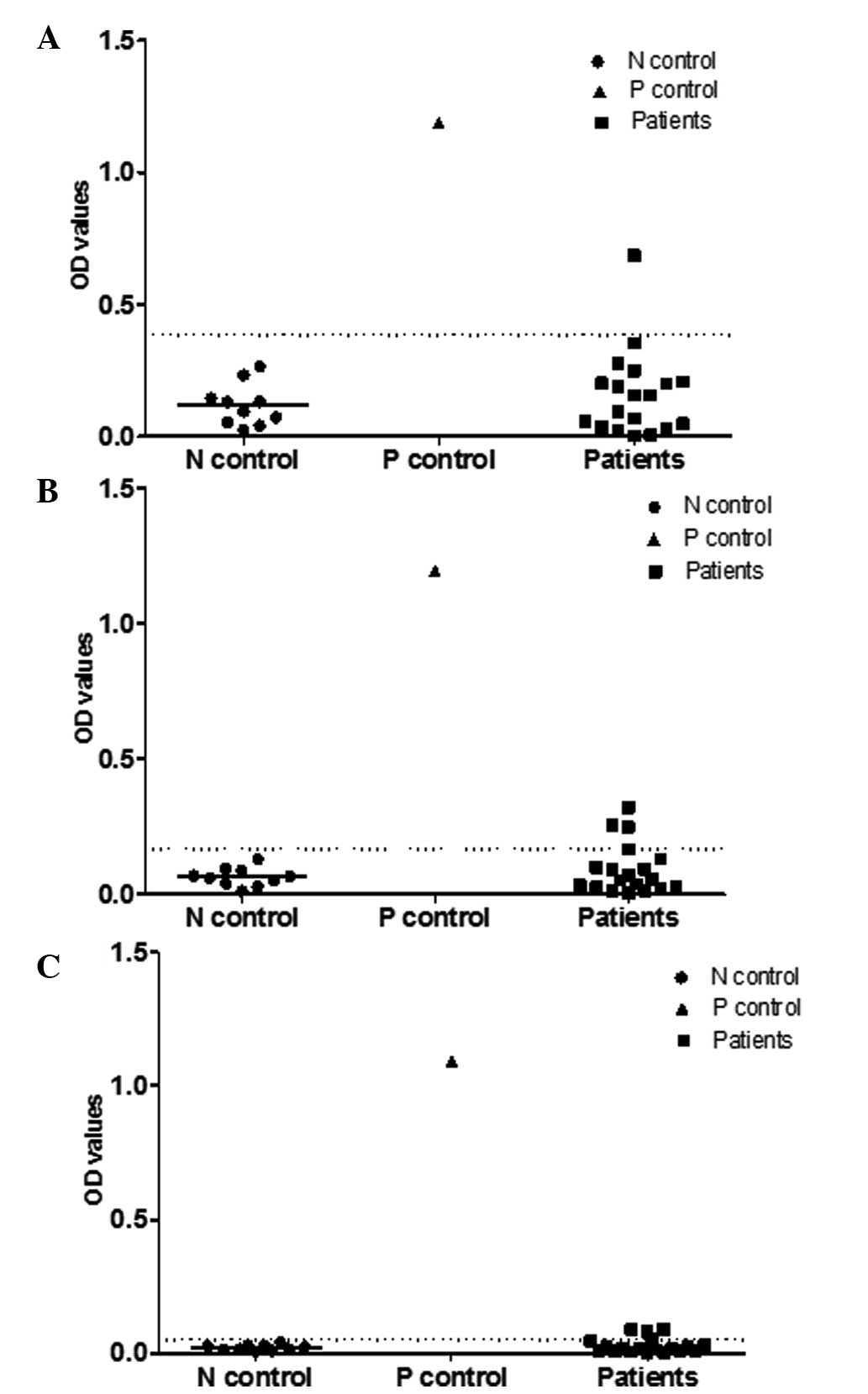
Detection of NY-ESO-1 spontaneous Abs by
ELISA
Serum samples from 155 patients with CRC were
assayed for the presence of NY-ESO-1 Abs by ELISA, using
recombinant NY-ESO-1 protein as the antigen. In total, 38 of the
155 patients (24.5%) with CRC were serum Ab NY-ESO-1-positive, with
14 patients (9%) strong positive and 24 (15.5%) weak positive. In
order to further explain the criteria of judging the
positive/negative reactions, representative results of the ELISA
from 19 patients, along with the negative control (n=10) and
positive control (n=1), are provided (Fig. 1).
