Introduction
Oxidative stress has been implicated in the
pathophysiology of several types of cardiovascular disease (CVD),
including ischemic stroke, myocardial ischemia, myocardial
stunning, ischemia-reperfusion injury, hypertension and
atherosclerosis. It is also considered to play a role in the
progression of atherosclerosis (1–4).
Previous studies have demonstrated that the majority of patients
with CVD are likely to have chronic oxidative stress and that this
is associated with their diagnosed disease state (5–7).
H2O2 is an important byproduct of oxidative
metabolism and is a major contributor to oxidative stress-induced
functional and metabolic dysfunction.
The β-hydroxy-β-methylglutaryl coenzyme A (HMG-CoA)
reductase inhibitors (statins) are widely used to inhibit the
progression of atherosclerosis and reduce the incidence of CVD. As
well as their cholesterol-lowering effects, statins improve
endothelial function in normocholesterolemia (8,9).
Certain over-the-counter (OTC) products, including resveratrol,
also exhibit similar effects to statins. Resveratrol
(trans-3,5,4-trihydroxystilbene) is a polyphenol (phytoalexin) that
naturally occurs in red wine and in a variety of therapeutic
plants. In vitro experiments have revealed that the
cardiovascular protective effects of resveratrol may occur through
a number of mechanisms. Resveratrol inhibits the proliferation of
smooth muscle cells, platelet aggregation and the oxidation of
low-density lipoprotein cholesterol, and reduces the synthesis of
lipids and eicosanoids, which promote inflammation and
atherosclerosis (10). These
multiple protective effects of resveratrol increase its demand as
an OTC product, even for those undergoing treatment with
statins.
A number of studies have demonstrated the
aggravating effects of statins on oxidative stress in organisms
(11,12). Such aggravating effects of statins
on the myocardium have already been shown (13,14,15,16).
The aim of the present study was to evaluate the effects of
atorvastatin, resveratrol and resveratrol + atorvastatin (R+A)
pretreatment on myocardial contractions and endothelial function in
the presence of H2O2 as an experimental model
of oxidative stress in rats.
Materials and methods
Animals and experimental procedure
A total of 28 male Wistar albino rats, aged 8 weeks
and weighing 260–280 g, obtained from the Animal Care Facility of
Meram Medical Faculty (Konya, Turkey) were used in the present
study. Animals were housed identically in cages in an
air-conditioned room under a 12-h light/dark cycle. Temperature and
relative humidity were controlled within the limits of 21±2°C and
55±15%, respectively. All animals were acclimated for ≥7 days prior
to the onset of the study. A standard diet and tap water were
provided ad libitum. The experimental procedures were
approved by the Animal Ethics Committee of the Meram School of
Medicine (Konya, Turkey). All chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA) and stored according to the
manufacturers’ instructions unless otherwise specified. For 14
days, the control group (n=8) received 1.5 ml drinking water by
oral gavage and 1 ml 10% v/v dimethyl sulfoxide [DMSO;
intraperitoneal (i.p.)], the vehicle for resveratrol. The
atorvastatin group (n=6) received 40 mg/kg atorvastatin
(Lipitor®), which was prepared daily and dissolved in
drinking water, by oral gavage and 1 ml 10% v/v DMSO i.p. for the
same period of time. The resveratrol group (n=6) was treated with
30 mg/kg i.p. resveratrol and 1.5 ml drinking water by oral gavage
for the 14 days, and the R+A group (n=8) was treated with 40 mg/kg
atorvastatin by oral gavage and 30 mg/kg i.p. resveratrol. Rats
were weighed every five days for adjustments to the dosing schedule
and observed every day or as necessary. On day 15, the rats were
anesthetized with an i.p. injection of 50 mg/kg body weight sodium
pentobarbital. The heart and thoracic aorta were removed from each
rat and placed immediately into fresh, oxygenated, ice-cold
Krebs-Henseleit solution (KHS) composed of 119 mmol/l NaCl, 4.7
mmol/l KCl, 1.5 mmol/l MgSO4, 1.2 mmol/l
KH2PO4, 2.5 mmol/l CaCl2, 25
mmol/l NaHCO3 and 11 mmol/l glucose.
