Introduction
Rheumatoid arthritis (RA) is a chronic, disabling
and systemic autoimmune disease that leads to joint inflammation,
as well as progressive cartilage and bone erosion (1–3). RA
also causes tissue inflammation around the joints and other organs
of the body. The disease affects up to 1% of the adult population
worldwide (4).
Freund’s complete adjuvant (FCA)-induced arthritis
shares a number of characteristics with RA (5). FCA mirrors the pathology of RA, by
causing hyperplasia of the synovial tissues, inflammatory
infiltration of the joints, and the destruction of bone and
cartilage.
Asarum, a traditional Chinese medicine known
as ‘xixin’, is widely distributed in the north-east of China
(6). The herb has been used for
the treatment of colds, and as an analgesic, antitussive or
anti-allergic remedy. Modern pharmacological studies have shown
that Asarum species exhibit anti-inflammatory, antitussive,
anti-allergic, anti-hyperlipidemic and anti-myocardiac ischemia
properties by enhancing myocardial contractility, antiarrhythmic
activities and other mechanisms (7,8).
In the present study, the anti-arthritic activity of
Asarum extracts in rats with FCA-induced adjuvant arthritis
was evaluated and the anti-inflammatory mechanisms in
lipopolysaccharide (LPS)-treated RAW 264.7 macrophages were
explored. The underlying mechanism was also investigated.
Materials and methods
Materials
Unless otherwise specified, all chemicals were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s
modified Eagle’s medium (DMEM), fetal bovine serum and the
antibiotic-antimycotic solution were purchased from Gibco
(Auckland, New Zealand). The ELISA kits for TNF-α, IL-1β and IL-6
were obtained from Neobioscience (Beijing, China). Primary
antibodies against extracellular signal-regulated kinase (ERK),
phospho-ERK, p38, phospho-p38, c-Jun N-terminal kinase (JNK),
phospho-JNK, IκB-α, phospho-IκB-α, p65, phospho-p65, IKKβ and
phospho-IKKβ were purchased from Cell Signaling Technology
(Beverly, MA, USA). Horseradish peroxidase-conjugated secondary
antibodies were also obtained from Cell Signaling Technology.
Induction of adjuvant arthritis (AA)
Sprague-Dawley rats (250–300 g; obtained from Vital
River Laboratories, Beijing, China) were maintained under
conditions of standard lighting (an alternating 12 h light/dark
cycle), temperature (23–25°C) and humidity (40–70%). The rats were
immunized (day 0) with a single intradermal injection of 0.1 ml FCA
into the right hind paw. FCA was prepared by mixing 10 mg
heat-inactivated (58°C, 1 h) Bacillus Calmette-Guérin (BCG) with 1
ml sterile paraffin oil. Control animals received 0.1 ml saline
(0.9% NaCl solution). This study was performed in accordance with
the recommendations from the Guide for the Care and Use of
Laboratory Animals by the National Institutes of Health [Eighth
Edition (2011); Bethesda, MD, USA]. The animal use protocol was
reviewed and approved by the Institutional Animal Care and Use
Committee (IACUC) of Shandong University (Jinan, China).
Grouping
Treatment with the test agent began on day 7. All
treatments were orally administered to the rats. Rats were divided
into six groups: Group 1, normal control; Group 2, arthritis
control; Group 3, glycoside of Tripterygium wilfordii (GTW)
40 mg/kg/day; Group 4, Asarum extract-low dose (L) 20
mg/kg/day; Group 5, Asarum extract-medium dose (M) 40
mg/kg/day; and Group 6, Asarum extract-high dose (H) 80
mg/kg/day. GTW was purchased from Shanghai Fudan Forward
Pharmaceutical Co., Ltd. (Shanghai, China). Ansarum extract
was extracted and prepared in the Medical Experimental Center of
Shandong Qianfoshan Hospital (Jinan, Shandong).
