Introduction
Insulin autoimmune syndrome (IAS), or Hirata’s
disease, is a rare condition characterized by the combination of
recurrent, severe spontaneous hypoglycemia, high concentration of
total immunoreactive insulin (IRI) and the presence of
autoantibodies to insulin in patients who have not received insulin
injections. Since Hirata et al first described the disease
(1), there have been a total of
330 reported cases of IAS over the last 37 years in Japan (2), where IAS is the third leading cause
of spontaneous hypoglycemia, following insulinoma and diffuse
islet-cell hyperplasia (nesidioblastosis). IAS is more common among
patients aged >40 years, with reports of this sundrome in the
pediatric age group being extremely rare. The peak age of onset was
reported to be 60–69 years for both genders (2) and the incidence of IAS is lower in
countries outside Japan. The number of reports of this disorder in
China and, particularly, in adolescents, is limited. In addition,
reports on IAS-related human leukocyte antigen (HLA) genotype are
rare, with only 84 cases reported in China by late 2012 (3). This is the rare case report of a
methimazole (MTZ)-associated IAS in a Chinese female adolescent
patient.
Case report
A 17-year-old female patient from Southwest China
was referred to the local hospital due to recurrent episodes of
weariness, sweating, palpitations and weight loss of ~5 kg within a
few months. The patient’s thyroid-stimulating hormone was 0.011
mU/l (reference range, 0.27–4.2 mU/l) and her free thyroxine was
100 pmol/l (reference range, 12.0–22.0 pmol/l). The patient was
diagnosed with hyperthyroidism and antithyroid treatment with MTZ
(10 mg, three times daily) was initiated. After 2 weeks of
continuous MTZ administration, the patient experienced dizziness,
weakness, cold sweats, palpitations and tremor ~2 h after
breakfast. The symptoms disappeared following ingestion of food
(bread, milk). Four weeks later, the patient experienced
palpitations and sweating followed by unconsciousness during the
late postprandial phase (3 h after the ingestion of food). The
patient was immediately admitted to a local hospital and
hypoglycemia was diagnosed on the basis of a blood glucose
concentration of 2.88 mmol/l and symptom relief following
intravenous glucose administration; however, the cause of
hypoglycemia had not been identified. The patient was referred to
our hospital for further assessment and treatment. The physical
examination on admission revealed a chronically ill appearance,
with a weight of 65 kg, a height of 170 cm (BMI, 22.5
kg/m2) and blood pressure of 110/75 mmHg. There was no
exophthalmos and no thyroid enlargement, although the thyroid was
diffusely tender on palpation. The other systematic examinations
were normal. The patient had no history of diabetes mellitus,
infectious diseases, insulin usage or intake of oral hypoglycemic
agents. The laboratory tests revealed that the thyroid-stimulating
hormone level was 0.010 mU/l (reference range, 0.27–4.2 mU/l), the
free thyroxine was 91.54 pmol/l (reference range, 12.0–22.0 pmol/l)
and the thyrotrophin receptor antibody was 27.48 IU/l (reference
range, <3 IU/l), reflecting uncontrolled Graves’ disease. The
patient’s fasting plasma glucose was 4.62 mmol/l, the glycosylated
hemoglobin was 5.6%, the fasting serum total IRI was >1,000
mU/ml (reference range, 1.5–15 mU/l) and the free serum C-peptide
was 2.04 nmol/ml (reference range, 0.48–0.78 nmol/ml). The
haemoglobin concentration, leukocyte count, platelet count,
erythrocyte sedimentation rate and liver, renal and adrenal
function tests were all normal. There was no space-occupying lesion
in the pancreas on abdominal computed tomography.