Association between serum Abs and the
clinicopathological parameters
No significant correlations were detected between
the expression rate of NY-ESO-1 serum Abs and the
clinicopathological parameters, including the age and gender of the
patients, tumor location, surgical history, grading, vessel
emboli/nerve invasion, local infiltration, lymph node status,
metastatic status and K-ras mutation status (P>0.05). In
addition, there was no statistically significant difference in the
expression of anti-NY-ESO-1 Abs between stage III and IV patients
due to the uneven distribution of sample size (Table II).
 | Table IIAssociation between the expression of
NY-ESO-1 serum antibodies and clinicopathological parameters. |
Table II
Association between the expression of
NY-ESO-1 serum antibodies and clinicopathological parameters.
| Parameter | Patients, n | Weak positive, n
(%) | Strong positive, n
(%) | Positive, n (%) | P-valuea |
|---|
| Gender (n=155) | | | | | |
| Male | 93 | 15 (16.1) | 10 (10.8) | 25 (26.9) | 0.640 |
| Female | 62 | 9 (14.5) | 4 (6.5) | 13 (21.0) | |
| Location (n=155) | | | | | |
| Colon | 83 | 11 (13.3) | 7 (8.4) | 18 (21.7) | 0.659 |
| Rectum | 72 | 13 (18.1) | 7 (9.7) | 20 (27.8) | |
| Surgical history
(n=155) | | | | | |
| None | 28 | 4 (14.3) | 1 (3.6) | 5 (17.9) | 0.254 |
| Palliative | 26 | 5 (19.2) | 0 (0.0) | 5 (19.2) | |
| Radical | 101 | 15 (14.9) | 13 (12.9) | 28 (27.7) | |
| Gradingb (n=134) | | | | | |
| G1/G1–2 | 11 | 2 (18.2) | 0 (0.0) | 2 (18.2) | 0.755 |
| G2 | 79 | 10 (12.7) | 9 (11.4) | 19 (24.1) | |
| G3/G2–3 | 44 | 8 (18.2) | 3 (6.8) | 11 (25.0) | |
| Vessel emboli/nerve
invasion (n=112) | | | | | |
| Yes | 35 | 8 (22.9) | 2 (5.7) | 10 (28.6) | 0.278 |
| No | 77 | 9 (11.7) | 9 (11.7) | 18 (23.4) | |
| Local infiltration
(n=113) | | | | | |
| 1 | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.052 |
| 2 | 9 | 3 (33.3) | 3 (33.3) | 6 (66.6) | |
| 3 | 10 | 2 (20.0) | 1 (10.0) | 3 (30.0) | |
