Introduction
Transient global cerebral ischemia (TGCI) leads to
chronic memory impairment in clinical and animal models (1,2).
TGCI in rats is a common animal model used for the study of
cerebral ischemia-reperfusion injury (3). In the animal model, TGCI leads to
severe neuronal damage in selectively vulnerable brain areas, such
as the hippocampal CA1 region (4).
With the aim to find an effective treatment for cerebral ischemia,
research has been conducted using animal models to identify drugs
that reduce the extent of the brain injury and cognitive impairment
induced by cerebral ischemia-reperfusion injury (5). However, to date, no drug has been
demonstrated to be effective in clinical trials (5).
Pinocembrin (5,7-dihydroxyflavanone) is a flavonoid
compound that is abundant in propolis. Pinocembrin exhibits a
variety of biological effects, including antitumor, antimicrobial,
anti-inflammatory and vasorelaxative activity (6), and is easily transported through the
blood-brain barrier (7). Previous
studies revealed that pinocembrin protected against cerebral
ischemic injury in rat models of middle cerebral artery occlusion
and TGCI (8,9). Pinocembrin has also been shown to
alleviate the damage of primary cultured cortical neurons and
cerebral microvessel endothelial cells in oxygen-glucose
deprivation/reoxygenation (10).
Furthermore, the flavonoid has been demonstrated to protect the
structure and function of cerebral mitochondria in a rat model of
chronic cerebral hypoperfusion (11).
However, to the best of our knowledge, there are no
behavioral studies on the therapeutic potential of pinocembrin in a
TGCI model, where the rats have been subjected to global cerebral
ischemia for 20 min, followed by reperfusion for two weeks. In the
present study, the hypothesis that pinocembrin protects against
cognitive impairment induced by TGCI was analyzed. The effects of
pinocembrin on neurological scores, neuronal loss and the glial
fibrillary acidic protein (GFAP)-positive cell count in the
hippocampus were investigated with the aim of identifying the
underlying mechanisms.
Materials and methods
Animals, surgical procedures and
neurological scores
Adult male Sprague-Dawley rats (body weight, 300±30
g) were obtained from the Vital River Company (Beijing, China).
Prior to surgery, the rats fasted for 4 h and had free access to
water. All the procedures obeyed the Chinese Academy of Medical
Sciences and Peking Union Medical College (Beijing, China)
guidelines and ethics for animal experiments.
The rats were divided into five experimental groups
(n=50). The sham group (n=10) consisted of rats that had not been
exposed to ischemia, whereas the other four groups (n=10 per group)
comprised rats that had been exposed to TGCI. The sham and control
TGCI groups were administered a vehicle
(hydroxypropyl-β-cyclodextrin) intravenously immediately after
reperfusion. The other three groups were intravenously treated with
1, 5 and 10 mg/kg pinocembrin, respectively. Pinocembrin was
synthesized and processed as sterile injection powder at the
Department of New Drug Development, Institute of Materia Medica,
Chinese Academy of Medical Sciences. The rats continued to be
administered pinocembrin or the vehicle intravenously once a day at
the same time until they were sacrificed (9).
The rats were anesthetized with 10% chloral hydrate
(4 ml/kg) and the surgical procedures were performed as described
in previous studies (4,9). The two common carotid arteries were
isolated from the surrounding tissue, and the vertebral arteries
were occluded by electric coagulation. After recovery for 24 h, the
rats were anesthetized with ether, and the common carotid arteries
were occluded for 20 min and reperfused. The body temperature of
the rats was maintained at 37.0±0.5°C. Rats in the sham group were
treated similarly to the ischemic groups, but without blocking the
arteries. Immediately after reperfusion, the rats were given
neurological scores on a scale up to 25 (4,9).
Rats with a neurological score of ≥10 were considered successful;
thus, participated in the following experiments. The rats were
scored again at 24 h following reperfusion.