Detection of myocardial contraction
Myocardial strips (10–13 mm long and 2–3 mm wide)
were prepared from the left ventricle using a previously described
method (17). The strips were
placed in a 10-ml chamber containing oxygen-enriched (0.5 l/min)
KHS at 37°C. One end of the strip was attached to a force
transducer (MP30; Biopac Systems, Inc., Santa Barbara, CA, USA) by
a thin silk thread and the other end was attached to a hook in the
tissue bath. The strips were left for 30 min for stabilization.
Following the stabilization period, the maximum contractions to
electrical stimulation (ES) were recorded [frequency, 0.5 Hz;
duration, 5 msec; and voltage, 30–40 V (20% above threshold)]. The
effects of H2O2 on myocardial contractions
were then evaluated at concentrations of 1×10−7,
1×10−6 and 1×10−5 M. Following each dose, the
organ bath was washed with KHS and the next dose was applied after
20 min resting time. The same procedure was conducted for all four
groups. Contractions are given as a percentage of the initial
contractions.
Detection of thoracic aorta
responses
Thoracic aorta rings (2–3 mm wide) were placed into
a 10-ml chamber containing oxygen-enriched (0.5 l/min) KHS at 37°C.
One end of the strip was attached to a force transducer (MP30;
Biopac Systems, Inc.) by a thin silk thread, while the other end
was pinned to a hook in the tissue bath. The strips were left for
15 min to spontaneously recover their isometric tension, following
which they were gradually stretched to a resting force of 1 × g.
The tissues were allowed to equilibrate for 30 min with repeated
washing every 10 min with KHS. Following the equilibration period,
the thoracic rings were contracted with 80 mM KCl. After the 30-min
wash-out period in which the tissues were repeatedly washed every
10 min with KHS, 1×10−8, 1×10−7,
1×10−6, 1×10−5 and 1×10−4 M
H2O2 were cumulatively added to the organ
bath. Once the contractions reached a plateau, the tissues were
washed twice every 15 min and incubated with 1×10−4 M N
(G)-nitro-L-arginine methyl ester (L-NAME), an inhibitor of nitric
oxide (NO) formation, for 30 min to evaluate the effect of the
vascular endothelium on the H2O2 results.
Following incubation, 1×10−8, 1×10−7,
1×10−6, 1×10−5 and 1×10−4 M
H2O2 were cumulatively added to the organ
bath once more. All results are expressed as a percentage of the
previous contraction induced by 80 mM KCl.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The statistical significance of differences between the
groups was analyzed by one-way analysis of variance or the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
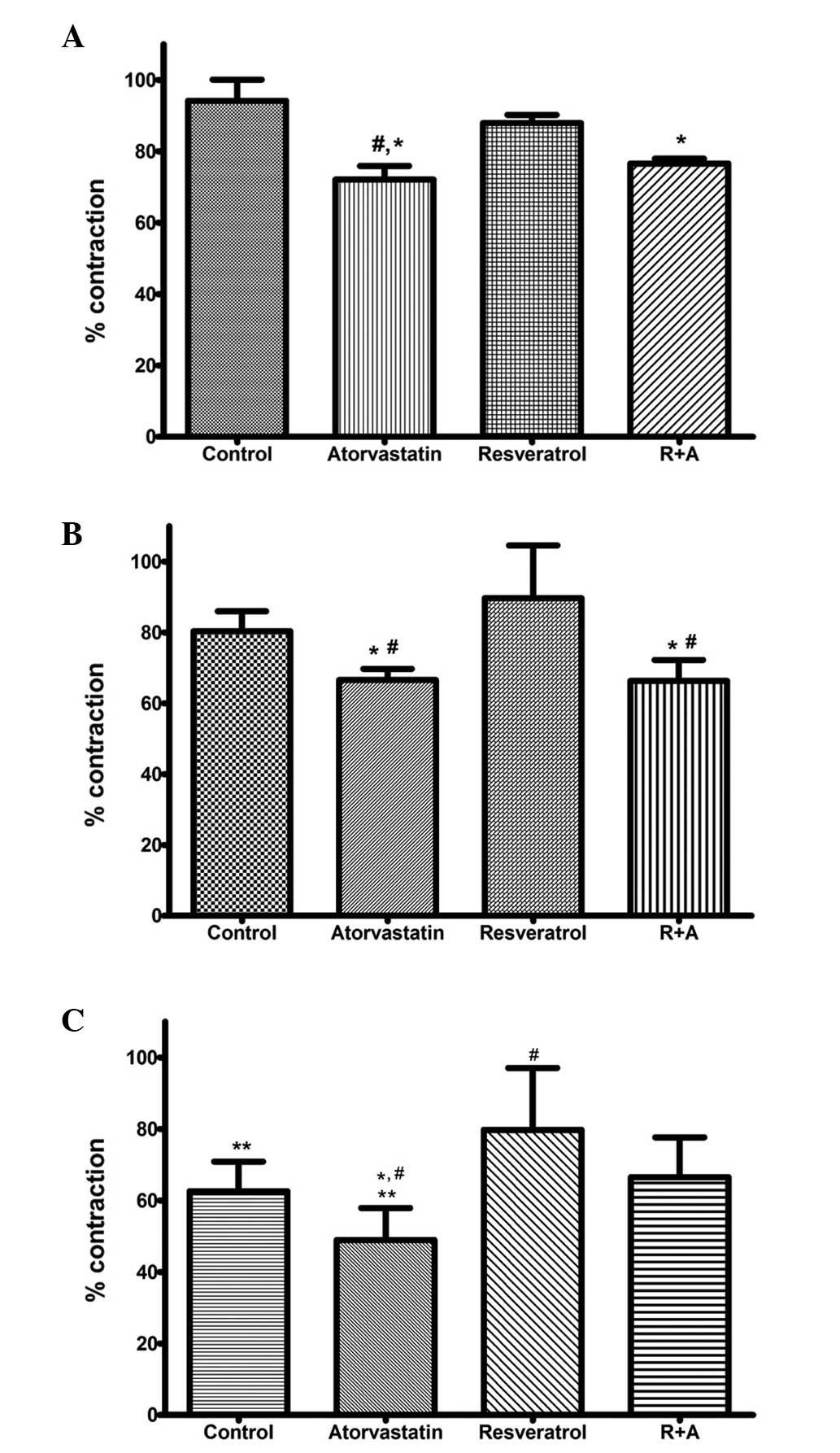
Intragroup myocardial results
H2O2 was applied to the organ
bath at doses of 1×10−7, 1×10−6 and
1×10−5 M. To observe the effects of increasing doses of