Evaluation of AA
Paw volumes were recorded on days 14, 18, 22, 26 and
30. The arthritis index (AI) was classified using a five-value
scale: 0, no swelling; 1 point, swelling on the joint of the little
toe; 2 points: swelling on the metatarsal phalange joint and foot;
3 points, swelling on the hind paw excluding the ankle; and 4
points, swelling on the hind paw and ankle. The sum of points for
each rat was then calculated.
The hind paw volume (ml) of all animal groups was
measured by a plethysmometer on days 14, 18, 22, 26 and 30 after
the injection of FCA emulsion. The paw swelling rate (%) was
expressed as increased multiples of right hind paw volume by
subtraction of the basic paw volume, as a proportion of the basic
paw volume.
The ratio of spleen weight to rat body weight
represented the spleen index. Blood was collected from the
retro-orbital plexus for measurement of biochemical and
hematological parameters, namely tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-6.
Cell cultures
The RAW 264.7 murine macrophage/monocyte cell line
was maintained at 37°C and at 5% CO2 in Dulbecco’s
modified Eagle’s medium (Gibco-BRL, Gaithersburg, MD, USA) with 10%
fetal bovine serum (Gibco-BRL). The RAW 264.7 cells were plated at
a density of 2.0×105 cells/well and incubated overnight.
Asarum extracts (2, 10 and 50 μM) or aspirin (50 μM) were
then added. Following 2 h of exposure, lipopolysaccharide (LPS) was
added to the treated cells at a final concentration of 0.5 μg/ml.
The resultant cell lysates were immunoblotted using
affinity-purified antibodies against IKKβ, phospho-IKKβ, IκB,
phospho-IκB, P65, P65, phospho-P65, phospho-ERK and phospho-JNK
(9,10).
Western blot analysis
Following treatment, the cells were washed three
times with phosphate-buffered saline (PBS), transferred into a 100
μl loading buffer [10 mm Tris-HCl, pH 6.8, glycerol 2%, bromophenol
blue 2%, sodium dodecyl sulfate (SDS) 0.4% and mercaptoethanol
0.14%] and incubated on ice for 30 min. For western blotting, the
protein of the cell lysate was separated in 10% SDS-PAGE gel and
transferred onto nitrocellulose membranes. After blocking with 5%
bovine serum albumin (BSA) for 2 h at room temperature, the
membranes were washed three times with Tris-HCl buffer solution
containing Tween-20 (TBST). The membranes were then incubated with
the primary antibodies overnight at 4°C. The membranes were washed
and then incubated with horseradish peroxidase-conjugated secondary
mouse or rabbit antibodies. The BSA and antibodies were suspended
in TBST.
Statistical analysis
Values are presented as the mean ± standard
deviation. Statistical analysis was performed using a two-tailed
Student’s t-test, and P<0.05 was considered to indicate a
statistically significant difference. Calculations were performed
by using SPSS software, version 13.0 (SPSS, Inc., Chicago, IL,
USA).
Results
AI
From day 14, a statistically significant (P<0.05)
increase in AI was observed in the FCA-induced arthritic animals in
the disease control group compared the normal control group, as in
previous study (11). Treatment
with Asarum extract at 40 and 80 mg/kg yielded significant
(P<0.05) reductions in the swelling pain scores compared with
those in the disease control group after 30 days, as shown in
Table I.
 | Table IEffect of Asarum extracts on
swelling scores in rats with adjuvant-induced arthritis (mean ±
standard deviation). |
Table I
Effect of Asarum extracts on
swelling scores in rats with adjuvant-induced arthritis (mean ±
standard deviation).