Behavioral assessment
After reperfusion for 14 days, the spatial learning
and memory of the rats were tested in an open-field Morris water
maze (diameter, 150 cm; height, 50 cm). The pool was filled with
water at a temperature of 25±1°C, and a circular platform
(diameter, 10 cm; position, 1.5 cm below the water surface) was
placed in the first quadrant. During a training trial, each rat had
to escape the water by climbing onto the circular platform, which
was not visible to the rat. The time the rat took to locate the
platform was recorded. If the rat failed to locate the platform
within 90 sec, the trial was terminated and the unsuccessful rat
was guided onto the platform. All the rats were allowed to remain
on the platform for 20 sec. After a final training trial on day 5,
the rats were subjected to a probe trial (60 sec) in the maze
without the presence of a platform. The length of time that the
rats remained in the first quadrant was recorded. All the trials
were recorded for analysis with a computer-assisted image analyzer
(11,12).
Nissl staining
Following the behavioral test, the rats were
anesthetized, perfused with 0.9% NaCl (1,000 ml/kg) and reperfused
with 4% paraformaldehyde (1,000 ml/kg; n=4 per group). The brains
were removed and fixed in the same solvent (4% paraformaldehyde),
and embedded in paraffin. Coronal sections with a thickness of 5 μm
were cut for Nissl staining, as described previously (4). The samples were photographed under a
light microscope (Olympus Corp., Tokyo, Japan) (magnification, ×40
and ×400). The neuronal density was determined by the average
number of surviving hippocampal CA1 and CA4 neurons per 1-mm
section, with three sections of bilateral hippocampal slices
(4,7).
Immunohistochemistry assay
Brain samples embedded in paraffin were cut into
5-μm thick coronal sections for the immunohistochemistry assay.
Free floating sections were incubated at 4°C for 24 h in a mixture
of 0.05 M phosphate-buffered saline (PBS), containing mouse
anti-GFAP (1:1,000 dilution; Santa Cruz Bioctechnology, Inc., Santa
Cruz, CA, USA), 0.3% Triton X-100 and 1% bovine serum albumin, as
described previously (13). The
sections were subsequently incubated for 90 min with a secondary
antibody labeled with biotin (1:200), following which the sections
were treated with 0.02% 3,3′-diaminobenzidine and 0.01%
H2O2 for 3 min. Next, the sections were
washed three times with PBS for 5 min. Finally, the sections were
mounted on gelatin-coated slides, dehydrated in an ascending
alcohol series, cleared in xylene (13) and photographed under a light
microscope (magnification, ×400).
Statistical analysis
All data are presented as the mean ± standard error
of mean. The behavioral studies performed in the Morris water maze
were evaluated with two-way analysis of variance (ANOVA) and the
Bonferroni post hoc test. Other data were evaluated with one-way
ANOVA and the Newman-Keuls post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Pinocembrin decreases the neurological
scores of TGCI rats
As shown in Fig. 1,
the neurological scores of the sham rats were zero, indicating no
neurological deficits. However, the scores of the other four groups
increased following global cerebral ischemia-reperfusion compared
with those of the sham group (P<0.001), and there were no
statistically significant differences among the ischemic group
scores (Fig. 1A). The scores of
the TGCI group at 24 h after reperfusion increased compared with
the sham group (P<0.001), while the scores of the pinocembrin 5
and 10 mg/kg groups decreased when compared with the control group
(P<0.05; Fig. 1B). Since the
neurological score indicates the extent of brain damage, the data
indicated that pinocembrin reduced the neurological symptoms in the
TGCI rats.