| 4 | 93 | 13 (14.0) | 7 (7.5) | 20 (21.5) | |
| Lymph node
statusc (n=109) | | | | | |
| 0 | 21 | 4 (19.0) | 2 (9.5) | 6 (28.6) | 0.786 |
| 1 | 53 | 6 (11.3) | 4 (7.5) | 10 (18.9) | |
| 2 | 35 | 6 (17.1) | 4 (11.4) | 10 (28.6) | |
| Metastatic
statusd (n=153) | | | | | |
| 0 | 32 | 3 (9.4) | 3 (9.4) | 6 (18.7) | 0.585 |
| 1 | 44 | 8 (18.2) | 6 (13.6) | 14 (31.8) | |
| 2 | 41 | 9 (22.0) | 3 (7.3) | 12 (29.3) | |
| ≥3 | 36 | 4 (11.1) | 2 (5.6) | 6 (16.7) | |
| Stage (n=153) | | | | | |
| III | 32 | 3 (9.4) | 2 (6.3) | 5 (15.6) | 0.493 |
| IV | 121 | 21 (17.4) | 12 (9.9) | 33 (27.3) | |
| K-ras status
(n=37) | | | | | |
| Wild type | 20 | 1 (5) | 1 (5) | 2 (10) | 0.159 |
| Mutant type | 17 | 4 (23.5) | 0 (0.0) | 4 (23.5) | |
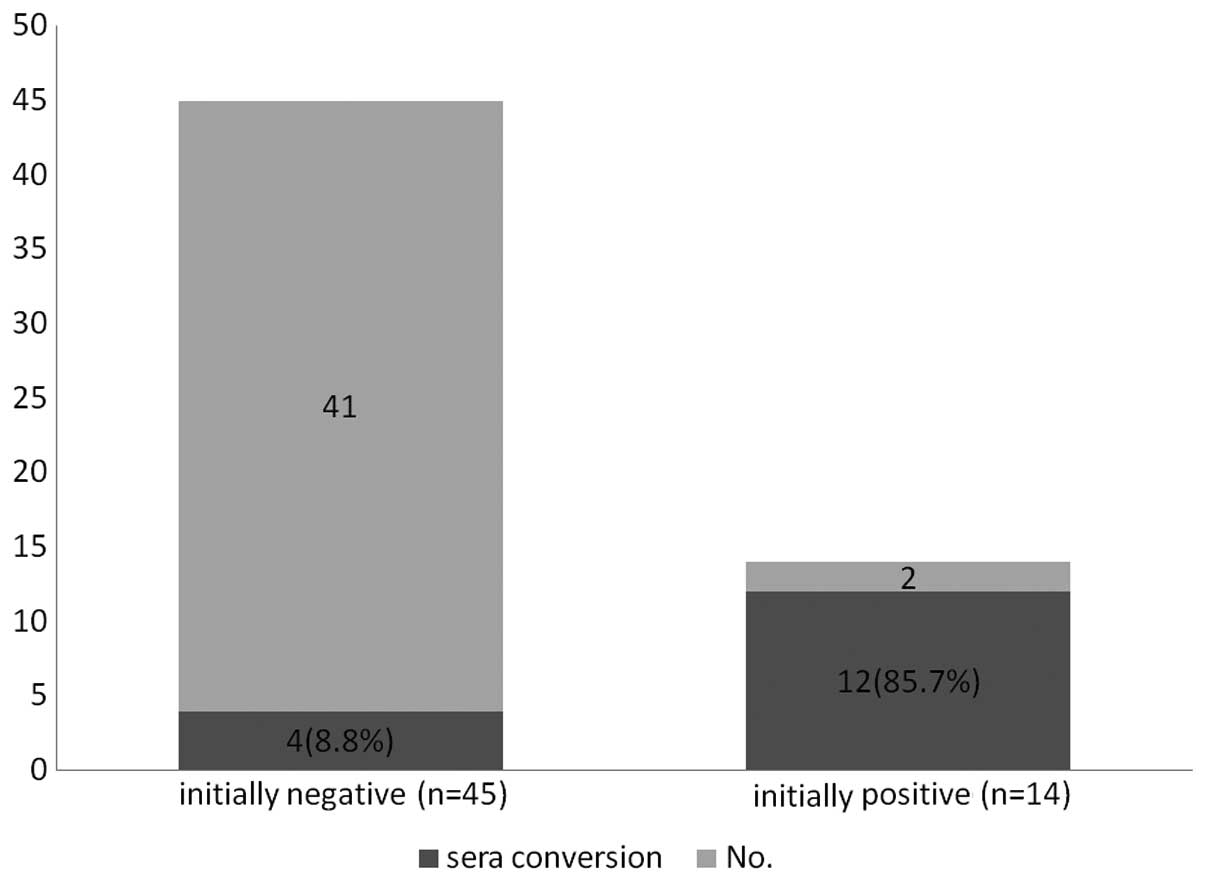
Kinetic expression of serum Abs against
NY-ESO-1
Serum samples were randomly collected from 59
patients with different clinical statuses (range, 2–6 times; mean,
2.4 times) in order to investigate the dynamic change in
NY-ESO-1-specific Ab expression and the possible influencing
factors. In total, 84 serum samples were collected. There were 14
(23.7%) NY-ESO-1-positive patients initially, and there was no
statistically significant difference in the rate of
NY-ESO-1-positive expression when compared with the total 155
patients (24.5%). Notably, 16/59 (27.1%) patients demonstrated
NY-ESO-1 sera conversion, including mutual transformation between
negative and positive or between strong positive and weak positive
(Table III). In addition, among
the 16 patients, 12 were initially NY-ESO-1-positive, while four
patients were initially negative. This observation indicated that
the initially positive patients were more likely to undergo sera
conversion compared with the initially negative patients, with the
incidence of 85.7 vs. 8.8% (P<0.001; Fig. 2). Similarly, sera conversion was
not shown to correlate with other clinicopathological parameters,
including tumor location, lymph nodes status and stage
(P>0.05).