| | Scores |
|---|
| |
|
|---|
| Groups | n | Day 14 | Day 18 | Day 22 | Day 26 | Day 30 |
|---|
| Control | 12 | 0 | 0 | 0 | 0 | 0 |
| Model | 12 | 0.67±0.65 | 1.25±0.75 | 2.5±1.51 | 2.42±1.51 | 2.25±1.22 |
| GTW, 40 mg/kg | 12 | 0.58±0.67 | 1±0.85 | 1.42±1.31 | 1.25±1.22b | 1.17±0.94b |
| Asarum
extract |
| 20 mg/kg | 12 | 0.67±0.65 | 1.17±0.58 | 1.67±0.89 | 1.67±1.15 | 1.42±0.99 |
| 40 mg/kg | 12 | 0.58±0.67 | 1.08±0.79 | 1.58±1.08 | 1.5±1.17 | 1.33±0.89b |
| 80 mg/kg | 12 | 0.58±0.67 | 1±0.60 | 1.5±1 | 1.33±0.98b | 1.25±0.87b |
Rate of swelling
Table II shows the
rate of swelling. Right hind paw swelling was found to be
significantly increased in AA rats compared with normal animals.
Asarum extract at 40 and 80 mg/kg diminished the rate of
swelling.
 | Table IIEffect of Asarum extract on the
rate of swelling in rats with adjuvant-induced arthritis (mean ±
standard deviation). |
Table II
Effect of Asarum extract on the
rate of swelling in rats with adjuvant-induced arthritis (mean ±
standard deviation).
| | Rate of swelling
(%) |
|---|
| |
|
|---|
| Groups | n | Day 14 | Day 18 | Day 22 | Day 26 | Day 30 |
|---|
| Control | 12 | 1.10±2.41 | 2.21±1.75 | 1.38±3.13 | 2.98±2.39 | 0.14±1.44 |
| Model | 12 | 70.73±12.08a | 79.28±15.17a | 72.25±15.37a | 70.08±13.38a | 66.72±11.90a |
| GTW, 40 mg/kg | 12 | 66.26±13.02 | 70.40±12.93 | 62.33±9.76 | 57.09±11.36b | 53.26±11.42c |
| Asarum
extract |