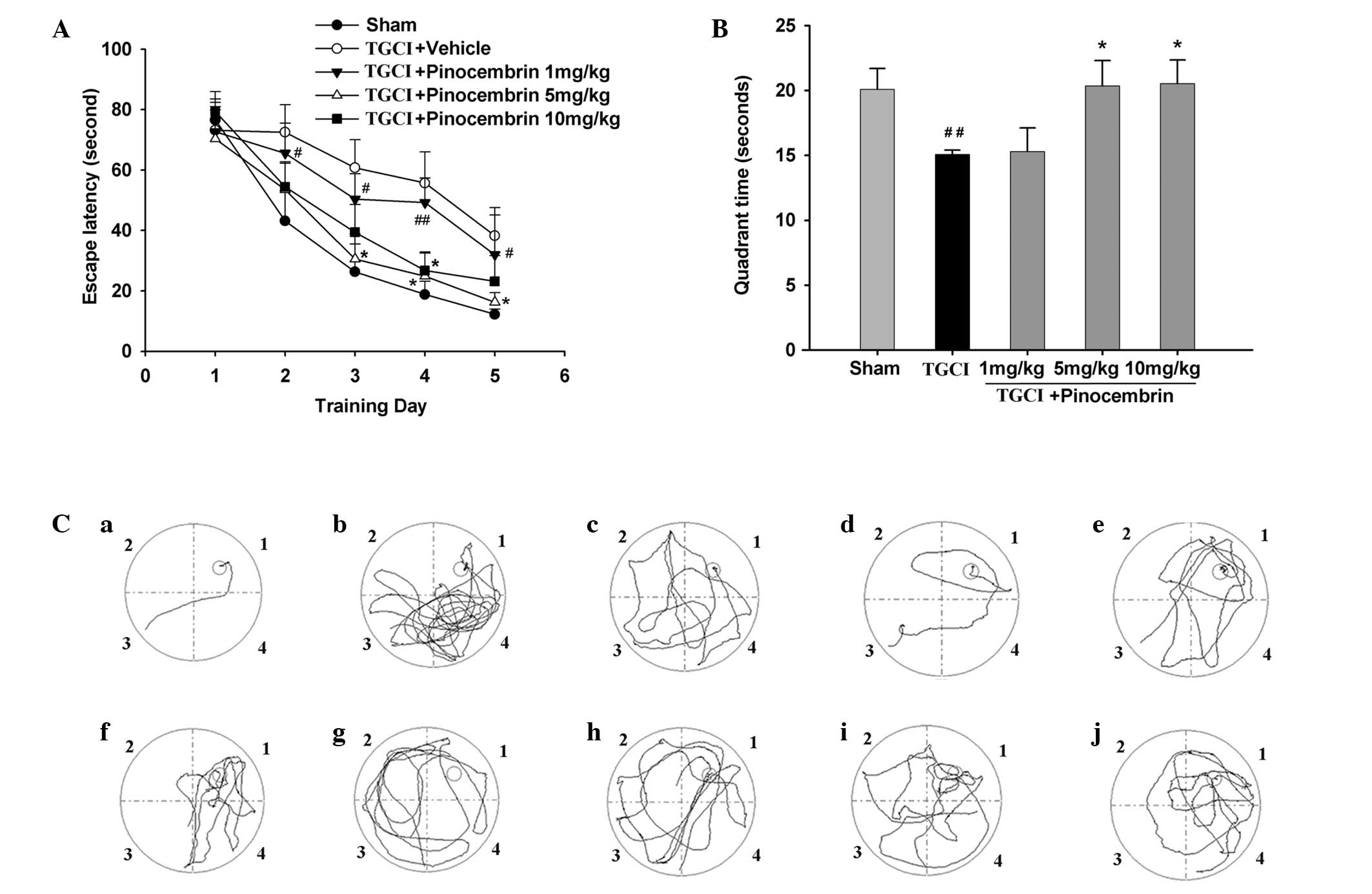
Pinocembrin reduces cognitive impairment
in TGCI rats
Behavioral deficits are the major sequela in
patients that have suffered a stroke, particularly impairment in
learning and memory after cerebral ischemia. In order to
investigate the effects of pinocembrin on the memory damage caused
by the TGCI procedure, the learning and memory of the rats were
assessed in the Morris water maze 14 days after TGCI. During the
five-day training period of the hidden platform test, the mean
latency to locate the platform (escape latency) decreased
progressively in all the groups. Rats in the TGCI group required
additional time to locate the platform when compared with the rats
in the sham group and those that had been administered 5 and 10
mg/kg pinocembrin (Fig. 2A). In
the probe test, the time the control group rats stayed in quadrant
1 (where the platform was located) decreased compared with the sham
group rats, while the time that the pinocembrin-treated rats spent
in quadrant 1 increased (Fig. 2B).
