Introduction
Malignant ascites, a common complication of
abdominal malignancies, is one of the main causes of mortality in
patients with cancer. The treatment of malignant ascites is
important for improving the prognosis of abdominal malignancies.
There are numerous methods for the treatment of malignant ascites;
at present, the frequently used methods include intraperitoneal
chemotherapy, intraperitoneal injection of radioisotopes or
biological response modifiers, systemic chemotherapy and
radiotherapy (1–3). However, specifically effective
therapies remain lacking, and existing problems include short
efficacy and drug resistance, as well as questions on how to
maintain high levels of abdominal local drug concentration. With
the development of modern medical technology in gene therapy and
immunotherapy, immunogene therapy has become one of the most
promising anti-tumor therapeutic strategies (4,5).
Mononuclear cells, important members of the body
immune response, have the potential to differentiate into
macrophages and dendritic cells. Interleukin-12 (IL-12) is an
interleukin that is naturally produced by dendritic cells and
macrophages in response to anti-tumor immune stimulation. IL-12
exhibits certain anti-tumor effects by inhibiting tumor growth and
prolonging the survival time of tumor-bearing animals (6,7).
However, in practical application, numerous problems remain with
IL-12 treatment, including large doses, repeated injections and a
number of side-effects (8). It is
important to select an improved therapy carrier to limit IL-12
entering the tumor tissue and to maintain its therapeutic dose for
tumor therapy. Among the potential carriers, mesenchymal stem cells
play an important role in the targeted release of therapeutic gene
expression products into the tumor tissue to inhibit tumor growth
and metastasis. In the present study, the mouse interleukin
(mIL)-12 gene was introduced into mesenchymal stem cells (MSCs) via
the mediation of lentivirus, and highly efficient and stable MSCs
were obtained through screening. Using MSCs as the targeted vehicle
of gene therapy, the anti-ascites effect of lentivirus-mediated
mIL-12 MSCs (Lenti-mIL-12-MSCs) was explored.
Materials and methods
Cells and animals
The MethA fibrosarcoma and the transplanted H22
hepatoma cell lines were purchased from the American Type Culture
Collection (Manassas, VA, USA). The Lenti-mIL-12-MSC stable cell
line was constructed and preserved by the State Key Laboratory of
Biotherapy at the West China Hospital (Sichuan University, Chengdu,
China). Female BALB/c mice (6–8 weeks old) were purchased from the
West China Experimental Animal Center of Sichuan University
(Chengdu, China). All animal experiments were conducted according
to the ethical guidelines of Sichuan University. The present study
was approved by the Institutional Review Board and Ethics committee
of the Medical College of Yan’an University (Yan’an, China).
Isolation and chemotaxis of mouse bone
marrow MSCs
Bone marrow cells were washed out by RPMI-1640
medium from the femur of BALB/c mice isolated in a sterile
environment. Subsequent to centrifugation and the disposal of
supernatants, sterile Tris-NH4Cl was added prior to
another centrifugation that lysed the red blood cells.
Tris-NH4Cl was then rinsed off and the cells were
pelleted by centrifugation. The cells were subsequently suspended
in RPMI-1640 medium containing 10 ng/ml granulocyte-macrophage
colony-stimulating factor, IL-4 and 10% fetal calf serum. The cell
suspension was transferred into six-well culture plates for
incubation at 37°C in 5% CO2. After 48 h, the medium was
changed, during which time the suspended cells were discarded. The
cells were collected after five days of culture.
A chemotaxis chamber (96-well format, Neuro Probe,
Inc., Gaithersburg, MD, USA) was placed into 0.5 mol/l acetic acid
for 2 min and then phosphate-buffered saline (PBS) was used to wash
off the acetic acid. Subsequent to soaking with 0.1% gelatin for 2
h, the chamber was naturally dried. The cells were divided into MSC
(untreated MSCs), Null (MSCs screened following the transfection
with empty virus) and Lenti-mIL-12-MSC (MSCs screened following the
transfection with Lenti-mIL-12) groups. The supernatant of
serum-free cell culture was centrifuged and the volume was reduced
to one-fifth of the original volume. The concentrated supernatant
from each group (50 μl) was added into the lower layer of the wells
in triplicate. Dendritic cells (30 μl, ~1×106/ml) were
subsequently added above the polyvinylpyrrolidone (PVP) membrane
and incubated at 37°C for 2 h. Following incubation, the upper
layer of the PVP membrane was washed with PBS and fixed by ethanol.
