Introduction
Asthma is a lung disease characterized by chronic
inflammation of the airways (1).
An oxidant/antioxidant imbalance in asthma is one of the important
mechanisms underlying the inflammation process. Therefore,
supplementation of antioxidants in order to maintain the
oxidant/antioxidant balance can significantly reduce airway
inflammation and improve ventilation and airway remodeling. In
traditional Chinese medicine, there are a large number of natural
antioxidant substances, including phenolic acids and saponins
(2). Over the years, the use of
traditional Chinese medicine in the treatment of asthma and other
respiratory inflammatory diseases has exhibited unique effects when
compared with conventional treatments, including the use of inhaled
corticosteroids and oral leukotriene receptor antagonists (3). Chinese medicine not only produces an
anti-inflammatory response, it also exhibits immunoregulatory
effects and reduces the release of oxygen free radicals (4). Xiao-qing-long-tang is a well-known
drug that has been shown to be effective for the treatment of
coughs and asthma and has a far-reaching impact in the history of
Chinese medicine development. Paeonia and Schisandra
extracts are administered as adjuvant drugs in xiao-qing-long-tang;
this combination has exhibited considerable success. However, the
anti-inflammatory mechanisms of the two herbs in the treatment of
asthma remain unclear. Previous studies have demonstrated that
Paeonia contain saponins, while Schisandra contain
other antioxidants, including schisandrin B. However, the effects
of Paeonia and Schisandra in an in vivo model
of asthma remain unclear (5–7).
Thus, the present study aimed to investigate the underlying
mechanisms of Paeonia and Schisandra in the treatment
of asthma.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of
the 324th Hospital of PLA (Chongqing, China). Animal care
guidelines were strictly followed for all experimental procedures
in the study.
Extract preparation
Schisandra and peony original drugs were
extracted from Chinese medicine solution, in accordance with the
ratio of 1:1 using ethanol extraction methods. The reflux time was
5 h and the extracts were placed in a sealed container at 4°C.
Preparation of asthmatic rats
Rats (n=200) were purchased from the Third Military
Medical University Laboratory Animal Center (Chongqing, China) and
were maintained under specific pathogen free conditions. The rats
were randomly divided into treatment (group A), asthma (group B)
and saline control groups (group C). For the rats in the asthma and
treatment groups, 200 mg aluminum hydroxide, (Hebei Xinhua
Pharmaceutical Company, Shijiazhuang, China) 1 mg ovalbumin (Hebei
Xinhua Pharmaceutical Company) and 0.2 ml saline (0.9%; Hebei
Xinhua Pharmaceutical Company) were administered subcutaneously.
Once every two days, an additional subcutaneous injection of 0.2 ml
inactivated Bordetella pertussis (Qilu Pharmaceutical Co.,
Ltd. Jinan, China) was administered. Following sensitization, 2%
ovalbumin inhalation was applied for stimulation once a day for one
week. In the control group, normal saline was used to replace the
subcutaneous injection of antigen solution. In the asthma and
treatment groups, the rats exhibited symptoms such as sneezing,
scratching heads, shortness of breath, abdominal muscle
contraction, agitation, hair removal and hair thinning. The
treatment group received 2 ml herbal extracts via daily gavage for
10 days. Blood samples (2 ml) were centrifuged for analysis prior
to treatment and at day 5 and 10 following treatment in the rats of
the three groups. At day 10 following treatment, the rats were
sacrificed by cervical dislocation and the trachea tissues were
removed for hematoxylin and eosin staining and NF-κB activity
analysis in the three groups.
In vitro hydroxyl radical, total
antioxidant activity and total phenolic content determination in
the herbal extracts
A total antioxidant activity assay was performed
with the herbal extracts using a kit from the Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Determining the hydroxyl
radical suppression of the herbal extracts was conducted according
to the Fenton reaction principle. The procedure was performed using
a hydroxyl radical assay kit, according to the manufacturer’s
instructions (Nanjing Jiancheng Bioengineering Institute).