H2O2, an intragroup comparison of the
myocardial results was carried out for each group. In the control
group, H2O2 significantly reduced the
contractions induced by ES at all doses (1×10−7 vs.
1×10−6 M and 1×10−6 vs. 1×10−5 M,
P<0.01). The results were 94.16±5.94, 80.35±5.66 and 62.61±8.28%
for doses of 1×10−7, 1×10−6 and
1×10−5 M, respectively. In the rats treated with
atorvastatin, H2O2 caused a significant
dose-dependent decrease in myocardial contractions (72.09±3.80,
66.59±3.14 and 48.96±8.93% for H2O2 doses of
1×10−7, 1×10−6 and 1×10−5 M,
respectively; 1×10−5 vs. 1×10−7 M and
1×10−6 M, P<0.01). In the resveratrol group, no
significant changes in contraction were observed following
H2O2 application at all doses (87.91±2.33,
89.66±14.91 and 79.77±17.33% for H2O2 doses
of 1×10−7, 1×10−6 and 1×10−5 M,
respectively; P>0.05). In the R+A group, the contraction results
following H2O2 application were 76.57±1.40,
66.34±5.91 and 66.55±11.10% for 1×10−7,
1×10−6 and 1×10−5 M
H2O2, respectively;
H2O2 application did not significantly
decrease the contractions when its concentration was increased.
Intergroup myocardial results
Intergroup comparisons of the myocardial results
were evaluated for all doses of H2O2. At
1×10−7 M H2O2 the atorvastatin
group showed a significantly lower contraction percentage when
compared with the control and resveratrol groups (P<0.01). The
R+A group also demonstrated a significant decrease in contraction
percentage when compared with the control group (P<0.01).
However, no significant difference was observed between the
contraction percentages of the resveratrol and control groups
(Fig. 1A). Following a 20-min
washing period, the organ bath was adjusted to 1×10−6 M
H2O2. The myocardial contractions of the
atorvastatin and R+A groups were significantly lower than those
control group (P<0.05). The resveratrol group tissues showed a
significantly higher percentage contraction than those of the
atorvastatin and R+A groups (P<0.01). No significant difference
was observed between the myocardial contractions in the resveratrol
and control groups (Fig. 1B). At
the final dose of H2O2 (1×10−5 M),
the atorvastatin group exhibited a significant decrease in
contraction compared with all the other groups (atorvastatin versus
control and R+A groups, P<0.05; atorvastatin versus resveratrol
group; P<0.01). The results of the present study demonstrated
that resveratrol treatment alone attenuated the decrease in
contraction percentage and that this effect was significant
compared with all groups at 1×10−5 M
H2O2 (P<0.01 vs. atorvastatin and control
and P<0.05 vs. R+A). In the R+A group, the contraction
percentages were higher than those in the atorvastatin group but
significantly lower than those in the resveratrol group (P<0.05)
(Fig. 1C).