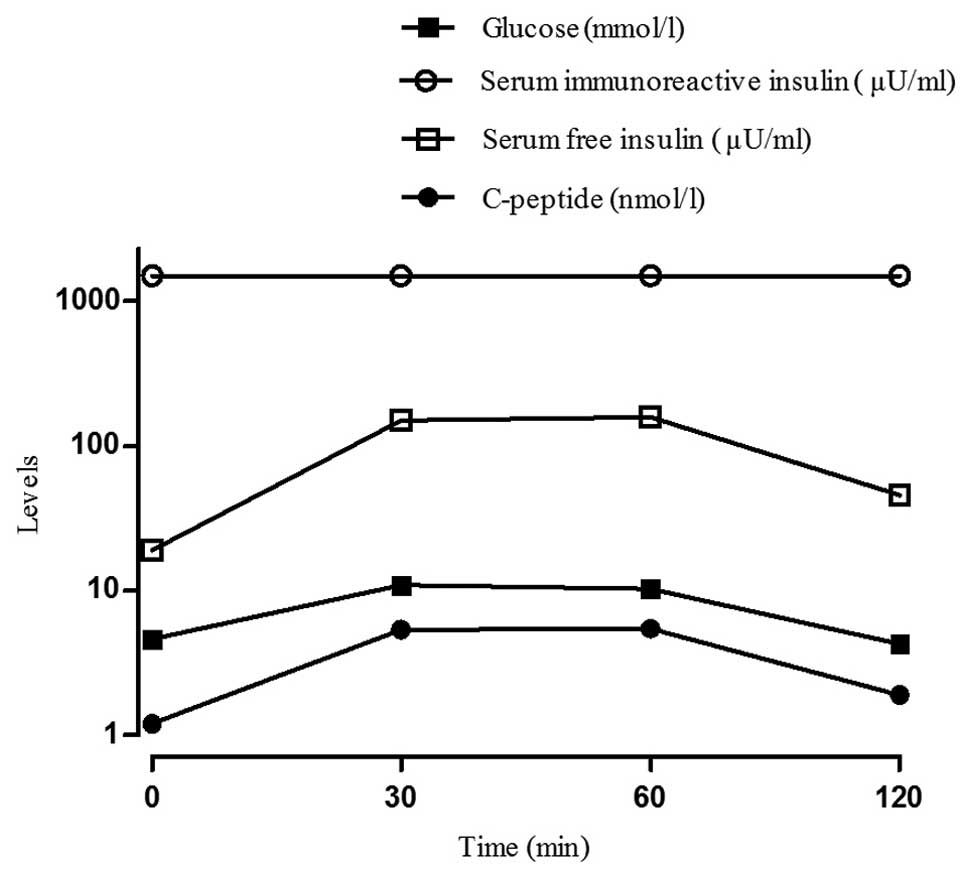
The laboratory tests revealed disproportionately
increased serum total IRI and C-peptide levels and anti-islet
β-cell autoantibodies were evaluated. The islet cell antibody (ICA)
and glutamic acid decarboxylase antibody (GADA) tests were
negative, but the insulin autoantibody (IAB) level was 2.40 U/ml
(reference range, negative). To further determine the association
between the serum concentrations of total IRI, free insulin,
glucose and C-peptide, the patient underwent an oral glucose
tolerance test, an insulin release test, the synchronized free
insulin and C-peptide levels were evaluated and the polyethelene
glycol (PEG) precipitation method was employed to remove IABs prior
to free insulin measurement to exclude interference. The results
revealed that total IRI levels were significantly elevated at all
time points, unlike C-peptide, which remained elevated while plasma
glucose began to decline during the oral glucose tolerance test,
suggesting a delayed clearance of insulin. Free insulin levels were
marginally increased at 30 min, but were significantly lower
compared to the total IRI, followed by a slow decline, beginning to
decline significantly by 90 min, with plasma glucose levels
fluctuating between 4.52 and 10.9 mmol/l after a 75 g oral glucose
load (Fig. 1). Serum total IRI and
free insulin levels were significantly increased
disproportionately, which indicated that hypoglycemia may be
secondary to MTZ-induced IAS. The patient was advised to
discontinue MTZ and was prescribed propylthiouracil (PTU) 100 mg
three times daily.