Determination of the total phenolic content in the herbal extracts
was measured using Lowry reagents (Sigma-Aldrich, St. Louis, MO,
USA).
Serum malondialdehyde (MDA), superoxide
dismutase (SOD) and glutathione peroxidase (GSH-Px)
measurement
These parameters were measured using ELISA kits,
according to the manufacturer’s instructions (Biovalue Co., Ltd.,
Shanghai, China.
Immunohistochemistry
Paraffin-embedded sections, 4-μm thick, were
obtained from the lung tissues. Following dewaxing, xylene and
graded ethanol dehydration was performed. Hydrogen peroxide (3%)
was used to inactivate endogenous peroxidase antigens. Following
serum sealing for 30 min, primary antibodies against NF-κB p65
(Beyotime Institute of Biotechnology, Haimen, China) were added and
incubated for 1 h. Staining was then performed using a
3,3′-diaminobenzidine staining kit (Biotek Co. Ltd. Beijing,
China). Phosphate-buffered saline was used in the negative control
group instead of primary antibody. A positive reaction was
indicated by brown staining in the cytoplasm and nuclei. In three
high magnification fields, the positive rate was calculated as the
ratio of positive cells/total cells.
Statistical analysis
Statistical analysis was performed using the
Statistical Package for Social Sciences computer software (version
17.0; SPSS, Inc., Chicago, IL, USA) for Windows. Analysis was
conducted using the χ2 test and the analysis of variance
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
Histology
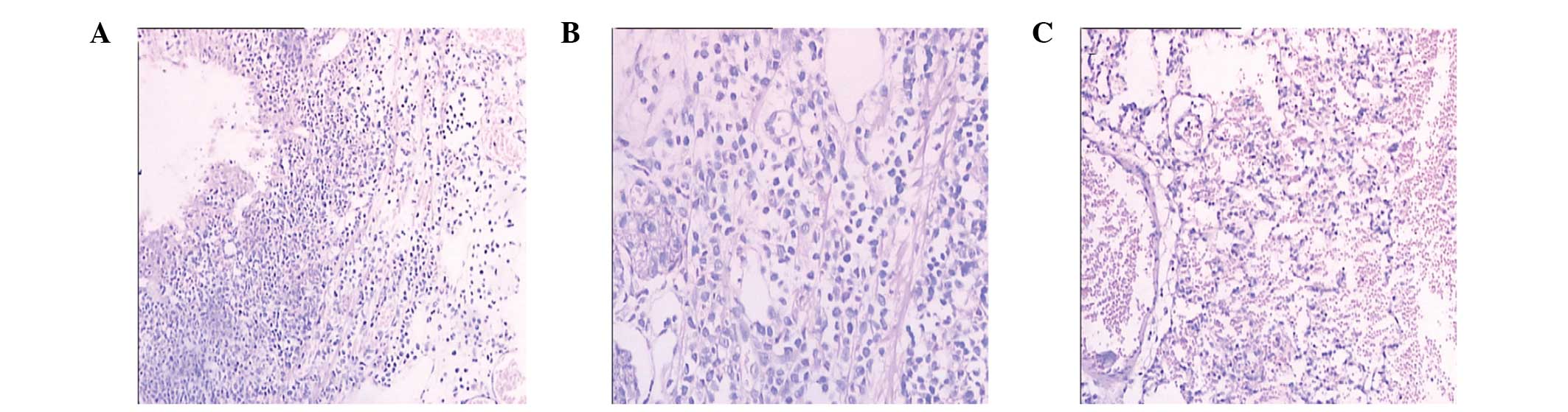
As shown in Fig.
1A, the tracheal mucosa and columnar epithelium underwent
exfoliation and evident eosinophil infiltration was observed prior
to treatment. As shown in Fig. 1B,
infiltration of a large number of eosinophils, neutrophils and
lymphocytes occurred at day 10 following saline treatment. However,
in the traditional Chinese medicine treatment group, the trachea
cilia and muscle layer were normal, and marked eosinophil
infiltration was not observed, as shown in Fig. 1C.