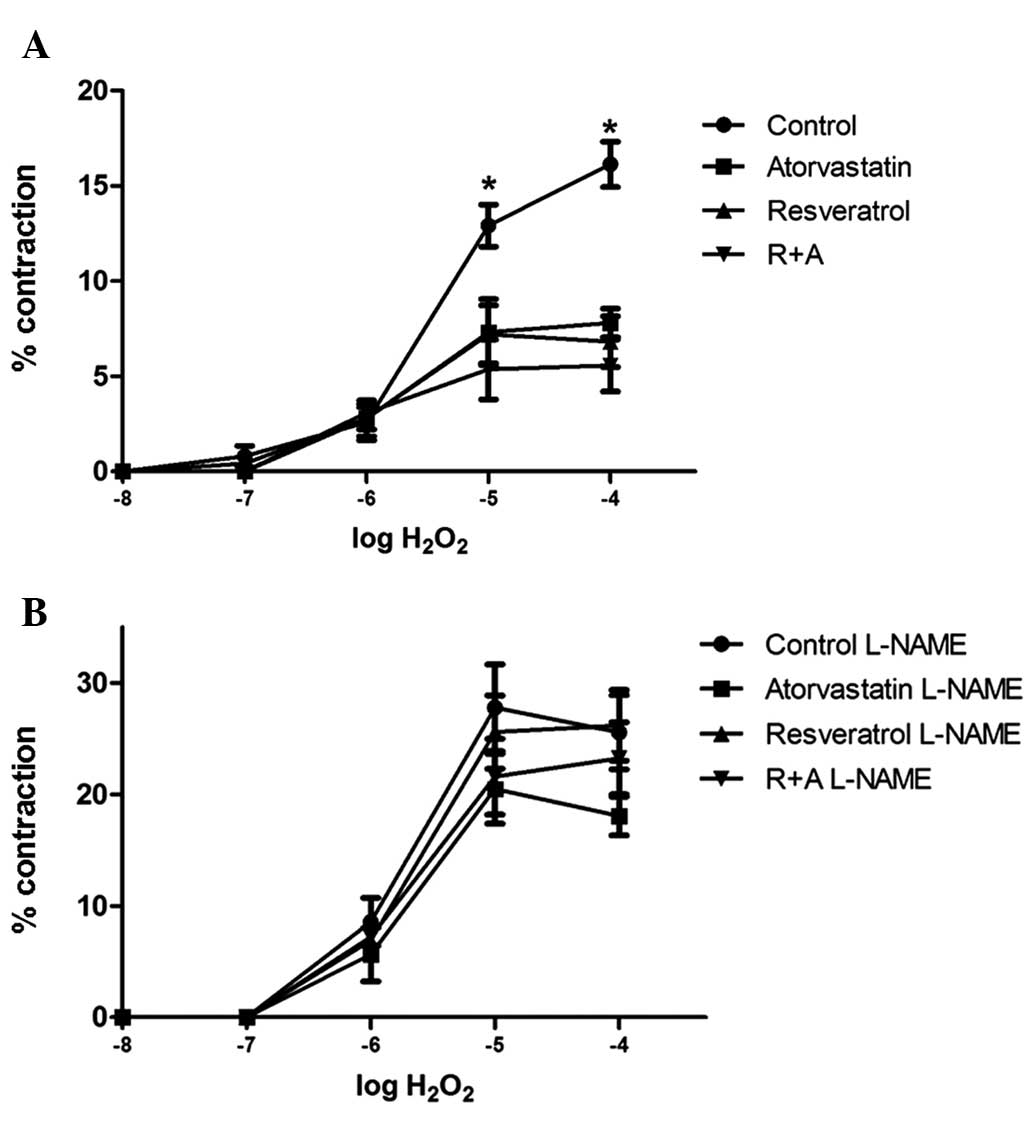
Results of thoracic aorta responses
Fig. 2 shows the
cumulative dose responses to H2O2 (between
1×10−8 and 1×10−4 M) in aortic segments for
the control, resveratrol, atorvastatin and R+A groups, with and
without 1×10−4 M L-NAME incubation. In all groups,
H2O2 caused vasoconstriction and the
contraction responses increased in a concentration-dependent
manner. The aortic rings reached their maximum contraction at
1×10−4 M H2O2, and the maximum
contraction responses were 16.14±1.09, 7.50±0.75, 6.82±1.33 and
5.58±1.37% for the control, atorvastatin, resveratrol and R+A
groups, respectively. The H2O2 responses were
significantly lower in the treatment groups than those in the
control group at 1×10−4 and 1×10−5 M
H2O2 (control versus atorvastatin and