The number of cells that migrated to the lower layer was counted
under high magnification following Wright-Giemsa’s staining. Six
fields of vision were used for the cell count for each well.
Enzyme-linked immunosorbent assay
(ELISA)
The level of IL-12 was measured using the ELISA kit
(BD Biosciences, Bedford, MA, USA). The procedure was carried out
according to the manufacturer’s instructions.
Observation of survival rate and duration
for tumor-bearing mice
Inbred female BALB/c mice (6–8 weeks old) were used
as hosts for transplanted H22 hepatoma and MethA fibrosarcoma
cells, and were fed in standardized sterile isolation cages.
Ascites models were constructed as follows (5): MethA cells (1×106
cells/mouse) were inoculated into the peritoneal cavity of 40 mice,
which were randomly divided into four groups of 10 mice on the
second day. The four groups were peritoneally injected on days 2
and 7 after inoculation with: i) 200 μl saline [normal saline (NS)
group]; ii) 200 μl uninfected MSCs (total cell number,
2×106; MSC group); iii) 200 μl infected Lenti-Null-MSCs
(total cell number, 2×106; Null group); iv) 200 μl
infected Lenti-mIL-12-MSCs (total cell number, 2×106;
Lenti-mIL-12-MSC group). The status of ascites development was
measured every two days and the survival rate (the percentage of
surviving mice in each group) was observed until day 40 (only the
Lenti-mIL-12-MSC group had surviving mice by this time). The
construction, grouping, treatment and inoculation for the H22 model
were performed in an identical manner.
Determination of ascites volume and red
blood cell number for tumor-bearing mice
The grouping and treatment were performed as
described above. On day 12 after the inoculation, the mice were
sacrificed and the ascites was collected to measure the volume,
followed by centrifugation. The supernatants and cells were
collected, and red blood cell number was counted under the
microscope.
Observation of toxicity and side
effects
During the treatment, the general conditions of the
activities, feeding, fur and body weight of the ascites-bearing
mice were observed. Following treatment, the mice were sacrificed
by cervical dislocation. The heart, liver, spleen, kidney,
pancreas, small intestines, brain, lungs and bone marrow of the
mice were visually inspected and prepared into biopsies for
observation under optical microscopes following hematoxylin and
eosin staining.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed by Student’s t-test
using SPSS 17.0 for Windows (StatSoft Inc., Tulsa, OK, USA). The
Kaplan-Meier curves for mice survival rates were analyzed by
log-rank test. The test level was α=0.05, and P<0.05 was
considered to indicate a statistically significant difference.
Results
mIL-12 exerts a strong chemotactic effect
on dendritic cells
To assess the chemotactic effect of mIL-12 on
dendritic cells, the number of dendritic cells that migrated
through the PVP membrane in the chemotaxis chamber was counted
under the microscope following Wright-Giemsa’s staining. The
average number of cells that passed through the PVP membrane and
were counted under each high magnification field was 8±0.4 for the
NS group, 14±0.9 for the MSC group, 12±0.8 for the Null group and
43±2.4 for the Lenti-mIL-12-MSC group (Fig. 1A). Statistical analysis indicated
that the dendritic cell chemotactic effect of supernatants from the
Lenti-mIL-12-MSC group cell culture was stronger than that of
supernatants from other groups (P<0.01). This result suggested
that mIL-12 exhibited a strong chemotactic effect on dendritic
cells.