| 20 mg/kg | 12 | 66.05±11.68 | 73.19±13.84 | 66.96±14.38 | 60.59±12.50 | 58.27±8.05 |
| 40 mg/kg | 12 | 65.92±13.15 | 72.82±9.90 | 66.78±11.12 | 61.79±11.92 | 56.74±8.19b |
| 80 mg/kg | 12 | 67.98±11.46 | 71.87±12.79 | 62.61±12.11 | 56.70±11.54b | 54.36±10.71b |
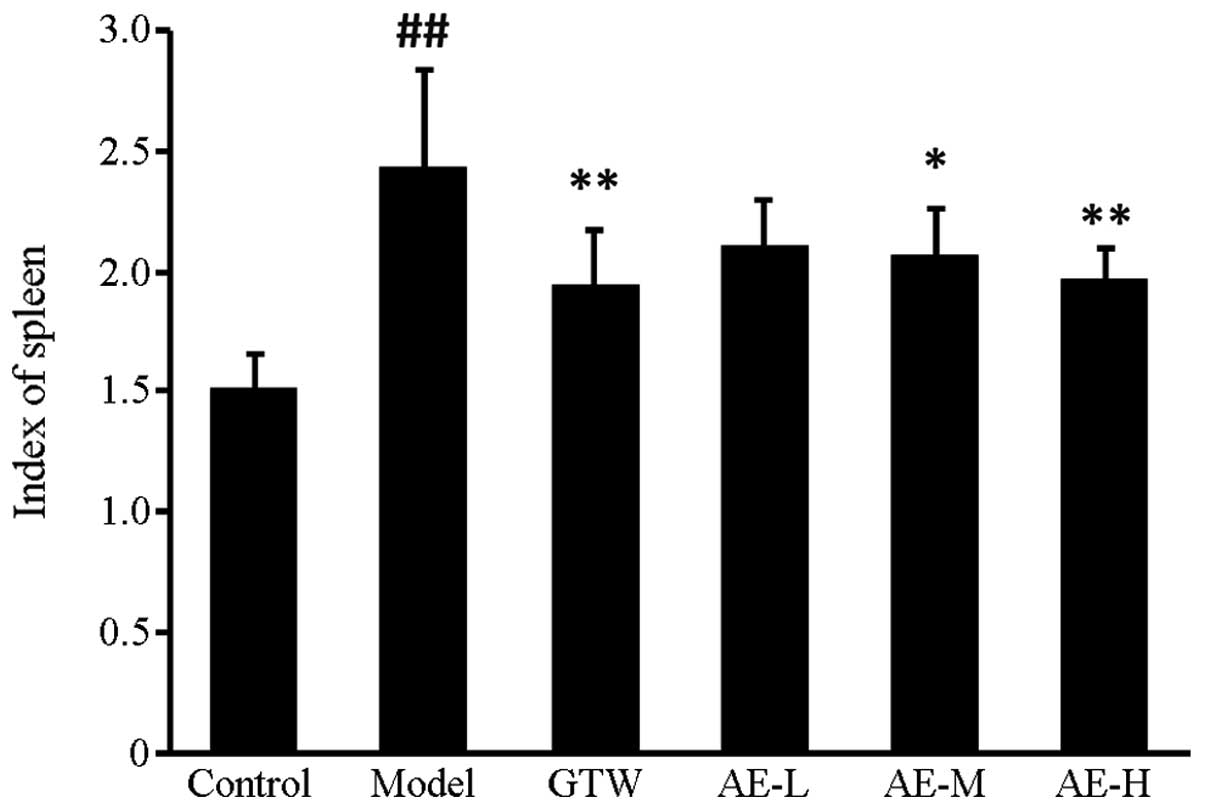
Spleen index
A significant (P<0.01) increase in the spleen
index of the FCA-induced arthritic animals was observed in the
disease control group compared with the normal control group.
Administration of Asarum extract caused a reduction in the
spleen index of FCA-induced arthritic rats. Specifically,
Asarum extract at 40 and 80 mg/kg significantly decreased
the spleen index compared with that in the disease control group
(P<0.05, P<0.01; Fig.
1).
IL-1β, IL-6 and TNF-α expression
levels
IL-1β, IL-6 and TNF-α expression levels were
detected using a standard ELISA (12–14),
and are presented in Table III.
Rats with FCA-induced arthritis had significantly (P<0.01)
increased IL-1β, IL-6 and TNF-α expression levels compared with
those in the normal control group. However, administration of
Asarum extract or GTW caused significant reductions in the
IL-1β, IL-6 and TNF-α expression levels.
 | Table IIIEffects of Asarum extracts on
IL-1β, IL-6 and TNF-α in rats with adjuvant-induced arthritis (mean
± standard deviation). |
Table III
Effects of Asarum extracts on
IL-1β, IL-6 and TNF-α in rats with adjuvant-induced arthritis (mean
± standard deviation).