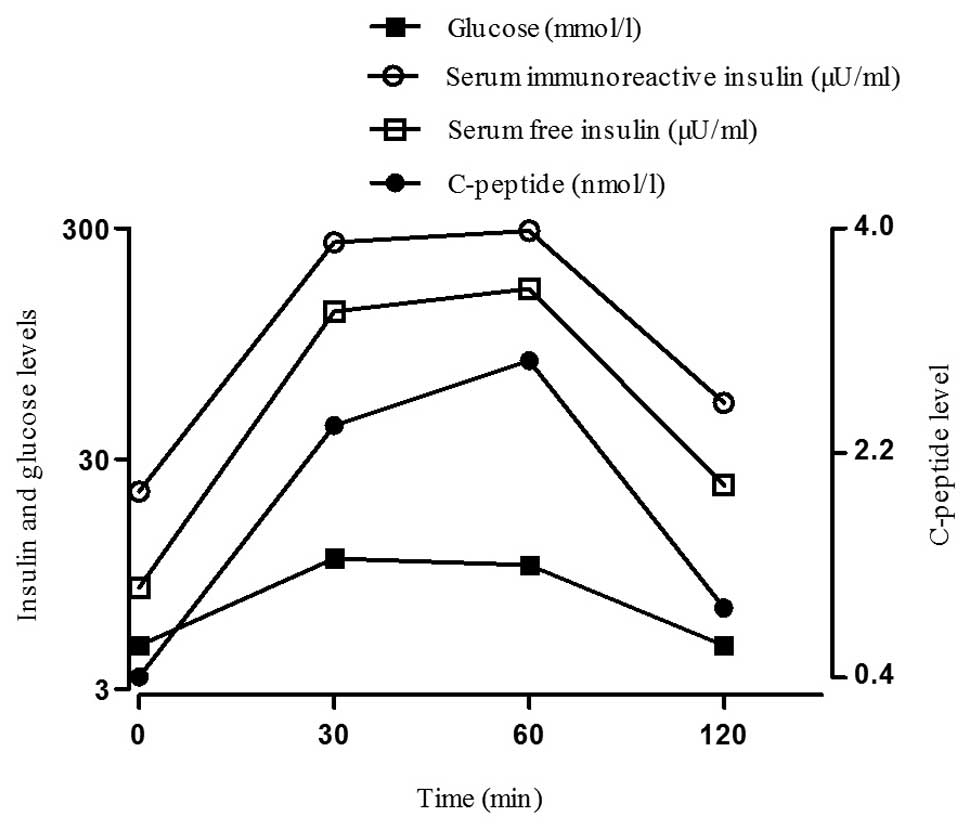
The patient continued the treatment protocol after
discharge and did not experience any further hypoglycemic episodes
over the following 5 months. The symptoms caused by hyperthyroidism
had also improved at the follow-up visit after 5 months, the
thyroid stimulating hormone level was 0.027 mU/l and the free
thyroxine level was 17.72 pmol/l. The oral glucose tolerance and
insulin release tests revealed that the total IRI levels had
decreased markedly compared to those on admission, but remained
elevated above baseline. The synchronized free insulin and
C-peptide levels had returned to normal (Fig. 2). During long-term follow-up, the
IAB levels gradually declined and were not detectable after 5
months of PTU administration (Table
I). The patient’s HLA typing by polymerase chain
reaction-sequence based typing revealed the
DRB1*0406/0901 genotype.
 | Table IAnti-islet β-cell autoantibody tests
on follow-up visits. |
Table I
Anti-islet β-cell autoantibody tests
on follow-up visits.