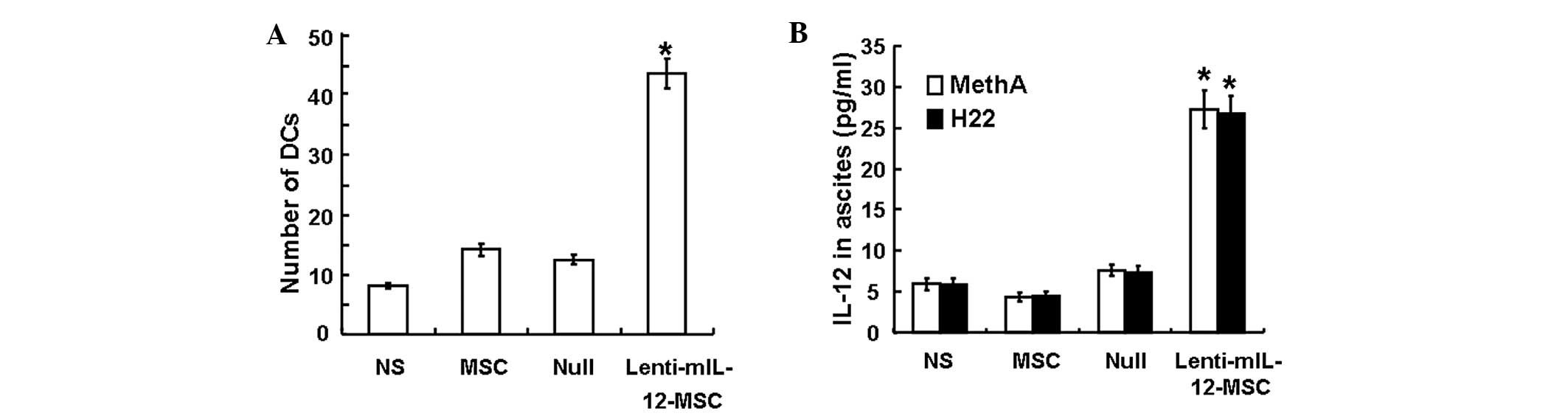 | Figure 1(A) In vitro chemotactic effect
of Lenti-mIL-12-MSC culture supernatant on DCs. The histogram shows
the number of DCs that passed through the membrane from the upper
layer of the chemotaxis chamber to the lower layer. Data are
presented as the mean ± SD (n=3). *Significant
difference from the NS, MSC and Null controls in the same model
(P<0.01). (B) Concentrations of mIL-12 in ascites in the MethA
and H22 models. Data are presented as the mean ± SD (n=10).
*Significant difference from the NS, MSC and Null
controls in the same model (P<0.01). NS, MSC, Null and
Lenti-mIL-12-MSC groups were peritoneally injected with 200 μl
saline, 200 μl uninfected MSCs (total cell number
2×106), 200 μl infected Lenti-Null-MSCs (total cell
number 2×106) or 200 μl infected Lenti-mIL-12-MSCs
(total cell number 2×106), respectively. Lenti-mIL-12,
lentivirus-mediated mouse interleukin-12; MSC, mesenchymal stem
cell; NS, normal saline; DC, dendritic cell; SD, standard
deviation. |
mIL-12 is highly expressed in ascites of
Lenti-mIL-12-MSC-treated mice
To assess the expression of IL-12 in mouse ascites,
IL-12 concentration was measured using ELISA. In both the MethA and
H22 tumor models, the concentration of IL-12 in the ascites of the
Lenti-mIL-12-MSC group (30 pg/ml) was significantly higher than
that of the control groups (<7.5 pg/ml) (P<0.05), whereas no
significant differences were observed among the control groups
(P>0.05) (Fig. 1B). The results
revealed that Lenti-mIL-12-MSCs promoted the expression of IL-12 in
ascites.