Fig. 2C demonstrates the
representative swimming paths of all the rats in a group,
indicating their training performance on day 5 of the trials in the
presence of the platform (a–e) and their probe trial performance in
the absence of the platform (f–j). The results revealed that the
memory injury of the rats treated with pinocembrin was alleviated
compared with the TGCI group rats.
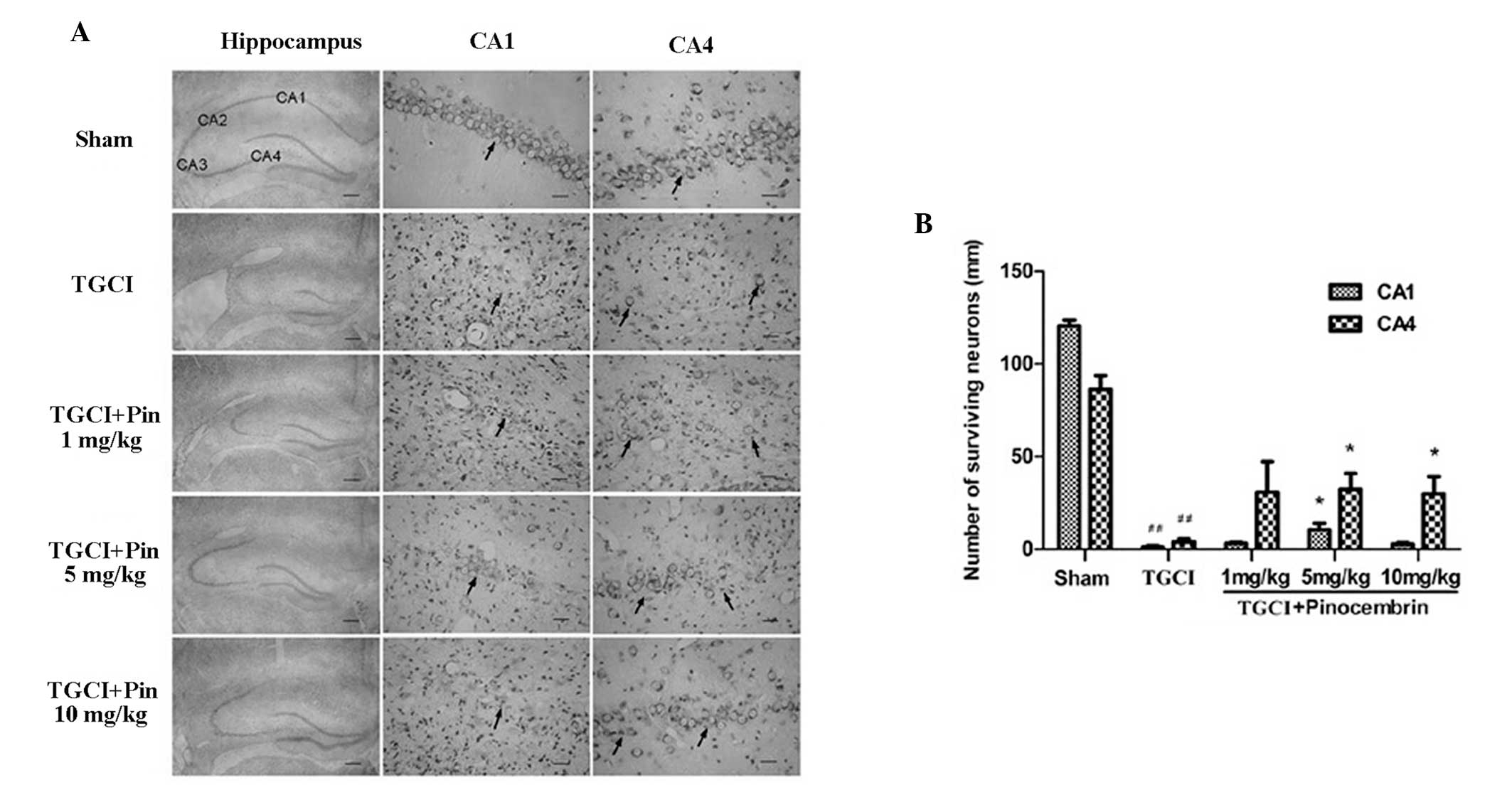
Pinocembrin reduces neuronal loss in TGCI
rats
Neurons are easily damaged during cerebral ischemia,
particularly in the hippocampal CA1 region. In this study, the
neurons of the sham group showed clear nuclei and nucleoli, while
the TGCI rats exhibited significant neuronal damage in the
hippocampal CA1 and CA4 regions when compared with the sham group.
In addition, neurons in the ischemic areas showed significantly
pyknotic nuclei (Fig. 3A). Neurons
were also counted in the hippocampal CA1 and CA4 regions, and TGCI
was shown to destroy ~99% of the neurons in the CA1 region and 75%
of the neurons in the CA4 region in the control group rats.