In vitro antioxidant activity
As shown in Table
I, the total antioxidant activity, hydroxyl free radical level
and total phenolic content in the mixed extract were higher than
those in the single herb extracts.
 | Table IAntioxidant activity of the herbal
extracts. |
Table I
Antioxidant activity of the herbal
extracts.
| Parameter | Schisandra
extract | Herbaceous peony
extract | Mixed extract |
|---|
| Total antioxidant
activity (mmol/g) | 1.482±0.115a | 0.556±0.031a | 1.963±0.211 |
| Hydroxyl free radical
(U/ml) | 316.24±1.46a | 89.09±3.76a | 368.55±1.27 |
| Total phenol content
(μg/ml) | 1138.26±6.53a | 1253.28±5.91a | 2055.32±4.65 |
Determination of erythrocyte antioxidant
activity
As shown in Table
II, serum and erythrocyte SOD activity and GSH-Px levels in
group A were higher than those in group B, while the level of MDA
in group A was lower than that in group B (P<0.05). Serum and
erythrocyte SOD activity and GSH-Px levels in group B were lower
than those in group C, and the MDA level in group B was higher than
that in group C (P<0.05).
 | Table IISerum antioxidant activity. |
Table II
Serum antioxidant activity.
| Parameter | Group A | Group B | Group C |
|---|
| SOD activity
(U/l) | 0.5±0.012a | 0.33±0.025a | 0.62±0.025 |
| GSH-Px (μmol/l) | 3166±252a | 1650±125a | 3250±203 |
| MDA (μmol/l) | 125.93±13.05a |
511.334±15.363a | 324.162±16.472 |
Determination of cytokine levels
IL-4, IL-6 and IL-13 levels in the serum (Table III), bronchoalveolar lavage fluid
(Table IV) and lung tissue
homogenates (Table V) were not
significantly different between groups A and B (P>0.05).
However, IFN-γ levels significantly increased in group A as
compared with group B, and IL-22 levels decreased significantly
(P<0.05). The levels of IL-4, IL-6, IL-13 and IL-22 in the lung
tissue, bronchoalveolar lavage fluid and serum of group B were
significantly higher than those in group C, while the IFN-γ level
decreased significantly (P<0.05).
 | Table IIICytokine levels in the serum. |
Table III
Cytokine levels in the serum.
| Cytokine (pg/ml) | Group A | Group B | Group C |
|---|
| IL-4 | 115.06±30.53a | 173.78±32.85a | 123.67±12.55 |
| IL-6 |
997.92±145.31a |
2005.23±125.62a | 759.54±102.47 |
| IFN-γ |
1434.07±115.63a |
766.83±84.05a | 1226.14±125.01 |
| IL-13 |
766.54±35.24a |
859.32±55.08a | 583.28±39.12 |
| IL-22 | 50.20±11.31a |
123.47±20.25a | 62.35±15.18 |
 | Table IVCytokine levels in the
bronchoalveolar lavage fluid. |
Table IV
Cytokine levels in the
bronchoalveolar lavage fluid.
| Cytokine
(pg/ml) | Group A | Group B | Group C |
|---|
| IL-4 | 40.81±8.46a | 39.75±8.35a | 28.09±7.66 |
| IL-6 | 83.52±12.65a |
187.19±13.62a | 51.09±10.21 |
| IFN-γ |
259.96±25.31a |
148.69±19.34a | 235.61±22.04 |
| IL-13 | 46.64±11.22a | 45.32±10.07a | 28.96±9.62 |
| IL-22 | 92.68±15.24a |
125.96±20.34a | 82.95±10.21 |
 | Table VCytokine levels in the lung
homogenates. |
Table V
Cytokine levels in the lung
homogenates.