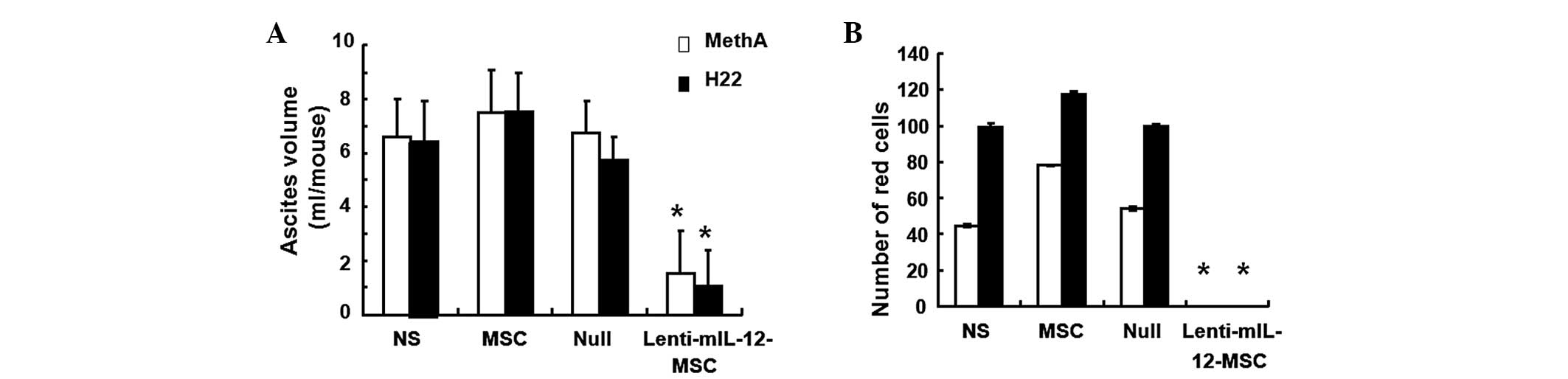
Lenti-mIL-12-MSCs reduce the volume of
ascites and the number of red blood cells
To investigate how Lenti-mIL-12-MSCs affect ascites
volume and red blood cell number, ascites was collected for volume
measurement and red blood cells were counted. The observations
indicated that the control groups of mice not treated with
Lenti-mIL-12-MSCs showed reduced food and water intake, dullness,
inactivity, poor reactivity and rapidly growing ascites in the
early stage, and had extreme abdominal distension, emaciation,
cachexia and a large amount of peritoneal viscous and bloody
ascites in the advanced stage. By contrast, the Lenti-mIL-12-MSC
group of mice exhibited good responsiveness, no abdominal
distension, low viscosity and blood-free ascites. In addition, the
data showed that the volume of ascites and the red blood cell count
in the Lenti-mIL-12-MSC group were significantly lower than those
in the control groups (P<0.01) (Fig. 2). These results demonstrated that
Lenti-mIL-12-MSCs inhibited the formation of ascites in the MethA
and H22 models, with significantly reduced severity of
malignancy.
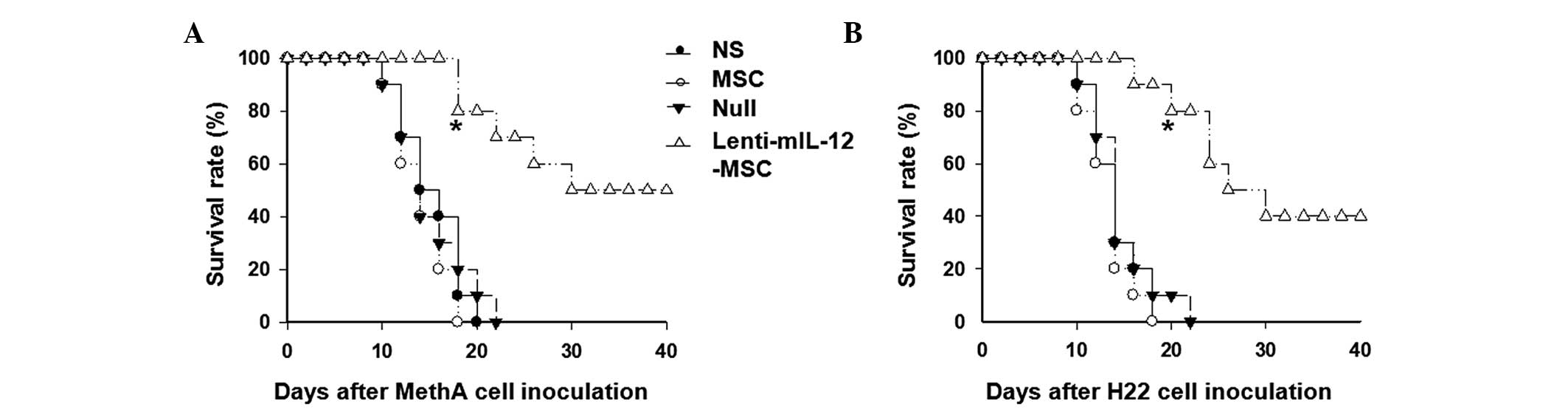
Lenti-mIL-12-MSCs increase the survival
rate and prolong the survival duration of the mice
To understand how Lenti-mIL-12-MSCs affect the
survival of mice, the survival rate was observed and calculated
until day 40 after the inoculation. During the experiment, the body
weight and abdominal circumference of the mice in the control
groups rapidly increased, and these mice started exhibiting high
mortality rates 10 days after the inoculation. However, the mice in
the Lenti-mIL-12-MSC group survived and lived healthily for a
longer time, with slow increases in body weight and abdominal
circumference. The survival time of the mice in the
Lenti-mIL-12-MSC group was prolonged compared with that in the
control groups. When all the mice in MSC group had died, 80% of the
mice in the Lenti-mIL-12-MSC group remained alive (P<0.01)
(Fig. 3). These results suggested
that Lenti-mIL-12-MSCs increased the survival rate and prolonged
the survival duration of the mice.
Lenti-mIL-12-MSCs have no toxicity and
side effects on tumor-bearing mice
To assess the toxicity and side effects of
Lenti-mIL-12-MSC injection, observations were made on the general
condition and hematoxylin and eosin-stained biopsies of internal
organs of the mice in each group. These observations revealed no