| Autoantibodies
(U/ml) | 2 weeks | 2 months | 5 months | 8 months |
|---|
| ICA (reference range,
negative) | Negative | Negative | Negative | Negative |
| GADA (reference
range, <1.05) | Negative | 1.02 | 1.01 | 0.94 |
| IAB (reference range,
negative) | 2.04 | 2.13 | Negative | Negative |
Discussion
Our patient had received MTZ for hyperthyroidism for
~2 weeks prior to the first hypoglycemic attack. There was no
exposure history to insulin or insulin-stimulating drug therapy;
therefore, oral hypoglycemic agents or exogenous insulin-induced
causes of hypoglycemia were excluded. The insulin release test
revealed that serum total IRI and free insulin levels were
significantly increased disproportionately, reflecting the
separation of the two insulins. The serum IRI and C-peptide levels
were not synchronized and serum IAB was increased. In addition,
there were no pancreatic abnormalities on abdominal computed
tomography and the ICA and GADA tests were negative. No underlying
autoimmune disorders or other causes of IAS could be identified,
excluding the possibility that the hypoglycemia was caused by
exogenous insulin increase. Therefore, it was concluded that the
hypoglycemia was due to IAB production induced by MTZ
treatment.
The cause of IAS is heterogeneous and has not been
fully elucidated. A previous study including 380 Japanese IAS
patients reported that ~50% of the patients had received drugs with
sulfhydryl groups (2), such as MTZ
(most common) (4), thiopronin,
penicillamine, glutathione, as well as other drugs, including
hydralazine, procainamide and isoniazid. It has been hypothesized
that sulfhydryl group drugs may cleave the disulfide bond of the
insulin molecule in vivo and enhance its immunogenicity,
which may lead to the production of IABs (5). In our patient, the history of
exposure to MTZ supported an etiological role for drugs with
sulfhydryl group in the development of IAS.
Previous studies have divided IAB into two types,
namely low-affinity/high-capacity and high-affinity/low-capacity
types (6,7). Eguchi et al (8) reported that the IAB produced in
patients with IAS is of the low-affinity/high-capacity type, as
demonstrated on the Scatchard plot. In our patient, the symptoms of
hypoglycemia occurred 2–3 h postprandially, as a large number of
insulin molecules released after food ingestion may bind to these
low-affinity/high-capacity IABs, which prevent insulin from acting.
Therefore, elevated blood glucose levels further stimulate islet β
cells to release more insulin, which also binds to the IABs. The
bound insulin may readily dissociate from the IABs, resulting in a
rapid increase in free insulin levels and consequent
hypoglycemia.
A characteristic of this patient’s presentation was
the strikingly high levels of total IRI (9). Insulin was measured by
radioimmunoassay; IAB and anti-IAB reagents competitively bound,
which caused spurious hyperinsulinemia. The PEG precipitation
method was employed to remove IABs prior to free insulin
measurement in order to exclude interference. The free insulin
levels were marginally increased, but were significantly lower
compared to total IRI, reflecting the separation of free insulin
and total IRI. Human proinsulin C-peptide is a cleavage product of
insulin secretion in the β cells of the islets of Langerhans and is
released in amounts equal to insulin into the portal circulation
(10). C-peptide levels may be
associated with the secretion of islet β cells (11). Since specific IABs may crossreact
with proinsulin and C-peptide levels were moderately elevated, the
serum C-peptide levels actually did not accurately reflect the
secretion of islet β cells. Despite the concordant elevation of
insulin and C-peptide levels in our tests, as the proportion of
proinsulin in the blood was low, a discrepancy between excessively
high IRI and only moderately elevated C-peptide levels was
obvious.
The majority of IAS cases described in Asians
exhibit a strong correlation with certain HLA systems, suggesting
the existence of a predisposing genetic component. It is noteworthy
that HLA-DRB1*0406 is quite prevalent among East Asian
patients, but Caucasian patients mainly express
HLA-DRB1*0403 (12). In
fact, Japanese IAS patients were found to be DR4-positive in 96%
(49/51) of cases and 82% (42/51) of cases possessed
DRB1*0406 in the polyclonal type (13). A previous study confirmed that the
product of DRB1*0406 is an insulin antigen-presenting
major histocompatibility complex molecule (14); therefore, populations with a higher
prevalence of DRB1*0406 are at higher risk of developing
IAS. The number of reports of the association of this disorder with
HLA genotype in China is limited, with a lack of large-scale
research data. By late 2012, only one case of a Chinese patient
with DRB1*0406 had been reported (15). The adolescent patient described in
this case report was HLA-DRB1*0406/0901, which supports
an common genetic and immunological basis of IAS.