| Cytokine
(pg/ml) | Group A | Group B | Group C |
|---|
| IL-4 | 43.29±3.06a | 47.35±5.33a | 30.57±4.31 |
| IL-6 | 89.62±8.69a |
187.19±10.08a | 59.61±9.64 |
| IFN-γ |
389.14±25.93a |
202.08±29.18a | 354.35±35.77 |
| IL-13 | 47.26±8.41a | 46.86±6.22a | 25.75±5.93 |
| IL-22 | 88.56±12.03a |
125.37±15.84a | 67.42±8.67 |
Discussion
When asthma occurs, airway inflammation results in
increased levels of proinflammatory cytokines, NADPH oxidase and
reactive oxygen species or reactive nitrogen radicals. Under normal
circumstances, antioxidants in the lung tissue can remove small
amounts of reactive oxygen species or reactive nitrogen radicals.
Only an excessive amount of reactive oxygen species or reactive
nitrogen radicals causes damage to airway proteins, lipids and DNA,
resulting in airway inflammation, airway hyperresponsiveness,
airway microvascular hyperpermeability and airway mucus
hypersecretion. However, continued inflammation increases the
levels of apoptosis and necrosis, which is followed by lung tissue
oxidative damage (8). Therefore,
oxidative stress is an important mechanism in the pathogenesis of
asthma. To reduce oxidative stress or increase the antioxidant
function, decreasing airway eosinophil infiltration, mucus
secretion, airway hyperresponsiveness and changes in airway
remodeling is required. Antioxidant supplementation has become a
new method for the treatment of asthma. Chinese traditional
medicine, including phenols, polysaccharides, alkaloids and
saponins, are recognized as strong antioxidants that exhibit a free
radical scavenging effect (9,10).
Water and ethanol extracts containing these Chinese medicinal
ingredients have been shown to exhibit strong anti-inflammatory and
anticytotoxic effects. In vitro experiments have
demonstrated that these extracts can inhibit nitric oxide (NO) and
tumor necrosis factor-α production (11). The antioxidant activity of Chinese
medicinal raw herbs is low (12),
however, a water or ethanol extract of these Chinese medicinal raw
materials exhibits significantly increased antioxidant activity.
Therefore, extracting the active ingredients of Chinese medicinal
raw materials is becoming increasing popular in the development of
Chinese medicine. In our preliminary study, an ethanol extract
reflux time of 5 h was shown to produce the highest antioxidant
activity in vitro.
The traditional Chinese medicinal formula,
xiao-qing-long-tang, has a strong therapeutic effect on asthma and
has been shown to regulate protein secretion in airway club cells
(13). In addition, the formula
has been shown to reduce the proportion of eosinophils in the serum
and thus, the levels of IL-5 and histamine release. The medicine
can stabilize mast cell membranes, inhibit mast cell degranulation
and also inhibit endothelin-1 levels and NO synthesis (14). Schisandra species, that are
in xiao-qing-long-tang, are a type of superior medicine that have
been used in China for almost one thousand years. Schisandra
has been shown to exhibit antioxidant, cough and asthma inhibiting
and liver function protective effects (15). Modern pharmacological studies have
shown that Schisandra exhibits biological and
pharmacological effects predominantly due to lignans (16). These lignans include schisandrin,
deoxyschisandrin, schisandrin B, schisandrin C, schisandrol B,
schisantherrin A, schisantherrin B and gomisin A. Levels of lignans
in Schisandra water extract can reach ~99% (17). Paeonia also has a very long
history of use in China and can significantly reduce eosinophil
chemokine expression and secretion. In addition, Paeonia can
inhibit the migration of eosinophils in A549 culture medium and the
activation of NF-κB. Paeonia species have a therapeutic
effect on asthma (18). A study of
44 types of traditional Chinese medicine revealed that there were
relatively high phenolic and flavonoid levels in Akebia,
Aster and water or ethanol extract of Paeonia.