| Groups | n | TNF-α (pg/ml) | IL-6 (pg/ml) | IL-1β (pg/ml) |
|---|
| Control | 12 | 69.5±8.86 | 73.4±10.32 | 14.225±3.98 |
| Model | 12 |
275.54±45.07a |
139.56±26.29a | 89.01±10.73a |
| GTW, 40 mg/kg | 12 |
186.01±34.03c | 86.58±10.13c | 60.43±14.44c |
| Asarum
extract |
| 20 mg/kg | 12 | 259.08±32.10 | 119.68±25.67 | 81.4±10.15 |
| 40 mg/kg | 12 |
224.21±52.02c |
107.73±31.03c | 75.23±19.99c |
| 80 mg/kg | 12 |
192.49±26.16c | 95.8±23.74c | 64.58±13.00c |
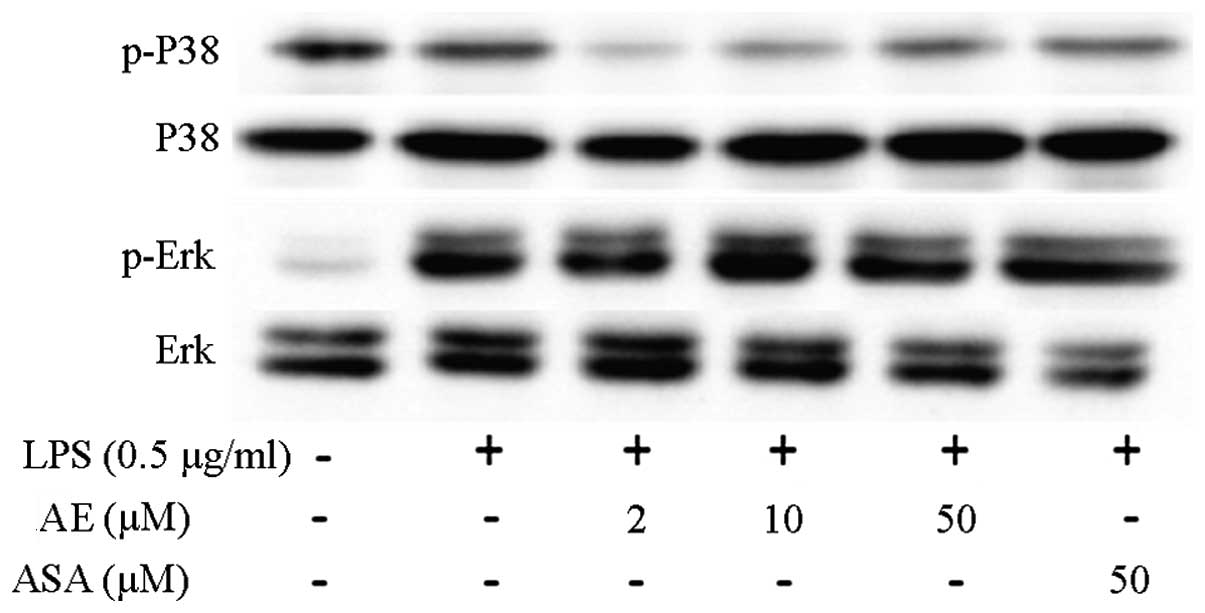
Mitogen-activated protein kinase (MAPK)
signaling pathway
Western blot analysis was performed to evaluate the
phosphorylation of MAPK (15,16).
Fig. 2 demonstrates that the
expression of phosphorylated ERK and p38-MAPK increased in the
LPS-induced RAW 264.7 cells. Asarum extract (10 and 50 μM)
significantly decreased the phosphorylation of ERK and p38, but not
JNK (data not shown). Aspirin administration did not cause
significant changes in the phosphorylation levels of ERK and
p38.
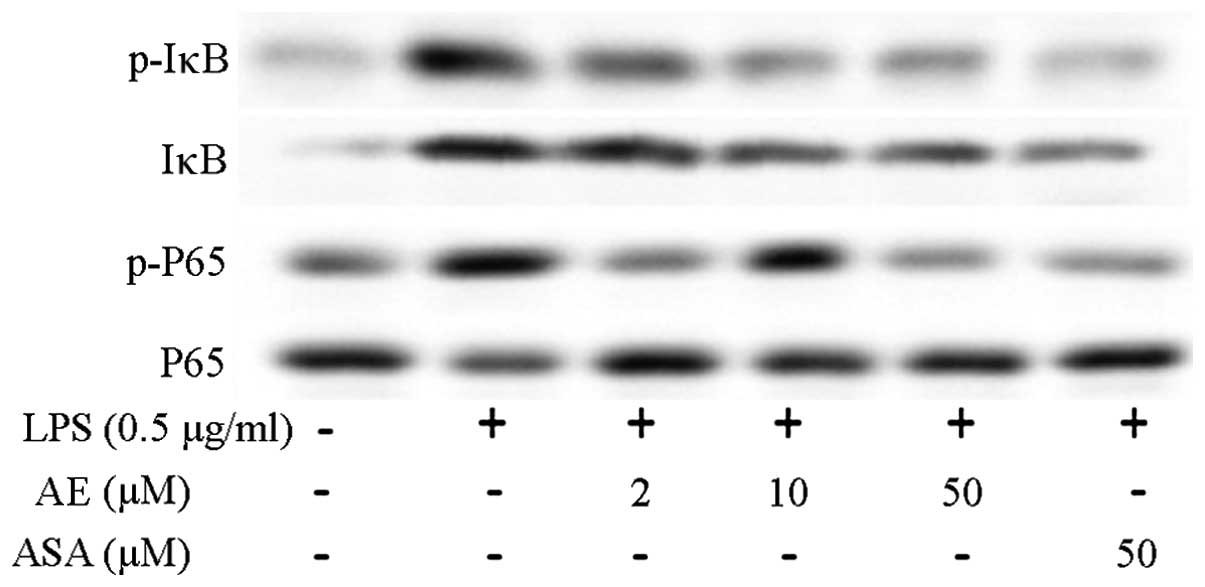
Nuclear factor (NF)-κB signaling
pathway
Phosphorylated IκB and P65 expression levels
increased in LPS-induced RAW264.7 cells (16–18).