In Japan, IAS is reportedly the third leading cause
of spontaneous hypoglycemia, in addition to insulinoma and diffuse
islet-cell hyperplasia (nesidioblastosis). It is generally
considered that insulinoma may also be characterized by low blood
glucose and high insulin levels (16). As in several cases of insulinoma
without a reliable tumor localization prior to surgery, small
tumors are often only identified by an experienced surgeon
(17), it was necessary to
evaluate IAB to avoid unnecessary surgery (5,18).
In addition, these findings almost certainly excluded the
possibility of occult insulinoma or nesidioblastosis. It was
previously suggested that insulin levels >1,000 pmol/l argue
against insulinoma and raise the suspicion of IAS (17). There were no pancreatic
abnormalities on abdominal computed tomography, the IAB test was
positive, an insulin release test exhibited the separating
phenomenon of free insulin and total IRI and the process of the
disease described in this case report was self-limiting. The
patient was advised to discontinue MTZ and supportive treatment was
provided. In addition, there was no other episode of hypoglycemia.
Therefore, insulinoma was excluded as the cause of
hypoglycemia.
Approximately 80% of the Japanese IAS patients
reported between 1970 and 2007 experienced a spontaneous remission
without special treatment (2).
Spontaneous remission may develop in <3 months. Following
emergency treatment of hypoglycemia, an α-glucosidase inhibitor may
be prescribed in combination with dietary control to decrease
endogenous insulin secretion (19). It has been reported that, in
patients with very high IAB concentrations, large dose of
chloroquine, cyclophosphamide and corticosteroids may significantly
lower plasma IAB concentrations. It has also been reported that
plasma exchange successfully decreased the IAB concentration in a
patient with severe, repeated episodes of hypoglycemia (20,21).
For refractory patients with frequent episodes of hypoglycemia,
partial pancreatectomy may be feasible, but is now rarely used in
clinical practice (6). In the
present case, antithyroid treatment with MTZ was discontinued after
admission and PTU was prescribed, with no further episodes of
hypoglycemia.
Although hypoglycemia caused by MTZ-induced IAS is
rare in adolescents with Graves’ disease, clinicians should be
aware of this possible etiology. For Graves’ disease patients
receiving antithyroid treatment with MTZ who experience
hypoglycemia and whose hypoglycemia cannot be explained by other
causes, IAS must be considered in order to avoid undue pancreatic
surgery and IAB levels should be assessed to exclude or confirm
this as the underlying cause. If MTZ-induced IAS is confirmed, the
patient should be advised to avoid the use of MTZ thereafter.
References
|
1
|
Hirata Y, Ishizu H, Ouchi N, et al:
Insulin autoimmunity in a case of spontaneous hypoglycemia. J Jpn
Diab Soc. 13:312–320. 1970.
|
|
2
|
Uchigata Y and Hirata Y: Insulin
autoimmune syndrome (Hirata disease). Immunoendocrinology:
Scientific and Clinical Aspects. Eisenbarth GS: 1st edition. Humana
Press; Totowa, NJ: pp. 343–367. 2011
|
|
3
|
Kai L, Xinguo H, Jun S, et al: Clinical
characteristics of insulin autoimmune syndrome in Chinese patients.