physiological and pathological abnormalities (data not shown),
suggesting that Lenti-mIL-12-MSCs exhibited neither toxicity nor
side effects on tumor-bearing mice.
Discussion
IL-12, a T-cell-stimulating factor, promotes the
differentiation and proliferation of CD4+, T and natural
killer cells, as well as interferon-γ production in these cells.
IL-12 also increases the activity of lymphokine-activated killer
cells (9) and induces
anti-angiogenic activities (10).
These properties indicate that IL-12 has potential as an
immunomodulatory factor for the treatment of malignant tumors. In
various tumor models, IL-12 has already shown promising anti-tumor
effects that prevent tumor formation, growth and metastasis
(11–13).
A key point in gene therapy is how to introduce the
target gene and highly express the target protein in the targeted
cells. Gene transduction can be achieved by physical, chemical and
biological techniques, including direct injection of plasmid DNA,
gene gun technology, electroporation and transfection by liposomes
or viruses. There has been a recent gradual increase in the use of
cell vehicles in gene therapies of tumors. Bone marrow MSCs
(BMMSCs) are a type of adult stem cell that mainly exists in adult
bone marrow. These cells have very low immunogenicity and are not
rejected in allografts or heterografts. In addition, BMMSCs exhibit
pluripotency and a strong proliferation ability, and can
directionally migrate to tumor lesions (7,14,15).
In the present study, the mIL-12 gene was introduced
into MSCs via the mediation of lentivirus, and highly efficient and
stable MSCs were obtained through screening (16). Using MSCs as the targeted vehicle
of gene therapy, the anti-ascites effect of Lenti-mIL-12-MSCs was
explored. In vitro chemotaxis experiments showed that the
culture supernatant of MSCs expressing mIL-12 had a strong
chemotactic effect on dendritic cells. Compared with mice in the
other groups, malignant ascites-bearing mice treated with
Lenti-mIL-12-MSCs exhibited a smaller ascites volume, and a lower
red blood cell number and viscosity, as well as a significantly
prolonged survival duration and rate, suggesting that this system
could inhibit ascites formation in the mice. These results
indicated that the chemotactic and maturity-inducing effect of
mIL-12 on dendritic cells effectively stimulated anti-tumor
immunity in the body. Therefore, the inhibition of ascites by
injected MSCs expressing mIL-12 was mediated by immune responses.