Asarum extract at 2, 10 and 50 μM significantly decreased
the phosphorylation of IκB and P65. Aspirin had no significant
effect on the phosphorylation of IκB and P65 (Fig. 3).
Discussion
RA is an autoimmune disease characterized by
multi-joint arthritis and joint erosion, which involves
inflammation and immunity, and may be inherited. However, the
mechanisms of RA have not been clearly elucidated. AA is an
experimental model induced by BCG that is recognized as 65 kDa
anti-heat shock protein (HSP65). HSP65 is similar to the
conservative sequences in AA rat auto-antigens as HSP65 introduces
an autoimmune response by activating T-cell clones. Therefore, AA
model rats, which have characteristics similar to those of RA, are
the ideal animal model for RA (19).
Joint swelling and pain are the initial
manifestations of RA. The podarthrum of the arthritis group animals
revealed swelling following injection, and symptoms developed with
time. Disease severity was evaluated objectively by measuring the
toe volume and by grading the swelling score. Fourteen days
following model establishment, the animals in the GTW and all the
Asarum extract groups demonstrated an alleviation of
secondary symptoms to varying degrees. Toe volume and swelling
score decreased, indicating an improvement in AA rats.
The AA model is an immune hyperfunctional model, and
spleen hyperplasia has been previously reported in AA model rats
(20). In the present study, 40
mg/kg/day GTW and 40 or 80 mg/kg/day Asarum extract were
found to significantly reduce spleen hyperplasia (P<0.05, versus
model group); however, spleen hyperplasia remained higher compared
with that in normal animals. This finding suggests that
Asarum extract may help in the recovery of the
hyperfunctioning of immune organs without causing damage.
Numerous cytokines, including TNF-α, IL-6 and IL-1β,
are released in RA and have an important role in the inflammation
and activation of synoviocytes, which induce joint injury (21). IL-1β is an important
pro-inflammatory cytokine that regulates immunity and inflammation.
IL-1β increases in acute inflammation and induces damage to cells
and tissues. Another pro-inflammatory cytokine, TNF-α, is produced
by monocytes and macrophages, as well as by T lymphocytes and
neutrophils. Release of TNF-α not only causes an inflammatory
response, but also induces the production of IL-1β and IL-6 by
macrophages, thereby aggravating local inflammatory responses. IL-6
transforms B lymphocyte precursors into antibodies, thus producing
mature B lymphocytes, promoting growth and differentiation of bone
marrow cells in cooperation with colony stimulating factors and
improving the degradation function of natural killer cells
(22). In the present study, the
expression levels of TNF-α, IL-6 and IL-1β in the plasma of the
model animals were higher compared with those in normal animals,
whilst Asarum extract decreased serum inflammatory cytokines
to varying degrees. These results indicate that Asarum
extract reduces the inflammatory response of AA model rats by
inhibiting pro-inflammatory factors, which in turn relieve joint
damage.