 | Table IIIConversion of NY-ESO-1 serum
antibodies in patients with a different clinical status (n=16). |
Table III
Conversion of NY-ESO-1 serum
antibodies in patients with a different clinical status (n=16).
| Condition of sera
conversion | Cases, n |
|---|
|
Positive→Negative | 6 |
|
Negative→Positive | 2 |
| Positive→Negative
and Negative→Positive | 2 |
| Strong/Weak
positive→Weak/Strong Positive | 6 |
Discussion
With the identification of a large number of TAAs,
antigen-specific immunotherapy has become an important and
promising therapy for cancer patients, in addition to surgery,
chemotherapy and radiotherapy. Thus far, the CT antigen, NY-ESO-1,
has been recognized as one of the most immunogenic TAAs, which was
initially identified by serological expression cloning of a
recombinant cDNA library obtained from a squamous cell carcinoma of
the esophagus (10,15). The CT gene, NY-ESO-1, is widely
expressed in malignant tumors of various types, including melanoma,
breast cancer, esophageal cancer, gastric cancer, hepatocellular
carcinomas and NSCLCs, with 10–40% of the aforementioned cancers
expressing the protein (16–19),
but not in normal tissues. Therefore, NY-ESO-1 has important
clinical significance for antigen-specific immunotherapy. Previous
studies have demonstrated that spontaneous Abs against NY-ESO-1 can
be detected in a number of NY-ESO-1-positive patients with
melanoma, ovarian cancer and other cancers (7,20,21).
However, the expression is negative in normal individuals and
NY-ESO-1-negative patients. Thus, the detection of
NY-ESO-1-specific Abs using a serological method may provide the
basis for NY-ESO-1-specific immunotherapy. In addition, only
limited clinical data are available with regard to the expression
pattern of CT genes and the associations with the pathological
characteristics of CRC. Therefore, in the present study, serum Abs
against NY-ESO-1 were analyzed by ELISA in 155 patients with
advanced-stage CRC (stages III and IV) to investigate the clinical
significance of NY-ESO-1 as a therapeutic target for CRC.
The present study detected NY-ESO-1 serum Abs in
~24.5% of patients with CRC, far higher than previous studies have
reported. Li et al (20)
reported that the frequency of NY-ESO-1 mRNA expression in CRC
tissues was 9.9%, with only one serum Ab positive patient in 12
patients with NY-ESO-1-positive tumors. Of the 12 patients, 11
individuals had advanced-stage (stages III and IV) CRC. In
addition, Scanlan et al (21) reported five autologous NY-ESO-1
Ab-positive CRC cases, in a total number of 74 patients, using a
serum Ab detection array method. These differences may have been
caused by a number of reasons. Firstly, the sample size in the
present study is larger than in previous studies. Secondly, the
present study only selected patients with stage III or IV CRC, and
several studies have hypothesized that Ab titers against NY-ESO-1
correlate with advanced stages of antigen-positive tumors (8,11–13,20).
Finally, serum specimens in the present study were under strict
preservation and test procedures, using a serum Ab detection method
with much higher sensitivity. In addition, all the samples were
assayed within 14 days to ensure freshness of the samples and
reduce Ab degradation. Overall, the higher expression of
NY-ESO-1-specific Abs in CRC cases indicates its value as a
potential target for immunotherapy. Notably, only certain patients
with advanced CRC can induce spontaneous humoral immunity to
NY-ESO-1, meaning that serum Ab detection misses a number of
patients as compared with tissue detection. However, serum Ab
detection remains a simple and quick screening method that may
benefit serum Ab NY-ESO-1-positive patients.
In a previous survey of sera from normal individuals
and cancer patients, Abs against NY-ESO-1 were found in ~10% of
patients with melanoma, ovarian cancer and other types of cancer
(7). Therefore, the expression
rate of NY-ESO-1-specific Abs in patients with advanced-stage CRC
is relatively high compared with other tumor types, dismissing the
previous hypothesis that CRC is not an evident target for
immunotherapeutic intervention due to the lack of frequently
expressed tumor-specific antigens in CRC tumor tissue (20). By contrast, the results of the
present study indicate that specific immunotherapy has great
application prospects in CRC.