resveratrol groups, P<0.05; control versus R+A group,
P<0.01). When the maximum vasoconstriction responses of the
treatment groups were examined, no statistical differences were
identified among the resveratrol, atorvastatin and R+A groups
(Fig. 2A). Following incubation
with 1×10−4 M L-NAME for 30 min, the contraction
responses of the tissues to H2O2
significantly increased when compared with their previous responses
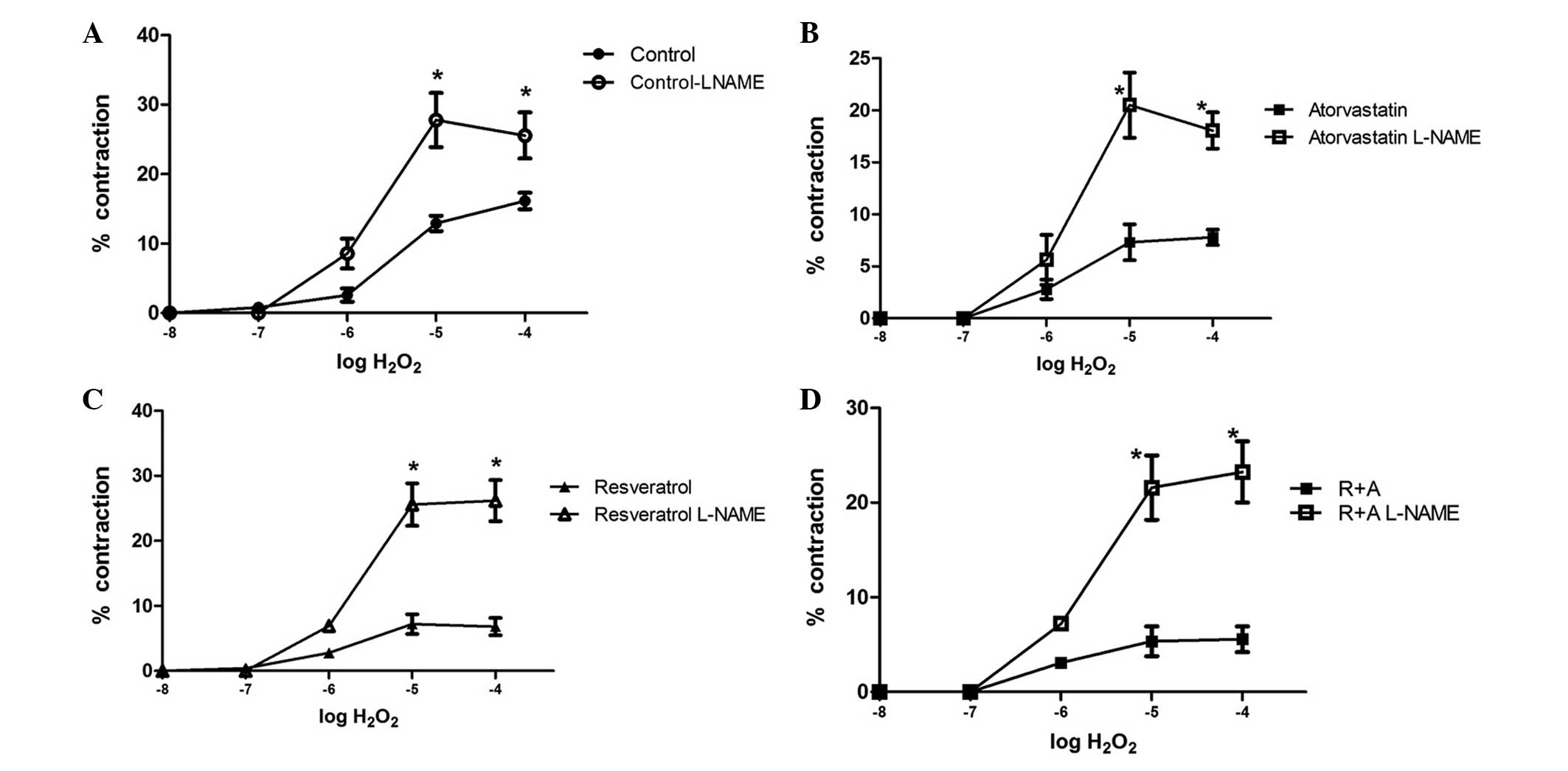
(Fig. 2B). In all groups, L-NAME
incubation significant augmented the H2O2
response at 1×10−5 and 1×10−4 M when compared
with the response without L-NAME incubation (Fig. 3). The maximum responses were
25.56±3.32, 18.06±1.74, 26.19±3.17 and 23.24±3.24% for the control,
atorvastatin, resveratrol and R+A groups, respectively.