However, pinocembrin reduced neuronal death in the hippocampal CA1
and CA4 regions in the TGCI rats (Fig.
3B).
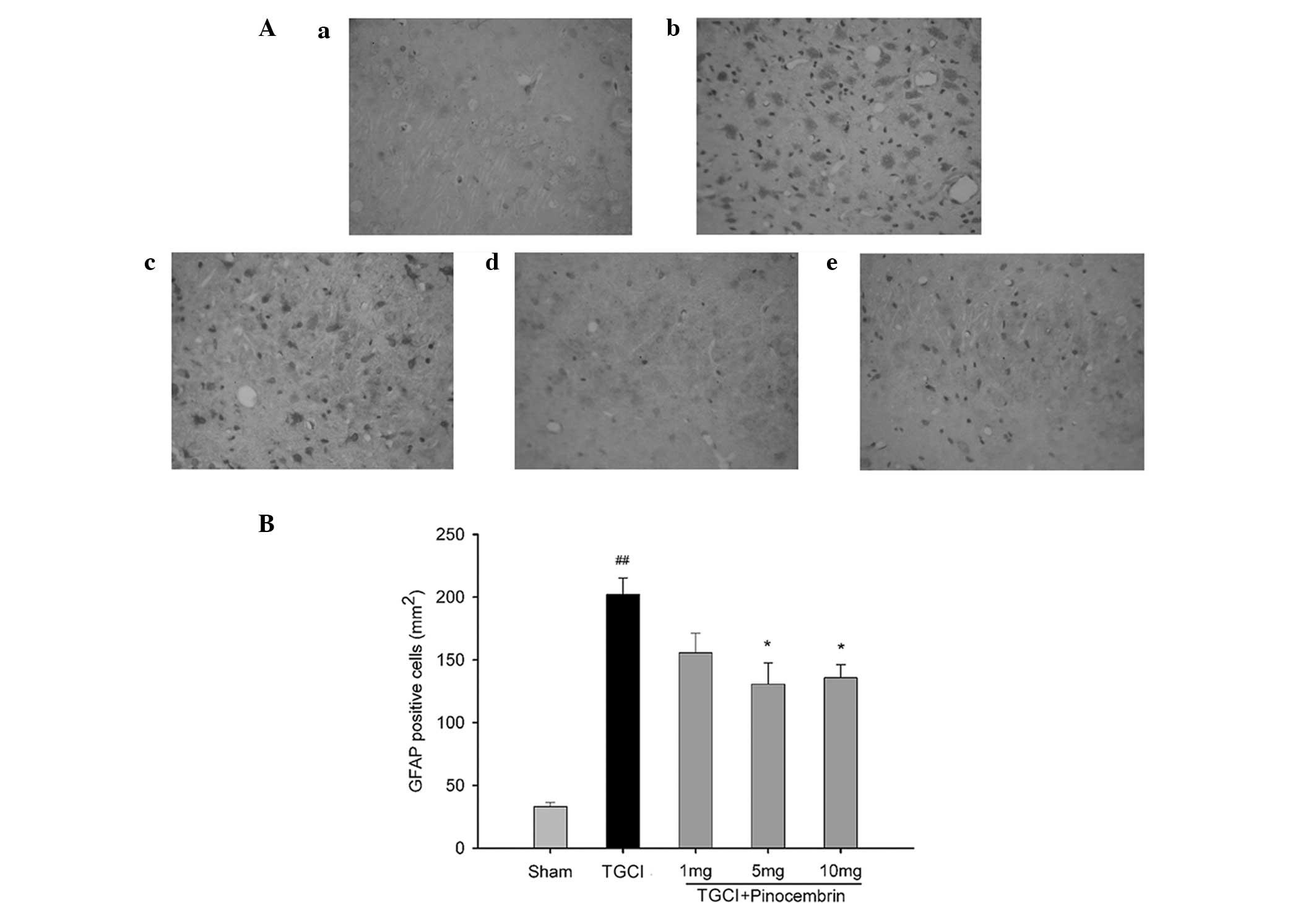
Pinocembrin reduces the amount of
GFAP-positive cells
Astrocyte cells are activated and an inflammatory
response is aggravated following cerebral ischemia-reperfusion
injury. The proliferation of astrocytes, as indicated by the count
of GFAP-positive cells, increased in the TGCI rats, while the
effects were decreased in the TGCI rats that were administered 5
and 10 mg/kg pinocembrin. Fig. 4A
shows representative images of the GFAP immunohistochemistry assay,
and Fig. 4B shows the
GFAP-positive cell count.
Discussion
Previous studies have demonstrated that pinocembrin
protects neurons against cerebral ischemia-reperfusion injury and
attenuates the disruption of the blood-brain barrier in TGCI rats
(13,14). In the present study, pinocembrin
was demonstrated to decrease memory impairment, neurological
scores, neuronal loss in the hippocampus and the number of
GFAP-positive cells in the hippocampal CA1 region of TGCI rats.
In an ischemic rat model, the rats develop TGCI
features due to the occlusion of the bilateral vertebral and common
carotid arteries, and ischemic damage in the hippocampus results in
memory impairment (15). A Morris
water maze test is often performed to evaluate the damage in the
hippocampus and spatial memory impairment (13,16).
Ischemic damage in the TGCI rats led to an impairment of spatial
learning and memory, as revealed by behavioral tests. The
observations of learning and memory impairment and the
morphological changes following TGCI injury were consistent with
previous studies (4,16,17).
Behavioral tests demonstrated that the TGCI rats treated with
pinocembrin performed better in the water maze when compared with
the untreated TGCI rats. It was also evident that pinocembrin
reduced the extent of ischemic neuronal damage in the hippocampal
CA1 region. Thus, the data demonstrated that pinocembrin treatment
resulted in marked behavioral protection against TGCI injury.