The association between NY-ESO-1 expression and
prognosis remains unclear. Certain studies have indicated that
NY-ESO-1 expression may be a poor prognostic factor since the
presence of lymph node metastases following curative resection is
one of the most important poor prognostic factors (22–24)
and there is significant correlation between NY-ESO-1 expression
and local lymph node metastasis (19), although the present study did not
find such correlation. By contrast, due to the induction of Ab and
T cell responses (7,25–27),
NY-ESO-1 expression may also favor the prognosis of patients with
lymph node metastasis. In the present study, the survival data were
not obtained. The patients will be followed-up to further validate
the correlation.
Integrated NY-ESO-1 Ab and CD8+ T cell
responses have been reported to correlate with the clinical benefit
in patients with advanced-stage melanoma treated with ipilimumab
(26). However, in the present
study, a correlation between the efficacy of certain
chemotherapeutic agents and NY-ESO-1-specific Ab expression was not
identified.
Furthermore, the present study included 59 patients
of different clinical status that underwent serum collection at
various time points. Kinetic monitoring of NY-ESO-1 expression
demonstrated that a number of patients underwent a change in their
clinical status, and notably, initial NY-ESO-1 serum Ab-positive
patients were more susceptible to the sera conversion (P<0.001).
However, the specific factors involved and the underlying
mechanisms remain unknown. In order to investigate potential
reasons, the present study analyzed the associations between
clinical parameters and sera conversion; however, the results were
not statistically significant (P>0.05). We hypothesize that
NY-ESO-1-positive patients with spontaneous humoral immunity to
NY-ESO-1 are more unstable; thus, specific immunotherapy to
NY-ESO-1 may be more effective in NY-ESO-1 seropositive patients
that are more susceptible to sera conversion.
In conclusion, the high expression rate of
NY-ESO-1-specific Abs in CRC indicates that NY-ESO-1-based specific
immunotherapy has great application potential in patients with CRC.
To the best of our knowledge, previous studies have primarily
concentrated on the expression of antigens in tumor tissue
specimens (17–19) in order to select appropriate
patients for NY-ESO-1-based antigen-specific immunotherapy. In the
present study, the expression levels of serum Abs against NY-ESO-1
were analyzed in peripheral blood samples, which provided a strong
basis for the clinical application of this methodology. The present
study analyzed the associations between the expression of serum Abs
against NY-ESO-1 and clinicopathological parameters. However, there
was no statistically significant difference that required further
investigation due to the uneven distribution of sample size in
these variables. Finally, humoral immunity to NY-ESO-1 changed in
patients with a different clinical status and the results indicated
that conversion was easier in patients who were NY-ESO-1 serum
Ab-positive. However, the specific underlying mechanisms require
further study.
Acknowledgements
The authors thank the staff at the Department of
Clinical Biochemistry, Chinese PLA General Hospital, for their
support and assistance during the sera sample collection.
References
|
1
|
Siegel R, DeSantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2007. CA Cancer J Clin. 57:43–66. 2007. View Article : Google Scholar
|
|
3
|
Lei T, Chen WQ, Zhang SW, et al:
Prevalence trend of colorectal cancer in 10 cities and counties in
China from 1988 to 2002. Zhonghua Zhong Liu Za Zhi. 31:428–433.
2009.(In Chinese).
|
|
4
|
Li HL, Gao YT, Zheng Y, et al: Incidence
trends of colorectal cancer in urban Shanghai, 1973–2005. Zhonghua
Yu Fang Yi Xue Za Zhi. 43:875–879. 2009.(In Chinese).
|
|
5
|
He J, Gu D, Wu X, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scanlan MJ, Gure AO, Jungbluth AA, et al:
Cancer/testis antigens: an expanding family of targets for cancer
immunotherapy. Immunol Rev. 188:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jager E, Chen YT, Drijfhout JW, et al:
Simultaneous humoral and cellular immune response against
cancer-testis antigen NY-ESO-1: definition of human
histocompatibility leukocyte antigen (HLA)-A2-binding peptide
epitopes. J Exp Med. 187:265–270. 1998. View Article : Google Scholar
|
|
8
|
Stockert E, Jager E, Chen YT, et al: A
survey of the humoral immune response of cancer patients to a panel
of human tumor antigens. J Exp Med. 187:1349–1354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mackensen A, Meidenbauer N, Vogl S, et al:
Phase I study of adoptive T-cell therapy using antigen-specific
CD8+ T cells for the treatment of patients with
metastatic melanoma. J Clin Oncol. 24:5060–5069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gnjatic S, Nishikawa H, Jungbluth AA, et
al: NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer
Res. 95:1–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jäger E, Stockert E, Zidianakis Z, et al:
Humoral immune responses of cancer patients against ‘Cancer-Testis’
antigen NY-ESO-1: correlation with clinical events. Int J Cancer.