Therefore, Paeonia is one of the three traditional Chinese
medicines with the highest anti-inflammatory and antioxidant
activities (19). In the present
study, following the joint use of Paeonia and
Schisandra, the total antioxidant activity was 1.963±0.211
mmol/g, hydroxyl radical inhibition was 368.55±1.27 U/ml and the
total phenolic content was 2055.32±4.65 μg/ml, which was
significantly improved as compared with the single herb extracts.
Levels of oxidative stress are closely associated with airway
inflammation (20).
The level of oxidative stress in vivo is
reflected through the oxide levels and antioxidant capacity in the
body. During an asthma attack, the vitality of inflammatory cells
increases, the function of airway epithelial cilia is impaired,
mucus secretion increases and a large number of free radicals are
produced. Active substances, including SOD, GSH-Px and vitamin C
and E, are involved in the pathophysiological process of asthma
(21). SOD exists in the cytoplasm
and lysosome and can transform O2 into H2O;
GSH-Px is also involved in this process. Free radicals induce body
injury mainly through lipid peroxidation, following the reaction of
unsaturated fatty acids, ethylene, pentane and MDA (22). Thus, following lipid injury of the
cells, the end products increase. Therefore, asthma treatment
should focus on increasing SOD and GSH-Px enzyme activity and
reducing the increase in oxidation products. During an asthma
attack, MDA levels increase, while GSH-Px levels decrease (23).
In the present study, serum and erythrocyte SOD
activity and GSH-Px levels were lower in group B than in group C,
while MDA levels in group B were higher than in group C
(P<0.05). These results further illustrate the effect of
oxidative stress on asthma. Paeonia and Schisandra
extracts were used in the treatment of asthmatic rats, and the
levels of serum and erythrocyte SOD activity and GSH-Px in the
treatment group were found to be higher than those in group B
(asthma group). In addition, the MDA level was lower than that in
group B (P<0.05). Therefore, Paeonia and
Schisandra extracts can significantly increase the
antioxidant levels and reduce oxidative damage.
Cytokines, inflammatory cells and inflammatory
mediators interact with each other, resulting in the infiltration
of a large number of airway eosinophils and CD4+ T
cells, as well as mucus hypersecretion, airway hyperresponsiveness,
airway remodeling and increased IgE production. An imbalance in the
cytokine levels produced by Th1, Th2 and Th17 cells leads to
asthma. Therefore, regulating cytokine levels is the focus for
asthma treatment (24). T
lymphocytes play a major regulatory role in asthmatic airway
inflammation, with Thl cell subsets secreting IFN-γ, which mediates
immune responses and delayed-type hypersensitivity. Th2 cell
subsets primarily produce IL-4, IL-5 and IL-13, which may induce
the production and aggregation of eosinophils, as well as stimulate
B cells to produce IgE and mediate humoral immune responses and
engineering-type hypersensitivity. IL-22 is a proinflammatory
cytokine that exhibits anti-inflammatory activity. IL-22 belongs to
the IL-10 cytokine family and has two functional receptors, IL-22R1
and IL-22R2 (25). IL-22 may be
activated by a variety of signaling pathways, the most important
being the signal transducer and activator of transcription 3
pathway (26). In an
antigen-sensitized rat model, IL-22 levels significantly increased
(27). Neutralizing IL-22
antibodies can increase the production of Th2 cytokines, causing
eosinophil infiltration and airway hyperresponsiveness (28). By contrast, IL-22 is a protective
agent for airway inflammation, and recombinant IL-22 can inhibit
eosinophilic airway inflammation and the production of Th2
cytokines (29,30). IL-22 also inhibits IFN-γ-inducible
expression of proinflammatory cytokines and human airway epithelial
cell adhesion (31). Therefore,
the function of IL-22 is closely associated with the external
environment and changes with the body disease state.
In conclusion, this study investigated the
antioxidant and anti-inflammatory effects of Schisandra and
Paeonia extract and showed that these effects could be
beneficial in alleviating the asthmatic condition. Therefore,
Schisandra and Paeonia extract could be used in the
future treatment of asthma.