Neuronal death following global ischemia occurs in a
delayed manner (18), which is
commonly referred to as delayed neuronal death. In the present
study, the extent of neuronal death in the hippocampal CA1 region
in the control and pinocembrin-treated groups was increased
compared with the neuronal death observed in a previous study
following reperfusion for 24 h (4). The production of reactive oxygen
species (ROS) is enhanced, and the subsequent oxidative stress and
inflammatory reactions may play an important pathological role in
ischemia-reperfusion (3,4). In TGCI, the increased formation of
ROS has been regarded as an underlying factor for mediating delayed
neuronal death, particularly for neurons in the hippocampal CA1
region (19). An increased level
of ROS damages the cell membranes and other cellular structures,
disturbs mitochondrial function and leads to the release of
mitochondrial cytochrome c and the activation of apoptotic
pathways (20,21). Pinocembrin alleviates oxidative
stress; therefore, reduces TGCI-induced injury (4). The present study demonstrated that
pinocembrin alleviated neuronal loss in the hippocampal CA1 region
in the TGCI rats.
Neuroprotection may be assessed by the neuron count
in the hippocampal CA1 region, and is essential for the evaluation
of neuroprotective effects using behavioral and histological
measures (22,23). The present study confirmed that the
application of global cerebral ischemia for 20 min in rats resulted
in damage to >99% of the neurons in the hippocampal CA1 region.
As revealed histologically, a significant impairment in learning
and memory in the TGCI rats was indicated by a poor performance in
the Morris water maze.
It has been established that inflammatory reactions
are responsible for neuronal damage in cerebral ischemia, including
focal and global ischemia (7,24). A
number of investigations have demonstrated the critical role of
inflammation after ischemia and reperfusion in stroke (7,8). The
inflammatory events occur at the blood-endothelium interface of the
cerebral capillaries, and aggravate the ischemic tissue damage in
order to improve the blood-brain barrier permeability (5,9). The
activation of microglia and astrocyte cells aggravates the injury
caused by cerebral ischemia. In the present study, astrocyte cells
were shown to proliferate in the TGCI rats; however, the
proliferation effect was reduced in the rats that were administered
5 and 10 mg/kg pinocembrin. Therefore, the effects of pinocembrin
on astrocyte cells of TGCI rats may reduce neuronal damage.
In conclusion, the present study revealed that
pinocembrin alleviated memory impairment in TGCI rats. This may be
attributed to the effect of pinocembrin against neuronal damage and
astrocyte proliferation in TGCI rats.
Acknowledgements
This study was supported by the Major Scientific and
Technological Special Project of China, ‘Significant New Drugs
Creation’ (2012ZX09301002-001). The authors thank Professor
Humphrey Rang, Head of the Department of Pharmacology, University
College London, for checking the manuscript and improving the
language.
Abbreviations:
|
TGCI
|
transient global cerebral ischemia
|
|
GFAP
|
glial fibrillary acidic protein
|
|
ROS
|
reactive oxygen species
|
|
PBS
|
phosphate-buffered saline
|
|
ANOVA
|
analysis of variance
|
References
|
1
|
O’Reilly SM, Grubb NR and O’Carroll RE:
In-hospital cardiac arrest leads to chronic memory impairment.
Resuscitation. 58:73–79. 2003.PubMed/NCBI
|
|
2
|
Kiryk A, Pluta R, Figiel I, et al:
Transient brain ischemia due to cardiac arrest causes irreversible
long-lasting cognitive injury. Behav Brain Res. 219:1–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kubo K, Nakao S, Jomura S, et al:
Edaravone, a free radical scavenger, mitigates both gray and white
matter damages after global cerebral ischemia in rats. Brain Res.
1279:139–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi LL, Chen BN, Gao M, Zhang HA, Li YJ,
Wang L and Du GH: The characteristics of therapeutic effect of
pinocembrin in transient global brain ischemia/reperfusion rats.
Life Sci. 88:521–528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green AR and Shuaib A: Therapeutic
strategies for the treatment of stroke. Drug Discov Today.