84:506–510. 1999.
|
|
12
|
Kurashige T, Noguchi Y, Saika T, et al:
Ny-ESO-1 expression and immunogenicity associated with transitional
cell carcinoma: correlation with tumor grade. Cancer Res.
61:4671–4674. 2001.PubMed/NCBI
|
|
13
|
Nakada T, Noguchi Y, Satoh S, et al:
NY-ESO-1 mRNA expression and immunogenicity in advanced prostate
cancer. Cancer Immun. 3:102003.PubMed/NCBI
|
|
14
|
Marzouk O and Schofield J: Review of
histopathological and molecular prognostic features in colorectal
cancer. Cancers (Basel). 3:2767–2810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YT, Scanlan MJ, Sahin U, et al: A
testicular antigen aberrantly expressed in human cancers detected
by autologous antibody screening. Proc Natl Acad Sci USA.
94:1914–1918. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Wu XJ, Zhao AL, et al:
Cancer/testis antigen expression and autologous humoral immunity to
NY-ESO-1 in gastric cancer. Cancer Immun. 4:112004.PubMed/NCBI
|
|
17
|
Chen HS, Qin LL, Cong X, et al: Expression
of tumor-specific cancer/testis antigens in hepatocellular
carcinoma. Zhonghua Gan Zang Bing Za Zhi. 11:145–148. 2003.(In
Chinese).
|
|
18
|
Kim SH, Lee S, Lee CH, et al: Expression
of cancer-testis antigens MAGE-A3/6 and NY-ESO-1 in non-small-cell
lung carcinomas and their relationship with immune cell
infiltration. Lung. 187:401–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ries J, Mollaoglu N, Vairaktaris E, et al:
Diagnostic and therapeutic relevance of NY-ESO-1 expression in oral
squamous cell carcinoma. Anticancer Res. 29:5125–5130.
2009.PubMed/NCBI
|
|
20
|
Li M, Yuan YH, Han Y, et al: Expression
profile of cancer-testis genes in 121 human colorectal cancer
tissue and adjacent normal tissue. Clin Cancer Res. 11:1809–1814.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scanlan MJ, Welt S, Gordon CM, et al:
Cancer-related serological recognition of human colon cancer:
identification of potential diagnostic and immunotherapeutic
targets. Cancer Res. 62:4041–4047. 2002.PubMed/NCBI
|
|
22
|
Wu AW, Gu J, Ji JF, et al: Role of COX-2
in carcinogenesis of colorectal cancer and its relationship with
tumor biological characteristics and patients’ prognosis. World J
Gastroenterol. 9:1990–1994. 2003.
|
|
23
|
Yarbro JW, Page DL, Fielding LP, et al:
American Joint Committee on Cancer prognostic factors consensus
conference. Cancer. 86:2436–2446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jass JR, Love SB and Northover JM: A new
prognostic classification of rectal cancer. Lancet. 1:1303–1306.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jager E, Gnjatic S, Nagata Y, et al:
Induction of primary NY-ESO-1 immunity: CD8+ T
lymphocyte and antibody responses in peptide-vaccinated patients
with NY-ESO-1+ cancers. Proc Natl Acad Sci USA.
97:12198–12203. 2000.PubMed/NCBI
|
|
26
|
Yuan J, Adamow M, Ginsberg BA, et al:
Integrated NY-ESO-1 antibody and CD8+ T-cell responses
correlate with clinical benefit in advanced melanoma patients
treated with ipilimumab. Proc Natl Acad Sci USA. 108:16723–16728.
2011.PubMed/NCBI
|
|
27
|
Tsuji T and Gnjatic S: Split T-cell
tolerance as a guide for the development of tumor antigen-specific
immunotherapy. Oncoimmunology. 1:405–407. 2012. View Article : Google Scholar : PubMed/NCBI
|