Chin J Intern Med. 50:690–691. 2011.
|
|
4
|
Lupsa BC, Chong AY, Cochran EK, et al:
Autoimmune forms of hypoglycemia. Medicine (Baltimore). 88:141–153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchigata Y and Hirata Y: Insulin
autoimmune syndrome (IAS, Hirata disease). Molecular Mechanisms of
Endocrine and Organ Specific Autoimmunity. Eisenbarth GS: 1st
edition. Landes Company; Paris: pp. 245–253. 1999, PubMed/NCBI
|
|
6
|
Kim MR, Sheeler LR, Mansharamani N, et al:
Insulin antibodies and hypoglycemia in diabetic patients. Can a
quantitative analysis of antibody binding predict the risk of
hypoglycemia? Endocrine. 6:285–291. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brooks-Worrell BM, Nielson D and Palmer
JP: Insulin autoantibodies and insulin antibodies have similar
binding characteristics. Proc Assoc Am Physicians. 111:92–96. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eguchi Y, Uchigata Y, Yao K, et al:
Longitudinal changes of serum insulin concentration and insulin
antibody features in persistent insulin autoimmune syndrome
(Hirata’s disease). Autoimmunity. 19:279–284. 1994.PubMed/NCBI
|
|
9
|
Gomez Cruz MJ, Jabbar M, Saini N, et al:
Severe hypoglycemia secondary to methimazole-induced insulin
autoimmune syndrome in a 16 year old African-American male. Pediatr
Diabetes. 13:652–655. 2012.PubMed/NCBI
|
|
10
|
Rubenstein AH, Clark JL, Melani F, et al:
Secretion of proinsulin C-peptide by pancreatic beta cells and its
circulation in blood. Nature. 224:697–699. 1969. View Article : Google Scholar
|
|
11
|
Lohmann T, Kratzsch J, Kellner K, et al:
Severe hypoglycemia due to insulin autoimmune syndrome with insulin
autoantibodies crossreactive to proinsulin. Exp Clin Endocrinol
Diabetes. 109:245–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uchigata Y, Hirata Y, Omori Y, Iwamoto Y
and Tokunaga K: Worldwide differences in the incidence of insulin
autoimmune syndrome (Hirata disease) with respect to the evolution
of HLA-DR4 alleles. Hum Immunol. 61:154–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cavaco B, Uchigata Y, Porto T, et al:
Hypoglycaemia due to insulin autoimmune syndrome: report of two
cases with characterisation of HLA alleles and insulin
autoantibodies. Eur J Endocrinol. 145:311–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murakami M, Mizuide M, Kashima K, et al:
Identification of monoclonal insulin autoantibodies in insulin
autoimmune syndrome associated with HLA-DRB1*0401. Horm Res.
54:49–52. 2000.PubMed/NCBI
|
|
15
|
Jianlin D, Changc L and lin G: Case
report: insulin autoimmune syndrome induced by methimazole. Chin J
Intern Med. 40:438. 2001.
|
|
16
|
Moreira RO, Lima GA, Peixoto PC, Farias ML
and Vaisman M: Insulin autoimmune syndrome: case report. Sao Paulo
Med J. 122:178–180. 2004. View Article : Google Scholar
|
|
17
|
Virally ML and Guillausseau PJ:
Hypoglycemia in adults. Diab Metab. 25:477–490. 1999.
|
|
18
|
Basu A, Service FJ, Yu L, et al: Insulin
autoimmunity and hypoglycemia in seven white patients. Endocr
Pract. 11:97–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schlemper RJ, Uchigata Y, Frölich M,
Vingerhoeds AC and Meinders AE: Recurrent hypoglycaemia caused by
the insulin autoimmune syndrome: the first Dutch case. Neth J Med.
48:188–192. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yaturu S, DePrisco C and Lurie A: Severe
autoimmune hypoglycemia with insulin antibodies necessitating
plasmapheresis. Endocr Pract. 10:49–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greenfield JR, Tuthill A, Soos MA, et al:
Severe insulin resistance due to anti-insulin antibodies: response
to plasma exchange and immunosuppressive therapy. Diabet Med.
26:79–82. 2009. View Article : Google Scholar : PubMed/NCBI
|