The thoracic aorta responses demonstrated that
treatment of rats with resveratrol, atorvastatin and R+A resulted
in a significantly lower vascular contraction response to
H2O2 at a concentration 1×10−4 M
when compared with the control group. However, this result was
eliminated with 1×10−4 M L-NAME incubation.
Discussion
The cardiac results of the present study revealed
that oral administration of 40 mg/kg atorvastatin for 14 days
resulted in a more sensitive myocardial response to
H2O2 in rats. Treatment with 30 mg/kg i.p.
resveratrol showed a cardioprotective effect against
atorvastatin-aggravated and H2O2-induced
contractile dysfunction in the rat myocardium. Furthermore, the
study revealed that atorvastatin and resveratrol exhibited a
protective effect against H2O2-induced
vasoconstriction and that this protective effect was mediated by
NO.
Vasoconstriction induced by cumulative
concentrations of H2O2 was augmented with
L-NAME incubation. These results indicated that the
vasoconstriction elicited by high concentrations of
H2O2 was negatively modulated by the
endothelium. NO exerted a protective effect to counteract the
oxidative effect of H2O2 in the groups
without L-NAME incubation. The protective effect of NO on
H2O2 in endothelial monolayer permeability
has been previously demonstrated by McQuaid et al (18). In their study, it was concluded
that, although lower levels of NO may only give a small amount of
cytoprotection, the barrier dysfunction in the endothelium caused
by H2O2 may be partially reversed by NO
(13). It may be concluded from
the results of the present study that the protective effect of
resveratrol and/or atorvastatin treatment may be attributed to
increased NO production in the vascular endothelium.
Under normal conditions, the
H2O2 concentrations in human plasma, blood
and vascular cells are likely to be in the lower micromolar ranges
or below. However, in pathological states, including myocardial
ischemia and heart failure, it has been demonstrated that
H2O2 concentrations can increase to
millimolar levels (19–21). Atorvastatin impairs cholesterol
production by inhibiting the synthesis of mevalonate. In addition
to cholesterol-lowering effects, statins inhibit the biosynthesis
of the major natural antioxidants ubiquinone (ubiphenol) Q10 and
glutathione peroxidase (22–24).
As a result of this, statins may aggravate oxidative stress in the
organism. Such aggravating effects of statins on the myocardium
have previously been demonstrated (16). These changes may modulate
myocardial contractility. A previous study revealed that an
increase in reactive oxygen species (ROS) in the myocardium
resulted in ischemia, reperfusion injury and myocardial damage
(25).
Studies on the antioxidant effects of statins have
been performed using oxidative stress markers (OSMs) in body fluids
(26–29). Although OSMs are accepted to
reflect the levels of oxidative stress within tissues, Argüelles
et al (30) demonstrated
that OSMs were not correlated with the tissue levels of oxidative
stress; furthermore, they suggested that OSMs did not reflect the
local oxidative stress status of individual organs. This is
consistent with the results of the present study, in which
atorvastatin treatment aggravated the H2O2
response in the myocardial tissue and showed a protective effect on
H2O2-induced vascular contractions. Although
the current study did not assess the antioxidant capacity of the
myocardium, the diminished myocardial response may be attributed to
decreased levels of antioxidant agents in the myocardium, including
ubiquinone Q10, whose production is HMG-CoA reductase-dependent
(31). The protective effect of
resveratrol can be attributed to its inhibitory effect on ROS
production in the myocardium (32).