In addition, the use of lentivirus as a vehicle prolonged the
expression duration of IL-12 in the abdomen. This may provide novel
avenues for the treatment of malignant ascites.
Acknowledgements
This study was supported by a grant from the
Scientific Funds for Creative Research Groups of the National
Natural Science Foundation of China (no. 30221001).
References
|
1
|
Scheithauer W, Kornek GV, Raderer M, et
al: Randomized multicenter phase II trial of two different
schedules of capecitabine plus oxaliplatin as first-line treatment
in advanced colorectal cancer. J Clin Oncol. 21:1307–1312. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santini D, Massacesi C, D’Angelillo RM, et
al: Raltitrexed plus weekly oxaliplatin as first-line chemotherapy
in metastatic colorectal cancer: a multicenter non-randomized phase
ii study. Med Oncol. 21:59–66. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sartori S, Nielsen I, Tassinari D,
Trevisani L, Abbasciano V and Malacarne P: Evaluation of a
standardized protocol of intracavitary recombinant interferon
alpha-2b in the palliative treatment of malignant peritoneal
effusions. A prospective pilot study. Oncology. 61:192–196. 2001.
View Article : Google Scholar
|
|
4
|
Walther W and Schlag PM: Current status of
gene therapy for cancer. Curr Opin Oncol. 25:659–664. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshiji H, Kuriyama S, Hicklin DJ, et al:
The vascular endothelial growth factor receptor KDR/Flk-1 is a
major regulator of malignant ascites formation in the mouse
hepatocellular carcinoma model. Hepatology. 33:841–847. 2001.
View Article : Google Scholar
|
|
6
|
Cheema TA, Fecci PE, Ning J and Rabkin SD:
Immunovirotherapy for the treatment of glioblastoma.
Oncoimmunology. 3:e272182014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marchi LH, Paschoalin T, Travassos LR and
Rodrigues EG: Gene therapy with interleukin-10 receptor and
interleukin-12 induces aprotective interferon-γ-dependent response
against B16F10-Nex2 melanoma. Cancer Gene Ther. 18:110–122.
2011.PubMed/NCBI
|
|
8
|
Quetglas JI, Dubrot J, Bezunartea J, et
al: Immunotherapeutic synergy between anti-CD137 mAb and
intratumoral administration of a cytopathic Semliki Forest virus
encoding IL-12. Mol Ther. 20:1664–1675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dickerson EB, Akhtar N, Steinberg H, et
al: Enhancement of the antiangiogenic activity of interleukin-12 by
peptide targeted delivery of the cytokine to alphavbeta3 integrin.
Mol Cancer Res. 2:663–673. 2004.PubMed/NCBI
|
|
10
|
Wigginton JM, Gruys E, Geiselhart L, et
al: IFN-gamma and Fas/FasL are required for the antitumor and
antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest.
108:51–62. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Kerkar SP, Yu Z, et al: Improving
adoptive T cell therapy by targeting and controlling IL-12
expression to the tumor environment. Mol Ther. 19:751–759. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerkar SP, Muranski P, Kaiser A, et al:
Tumor-specific CD8+ T cells expressing interleukin-12
eradicate established cancers in lymphodepleted hosts. Cancer Res.
70:6725–6734. 2010.PubMed/NCBI
|
|
13
|
Parker JN, Meleth S, Hughes KB, Gillespie
GY, Whitley RJ and Markert JM: Enhanced inhibition of syngeneic
murine tumors by combinatorial therapy with genetically engineered
HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther. 12:359–368.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dai LJ, Moniri MR, Zeng ZR, et al:
Potential implications of mesenchymal stem cells in cancer therapy.
Cancer Lett. 305:8–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bexell D, Svensson A and Bengzon J: Stem
cell-based therapy for malignant glioma. Cancer Treat Rev.
39:358–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu YL, Fu YH, Tabata Y and Gao JQ:
Mesenchymal stem cells: a promising targeted-delivery vehicle in
cancer gene therapy. J Control Release. 147:154–162. 2010.
View Article : Google Scholar : PubMed/NCBI
|