11:681–693. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rasul A, Millimouno FM, Ali Eltayb W, Ali
M, Li J and Li X: Pinocembrin: a novel natural compound with
versatile pharmacological and biological activities. Biomed Res
Int. 2013:3798502013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao M, Zhu SY, Tan CB, Xu B, Zhang WC and
Du GH: Pinocembrin protects the neurovascular unit by reducing
inflammation and extracellular proteolysis in MCAO rats. J Asian
Nat Prod Res. 12:407–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao M, Liu R, Zhu SY and Du GH: Acute
neurovascular unit protective action of pinocembrin against
permanent cerebral ischemia in rats. J Asian Nat Prod Res.
10:551–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng F, Liu R, Gao M, et al: Pinocembrin
attenuates blood-brain barrier injury induced by global cerebral
ischemia-reperfusion in rats. Brain Res. 1391:93–101. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu R, Gao M, Yang ZH and Du GH:
Pinocembrin protects rat brain against oxidation and apoptosis
induced by ischemia-reperfusion both in vivo and in vitro. Brain
Res. 1216:104–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guang HM and Du GH: Protections of
pinocembrin on brain mitochondria contribute to cognitive
improvement in chronic cerebral hypoperfused rats. Eur J Pharmacol.
542:77–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He XL, Wang YH, Gao M, Li XX, Zhang TT and
Du GH: Baicalein protects rat brain mitochondria against chronic
cerebral hypoperfusion-induced oxidative damage. Brain Res.
1249:212–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JM, Kim S, Kim DH, et al:
Neuroprotective effect of forsythiaside against transient cerebral
global ischemia in gerbil. Eur J Pharmacol. 660:326–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang YB, Kan MY, Yang ZH, et al:
Neuroprotective effects of N-stearoyltyrosine on transient global
cerebral ischemia in gerbils. Brain Res. 1287:146–156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi LL, Qiang GF, Gao M, et al: Effect of
pinocembrin on brain mitochondrial respiratory function. Yao Xue
Xue Bao. 46:642–649. 2011.(In Chinese).
|
|
16
|
Ban JY, Kang SW, Lee JS, Chung JH, Ko YG
and Choi HS: Korean red ginseng protects against neuronal damage
induced by transient focal ischemia in rats. Exp Ther Med.
3:693–698. 2012.PubMed/NCBI
|
|
17
|
Kumaran D, Udayabanu M, Nair RU, R A and
Katyal A: Benzamide protects delayed neuronal death and behavioural
impairment in a mouse model of global cerebral ischemia. Behav
Brain Res. 192:178–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui M, Wang L, Liang X, et al: Blocking
TRAIL-DR5 signaling with soluble DR5 reduces delayed neuronal
damage after transient global cerebral ischemia. Neurobiol Dis.
39:138–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Xu J, Li L, et al: Neuroprotective
effect of morroniside on focal cerebral ischemia in rats. Brain Res
Bull. 83:196–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kalay S, Oztekin O, Tezel G, et al: Role
of immunoglobulin in neuronal apoptosis in a neonatal rat model of
hypoxic ischemic brain injury. Exp Ther Med. 7:734–738.
2014.PubMed/NCBI
|
|
21
|
Cao Y, Mao X, Sun C, et al: Baicalin
attenuates global cerebral ischemia/reperfusion injury in gerbils
via anti-oxidative and anti-apoptotic pathways. Brain Res Bull.
85:396–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramos AB, Vasconcelos-Dos-Santos A, Lopes
de Souza SA, et al: Bone-marrow mononuclear cells reduce
neurodegeneration in hippocampal CA1 layer after transient global
ischemia in rats. Brain Res. 1522:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Batti L, Taylor CT and O’Connor JJ:
Hydroxylase inhibition reduces synaptic transmission and protects
against a glutamate-induced ischemia in the CA1 region of the rat
hippocampus. Neuroscience. 167:1014–1024. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yasuda N, Ishii T, Oyama D, et al:
Neuroprotective effect of nobiletin on cerebral
ischemia-reperfusion injury in transient middle cerebral
artery-occluded rats. Brain Res. 1559:46–54. 2014. View Article : Google Scholar : PubMed/NCBI
|