Increased levels of pro-oxidants have been
associated with vascular diseases and they are considered to be an
important initial step in the development of vascular diseases,
including atherosclerosis and hypertension (33). The present study demonstrated that
atorvastatin treatment disrupted ES-induced myocardial function in
the presence of H2O2, but that its
co-treatment with resveratrol recovered this effect. R+A treatment
also exhibited a protective effect on
H2O2-induced vascular responses. From these
results, resveratrol appears to be a promising treatment for the
improvement of myocardial function in diseases associated with the
development of oxidative stress. Resveratrol has been a popular
choice in OTC products. Resveratrol is frequently used for the
prevention of atherosclerosis; thus, the indications for its
administration appear to be similar to those for the administration
of statins. The combined treatment of R+A provides a superior
treatment for CVD compared with treatment with either agent
alone.
References
|
1
|
Weiss N, Heydrick SJ, Postea O, Keller C,
Keaney JF Jr and Loscalzo J: Influence of hyperhomocysteinemia on
the cellular redox state - impact on homocysteine-induced
endothelial dysfunction. Clin Chem Lab Med. 41:1455–1461. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Landmesser U, Spiekermann S, Dikalov S, et
al: Vascular oxidative stress and endothelial dysfunction in
patients with chronic heart failure: role of xanthine-oxidase and
extracellular superoxide dismutase. Circulation. 106:3073–3078.
2002. View Article : Google Scholar
|
|
3
|
Forgione MA, Leopold JA and Loscalzo J:
Roles of endothelial dysfunction in coronary artery disease. Curr
Opin Cardiol. 15:409–415. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eyries M, Collins T and Khachigian LM:
Modulation of growth factor gene expression in vascular cells by
oxidative stress. Endothelium. 11:133–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harrison D, Griendling KK, Landmesser U,
Hornig B and Drexler H: Role of oxidative stress in
atherosclerosis. Am J Cardiol. 91:7A–11A. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prasad K and Lee P: Suppression of
oxidative stress as a mechanism of reduction of
hypercholesterolemic atherosclerosis by aspirin. J Cardiovasc
Pharmacol Ther. 8:61–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vassalle C, Petrozzi L, Botto N, Andreassi
MG and Zucchelli GC: Oxidative stress and its association with
coronary artery disease and different atherogenic risk factors. J
Intern Med. 256:308–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Futterman LG and Lemberg L: Statin
pleiotropy: fact or fiction? Am J Crit Care. 13:244–249.
2004.PubMed/NCBI
|
|
9
|
Bell RM and Yellon DM: Atorvastatin,
administered at the onset of reperfusion, and independent of lipid
lowering, protects the myocardium by up-regulating a pro-survival
pathway. J Am Coll Cardiol. 41:508–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soner BC, Murat N, Demir O, Guven H, Esen
A and Gidener S: Evaluation of vascular smooth muscle and corpus
cavernosum on hypercholesterolemia. Is resveratrol promising on
erectile dysfunction? Int J Impot Res. 22:227–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sinzinger H, Chehne F and Lupattelli G:
Oxidation injury in patients receiving HMG-CoA reductase
inhibitors: occurrence in patients without enzyme elevation or
myopathy. Drug Saf. 25:877–883. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parker RA, Huang Q and Tesfamariam B:
Influence of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase
inhibitors on endothelial nitric oxide synthase and the formation
of oxidants in the vasculature. Atherosclerosis. 169:19–29. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satoh K, Yamato A, Nakai T, Hoshi K and
Ichihara K: Effects of 3-hydroxy-3-methylglutaryl coenzyme A
reductase inhibitors on mitochondrial respiration in ischaemic dog
hearts. Br J Pharmacol. l16:1894–1898. 1995. View Article : Google Scholar
|
|
14
|
Ichihara K, Satoh K, Yamamoto A and Hoshi
K: Are all HMG-CoA reductase inhibitors protective against ischemic
heart disease? Nippon Yakurigaku Zasshi. 114(Suppl 1): 142P–l49P.