Numerous cytokines and inflammatory factors are
involved in the inflammatory response and have various roles
through different pathways, including the MAPK and NF-κB pathways
(23–25). The nuclear transcription factor
NF-κB is involved in inflammation. During the resting condition,
NF-κB is inhibited by the binding of IκB and remains in the
cytoplasm in default mode. IκB is a p65/p50 inhibitor, and
stimulation through LPS induces phosphorylation of IκB kinase,
leading to polyubiquitination and degradation of IκB, as well as
the release of p65/60 protein. P65/60 protein moves into the cell
nucleus and binds to DNA, activating gene transcription and
producing multiple inflammatory factors that are involved in RA.
The MAPK signaling pathway regulates the gene expression of a
number of cytokines, chemotactic factors, growth factors, adherence
factors and other enzymes. This pathway also participates in immune
and inflammation reactions and has a vital role in cell
proliferation, differentiation and apoptosis (26). The results obtained in the present
study demonstrated that Asarum extract significantly
increases the phosphorylation of IKKβ, IκB and p65, which results
in the activation of the NF-κB signaling pathway. Asarum
extract was also found to significantly inhibit the phosphorylation
of P38 and ERK, thereby blocking the activation of the MAPK
signaling pathway. The combined inhibition of the two pathways may
prevent the inflammatory response, which may be the mechanism by
which Asarum extract inhibits AA progression.
These findings indicate that Asarum extract
is a potential therapeutic agent for AA as it exerts an
anti-inflammatory effect by mediating the NF-κB and MAPK signaling
pathways.
References
|
1
|
Miossec P: Rheumatoid arthritis: still a
chronic disease. Lancet. 381:884–886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogrendik M: Rheumatoid arthritis is an
autoimmune disease caused by periodontal pathogens. Int J Gen Med.
6:383–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xue M, Jiang ZZ, Wu T, et al:
Anti-inflammatory effects and hepatotoxicity of Tripterygium-loaded
solid lipid nanoparticles on adjuvant-induced arthritis in rats.
Phytomedicine. 19:998–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stahl EA, Raychaudhuri S, Remmers EF, et
al: Genome-wide association study meta-analysis identifies seven
new rheumatoid arthritis risk loci. Nat Genet. 42:508–514. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saratha V and Subramanian SP: Lupeol, a
triterpenoid isolated from Calotropis gigantea latex
ameliorates the primary and secondary complications of FCA induced
adjuvant disease in experimental rats. Inflammopharmacology.
20:27–37. 2012.PubMed/NCBI
|
|
6
|
Jiangsu New Medicine College. Zhong Yao Da
Ci Dian (Dictionary of Chinese Materia Medica). Shanghai Scientific
and Technological Publishers; Shanghai, China: pp. p10341977, (In
Chinese).
|
|
7
|
Han J, Sun CL and Ji MS: Recent advance of
Chinese herb - Asarum. Chinese Agricultural Science
Bulletin. 27:46–50. 2011.(In Chinese).
|
|
8
|
Shi XH, Han L, Jia B, Shen T, Peng C, You
FM and Liu XL: Effect of serum containing Asarum
heterotropoids on Na+ transporter in cardiac myocyte in
rats. Zhejiang Journal of Integrated Traditional Chinese and
Western Medicine. 19:599–602. 2009.(In Chinese).
|
|
9
|
Cho EJ, An HJ, Shin JS, et al: Roxatidine
suppresses inflammatory responses via inhibition of NF-kappaB and
p38 MAPK activation in LPS-induced RAW 264.7 macrophages. J Cell
Biochem. 112:3648–3659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park HH, Kim MJ, Li Y, et al: Britanin
suppresses LPS-induced nitric oxide, PGE2 and cytokine production
via NF-kappaB and MAPK inactivation in RAW 264.7 cells. Int
Immunopharmacol. 15:296–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan AY, Lao L, Zhang RX, et al: Effects of
an acetone extract of Boswellia carterii Birdw.