Acknowledgements
The study was supported by grants from the 12th
Five-Year Plan of Chengdu (no. C1203) and the Chongqing Gongguan
Projects (no. cstc2012gg-yyjs0627).
References
|
1
|
Holm M, Torén K and Andersson E: Incidence
of chronic bronchitis: a prospective study in a large general
population. Int J Tuberc Lung Dis. 18:870–875. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dai Y, Tu FJ, Yao ZH, Ding B, Xu W, Qiu XH
and Yao XS: Rapid identification of chemical constituents in
traditional Chinese medicine fufang preparation xianling gubao
capsule by LC-linear ion trap/Orbitrap mass spectrometry. Am J Chin
Med. 41:1181–1198. 2013. View Article : Google Scholar
|
|
3
|
Li S, Wang Y, Shi Y, Yu J, Sun W, Hu H and
Zhang Y: Regulatory effects of stage-treatment with established
Chinese herbal formulas on inflammatory mediators in pediatric
asthma. J Tradit Chin Med. 33:727–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li XM: Complementary and alternative
medicine in pediatric allergic disorders. Curr Opin Allergy Clin
Immunol. 9:161–167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmad F and Tabassum N: Preliminary
phytochemical, acute oral toxicity and antihepatotoxic study of
roots of Paeonia officinalis Linn. Asian Pac J Trop Biomed.
3:64–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin SK and Park JH: Effect of the addition
of Schisandra chinensis powder on the physico-chemical
characteristics of sausage. Asian-Australas J Anim Sci.
26:1753–1761. 2013.
|
|
7
|
Wang ZF, Zhao Y, Pang X, Yu HS, Kang LP,
Gao Y and Ma BP: Analysis and identification of chemical
constituents in Siwu decoction by UPLC-Q-TOF-MS(E). Zhongguo Zhong
Yao Za Zhi. 38:3702–3708. 2013.(In Chinese).
|
|
8
|
Ho WE, Cheng C, Peh HY, et al:
Anti-malarial drug artesunate ameliorates oxidative lung damage in
experimental allergic asthma. Free Radic Biol Med. 53:498–507.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang M, Wang XJ and Yang K: Medicine
antioxidants and antioxidant activity in vitro evaluation
methods. Chongqing Keji Xue Yuan Xue Bao. 8:109–112. 2006.(In
Chinese).
|
|
10
|
Diaz P, Jeong SC, Lee S, Khoo C and
Koyyalamudi SR: Antioxidant and anti-inflammatory activities of
selected medicinal plants and fungi containing phenolic and
flavonoid compounds. Chin Med. 24:7–26. 2012.PubMed/NCBI
|
|
11
|
Zhang L, Ravipati AS, Koyyalamudi SR, et
al: Antioxidant and anti-inflammatory activities of selected
medicinal plants containing phenolic and flavonoid compounds. J
Agric Food Chem. 59:12361–12367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Wen XB, Qin CP, et al:
Salvia extracts antioxidant activity. Xi Bei Nong Ye Xue
Bao. 20:160–163. 2011.(In Chinese).
|
|
13
|
Fang XM, Li J, Li ZG and Dong XB:
Functional mechanism of pingchuanning decoction on adjustment of
Clara cell secretory protein in airway remodeling of asthmatic
rats. J Tradit Chin Med. 32:215–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Zhou DX, Liu Y, et al:
Xiaoqinglongtang on airway hyperresponsiveness and its mechanism of
neuromodulation. Zhejiang Zhong Yi Yao Da Xue Xue Bao. 4:32–36.
2011.(In Chinese).
|
|
15
|
Qu ZH, Liu XM, Xie N, et al:
Schisandra ginseng soup prevention of asthma and the
mechanisms in mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 15:201–204.