1999.(In Japanese).
|
|
15
|
März W, Siekmeier R, Müller HM, Wieland H,
Gross W and Olbrich HG: Effects of lovastatin and pravastatin on
the survival of hamsters with inherited cardiomyopathy. J
Cardiovasc Pharrnacol Ther. 5:275–279. 2000.PubMed/NCBI
|
|
16
|
Pisarenko OI, Studneva IM, Lankin VZ, et
al: Inhibitor of beta-hydroxy-beta-methylglutaryl coenzyme A
reductase decreases energy supply to the myocardium in rats. Bull
Exp Biol Med. 132:956–958. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sahin AS, Görmüş N and Duman A:
Preconditioning with levosimendan prevents contractile dysfunction
due to H2O2-induced oxidative stress in human
myocardium. J Cardiovasc Pharmacol. 50:419–423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McQuaid KE, Smyth EM and Keenan AK:
Evidence for modulation of hydrogen peroxide-induced endothelial
barrier dysfunction by nitric oxide in vitro. Eur J Pharmacol.
307:233–241. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barlow RS, El-Mowafy AM and White RE:
H(2)O(2) opens BK(Ca) channels via the PLA(2)-arachidonic acid
signaling cascade in coronary artery smooth muscle. Am J Physiol
Heart Circ Physiol. 279:H475–H483. 2000.PubMed/NCBI
|
|
20
|
Lacy F, O’Connor DT and Schmid-Schönbein
GW: Plasma hydrogen peroxide production in hypertensives and
normotensive subjects at genetic risk of hypertension. J Hypertens.
16:291–303. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halliwell B, Clement MV and Long LH:
Hydrogen peroxide in the human body. FEBS Lett. 486:10–13. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lankin VZ, Tikhaze AK, Kaminnaia VI,
Kaminnyĭ AI, Konovalova GG and Kukharchuk VV: Intensification in
vivo of free radical oxidation of low density lipoproteins in
plasma from patients with myocardial ischemia treated by
HMG-CoA-reductase pravastatin and suppression of lipid peroxidation
by ubiquinone Q10. Biull Eksp Biol Med. 129:176–179. 2000.(In
Russian).
|
|
23
|
Lankin VZ, Tikhaze AK, Kukharchuk VV, et
al: Antioxidants decreases the intensification of low density
lipoprotein in vivo peroxidation during therapy with statins. Mol
Cell Biochem. 249:129–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lankin VZ and Tikhaze AK: Atherosclerosis
as a free radical pathology and antioxidative therapy of this
disease. Free Radicals, Nitric Oxide, and Inflammation: Molecular,
Biochemical, and Clinical Aspects. Tomasi A, Özben T and Skulachev
VP: (NATO Science Series). 344. IOS Press; Amsterdam: pp. 218–231.
2003
|
|
25
|
Robicsek F and Schaper J: Reperfusion
injury: fact or myth? J Card Surg. 12:133–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thomas MK, Narang D, Lakshmy R, Gupta R,
Naik N and Maulik SK: Correlation between inflammation and
oxidative stress in normocholesterolemic coronary artery disease
patients ‘on’ and ‘off’ atorvastatin for short time intervals.
Cardiovasc Drugs Ther. 20:37–44. 2006.PubMed/NCBI
|
|
27
|
Wassmann S, Ribaudo N, Faul A, Laufs U,
Böhm M and Nickenig G: Effect of atorvastatin 80 mg on endothelial
cell function (forearm blood flow) in patients with pretreatment
serum low-density lipoprotein cholesterol levels <130 mg/dl. Am
J Cardiol. 93:84–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sugiyama M, Ohashi M, Takase H, Sato K,
Ueda R and Dohi Y: Effects of atorvastatin on inflammation and
oxidative stress. Heart Vessels. 20:133–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marketou ME, Zacharis EA, Nikitovic D, et
al: Early effects of simvastatin versus atorvastatin on oxidative
stress and proinflammatory cytokines in hyperlipidemic subjects.
Angiology. 57:211–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Argüelles S, Garcia S, Maldonado M,
Machado A and Ayala A: Do the serum oxidative stress biomarkers
provide a reasonable index of the general oxidative stress status?
Biochim Biophys Acta. 1674:251–259. 2004.PubMed/NCBI
|
|
31
|
Hargreaves IP, Duncan AJ, Heales SJ and
Land JM: The effect of HMG-CoA reductase inhibitors on coenzyme
Q10: possible biochemical/clinical implications. Drug Saf.
28:659–676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li YG, Zhu W, Tao JP, et al: Resveratrol
protects cardiomyocytes from oxidative stress through SIRT1 and
mitochondrial biogenesis signaling pathways. Biochem Biophys Res
Commun. 438:270–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: the role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|