(Burseraceae) gum resin on adjuvant-induced arthritis in Lewis
rats. J Ethnopharmacol. 101:104–109. 2005.PubMed/NCBI
|
|
12
|
Nagai N and Ito Y: Therapeutic effects of
gel ointments containing tranilast nanoparticles on paw edema
inadjuvant-induced arthritis rats. Biol Pharm Bull. 37:96–104.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei ZF, Jiao XL, Wang T, et al:
Norisoboldine alleviates joint destruction in rats with
adjuvant-induced arthritis by reducing RANKL, IL-6, PGE(2), and
MMP-13 expression. Acta Pharmacol Sin. 34:403–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu KT, Nuss G, Boyce R, et al: Inhibition
of IL-1 release from human monocytes and suppression of
streptococcal cell wall and adjuvant-induced arthritis in rats by
an extract of Tripterygium wilfordii Hook. Gen Pharmacol.
25:1115–1122. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu J, Luo C, Wang P, et al: Saikosaponin
A mediates the inflammatory response by inhibiting the MAPK and
NF-κB pathways in LPS-stimulated RAW 264.7 cells. Exp Ther Med.
5:1345–1350. 2013.PubMed/NCBI
|
|
16
|
Reddy DB and Reddanna P: Chebulagic acid
(CA) attenuates LPS-induced inflammation by suppressing NF-kappaB
and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res
Commun. 381:112–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park WS, Jung WK, Lee DY, et al:
Cilostazol protects mice against endotoxin shock and attenuates
LPS-induced cytokine expression in RAW 264.7 macrophages via MAPK
inhibition and NF-kappaB inactivation: not involved in cAMP
mechanisms. Int Immunopharmacol. 10:1077–1085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoon WJ, Moon JY, Song G, et al:
Artemisia fukudo essential oil attenuates LPS-induced
inflammation by suppressing NF-kappaB and MAPK activation in RAW
264.7 macrophages. Food Chem Toxicol. 48:1222–1229. 2010.
View Article : Google Scholar
|
|
19
|
Bevaart L, Vervoordeldonk MJ and Tak PP:
Evaluation of therapeutic targets in animal models of arthritis:
how does it relate to rheumatoid arthritis? Arthritis Rheum.
62:2192–2205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Issekutz AC and Sapru K: Modulation of
adjuvant arthritis in the rat by 2-methoxyestradiol: an effect
independent of an anti-angiogenic action. Int Immunopharmacol.
8:708–716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Md Yusof MY and Emery P: Targeting
interleukin-6 in rheumatoid arthritis. Drugs. 73:341–356.
2013.PubMed/NCBI
|
|
22
|
Joosten LA, Netea MG and Dinarello CA:
Interleukin-1β in innate inflammation, autophagy and immunity.
Semin Immunol. 25:416–424. 2013.
|
|
23
|
Li G, Zhang Y, Qian Y, Zhang H, et al:
Interleukin-17A promotes rheumatoid arthritis synoviocytes
migration and invasion under hypoxia by increasing MMP2 and MMP9
expression through NF-κB/HIF-1α pathway. Mol Immunol. 53:227–236.
2013.PubMed/NCBI
|
|
24
|
Wei Z, Wang F, Song J, et al:
Norisoboldine inhibits the production of interleukin-6 in
fibroblast-like synoviocytes from adjuvantarthritis rats through
PKC/MAPK/NF-κB-p65/CREB pathways. J Cell Biochem. 113:2785–2795.
2012.PubMed/NCBI
|
|
25
|
Yang M, Xiao C, Wu Q, et al:
Anti-inflammatory effect of Sanshuibaihu decoction may be
associated with nuclear factor-kappa B and p38 MAPK alpha in
collagen-induced arthritis in rat. J Ethnopharmacol. 127:264–273.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|