2008.(In Chinese).
|
|
16
|
Lu Y and Chen DF: Analysis of
Schisandra chinensis and Schisandra sphenanthera. J
Chromatogr A. 1216:1980–1990. 2009.
|
|
17
|
Chen ZY and Yang YJ: Schisandra
lignans extraction technology of new progress. Ji Lin Hua Gong Xue
Yuan Xue Bao. 153:124–126. 2013.(In Chinese).
|
|
18
|
Kim J, Lee H, Lee Y, Oh BG, Cho C, Kim Y,
Shin M, Hong M, Jung SK and Bae H: Inhibition effects of Moutan
Cortex Radicis on secretion of eotaxin in A549 human epithelial
cells and eosinophilmigration. J Ethnopharmacol. 114:186–193. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravipati AS, Zhang L, Koyyalamudi SR, et
al: Antioxidant and anti-inflammatory activities of selected
Chinese medicinal plants and their relation with antioxidant
content. BMC Complement Altern Med. 12:173–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jarjour NN and Calhoun WJ: Enhanced
productin of oxygen radicals in asthma. J Lab Clin Med.
123:131–136. 1994.PubMed/NCBI
|
|
21
|
Fabian E, Pölöskey P, Kósa L, et al:
Activities of antioxidant enzymes in relation to oxidative and
nitrosative challenges in childhood asthma. J Asthma. 48:351–357.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaleli S, Akkaya A, Akdogan M, et al: The
effects of different treatments on prolidase and antioxidant enzyme
activities in patients with bronchial asthma. Environ Toxicol
Pharmacol. 22:35–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petlevski R, Zuntar I, Dodig S, et al:
Malonaldehyde and erythrocyte antioxidant status in children with
controlled asthma. Coll Antropol. 33:1251–1254. 2009.PubMed/NCBI
|
|
24
|
Bartemes KR and Kita H: Dynamic role of
epithelium-derived cytokines in asthma. Clin Immunol. 143:222–235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sonnenberg GF, Fouser LA and Artis D:
Functional biology of the IL-22-IL-22R pathway in regulating
immunity and inflammation at barrier surfaces. Adv Immunol.
107:1–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pickert G, Neufert C, Leppkes M, Zheng Y,
Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S,
Ouyang W, Neurath MF and Becker C: STAT3 links IL-22 signaling in
intestinal epithelial cells to mucosal wound healing. J Exp Med.
206:1465–1472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi K, Hirose K, Kawashima S, Niwa
Y, Wakashin H, Iwata A, Tokoyoda K, Renauld JC, Iwamoto I, Nakayama
T and Nakajima H: IL-22 attenuates IL-25 production by lung
epithelial cells and inhibits antigen-induced eosinophilic airway
inflammation. J Allergy Clin Immunol. 128:1067–1076. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Besnard AG, Sabat R, Dumoutier L, Renauld
JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard
F, Warszawska K, Wolk K, Quesniaux V, Ryffel B and Togbe D: Dual
role of IL-22 in allergic airway inflammation and its cross-talk
with IL-17A. Am J Respir Crit Care Med. 183:1153–1563. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagome K, Imamura M, Kawahata K, et al:
High expression of IL-22 suppresses antigen-induced immune
responses and eosinophilic airway inflammationvia an
IL-10-associated mechanism. J Immunol. 187:5077–5089. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taube C, Tertilt C, Gyülveszi G, Dehzad N,
Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC,
Arnold-Schild D, Schild H, Buhl R and Becher B: IL-22 is produced
by innate lymphoid cells and limits inflammation in allergic airway
disease. PLoS One. 6:e217992011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pennino D, Bhavsar PK, Effner R, Avitabile
S, Venn P, Quaranta M, Marzaioli V, Cifuentes L, Durham SR, Cavani
A, Eyerich K, Chung KF, Schmidt-Weber CB and Eyerich S: IL-22
suppresses IFN-γ-mediated lung inflammation in asthmatic patients.
J Allergy Clin Immunol. 131:562–570. 2013.
|