Introduction
Allergic asthma affects 1–18% of the population in
different countries and therefore, represents one of the most
common diseases (1). Asthma is a
chronic inflammatory airway disease characterized by recurrent
episodes of wheezing, breathlessness, chest tightness and coughing
driven by an aberrant T helper 2 (Th2) immune response to
environmental allergens (2). In
addition to its classical nervous system domain, nerve growth
factor (NGF) is regarded as an important factor involved in the
pathogenesis of allergic diseases, including asthma (3). Studies in ovalbumin sensitized and
challenged asthmatic mice with anti-NGF antibody (4) or small interfering RNA (5) have shown that blocking NGF can
improve airway inflammation. In addition, transgenic mice
overexpressed NGF in the airways had more severe airway
inflammation compared with wild-type mice when ovalbumin sensitized
and challenged (4). However, the
mechanisms underlying the effects of NGF on inflammation in asthma
remain unclear.
Previous studies have hypothesized that NGF
functions as an amplifier for Th2 effector functions, playing an
important role in the development of airway hyperreactivity
(6). Recently, an in vivo
study demonstrated that anti-NGF treatment inhibited allergic
airway inflammation by modulating the balance of pro- and
anti-asthmatic T cell responses in the lungs of mice (7). However, the maturation and subtype of
lung dendritic cells (DCs) were not investigated in this study. DCs
can be found throughout the conducting airways, lung interstitium,
vasculature, pleura, and bronchial LN Under basal conditions
(8). Serving as innate sensors of
foreign antigens/pathogens, DCs recognize microbial patterns,
damage induced molecules and cytokines and then migrate to the
draining lymph nodes where they activate naive T lymphocytes,
affecting the nature of T-lymphocyte differentiation (9). Lambrecht et al have shown that
in vivo depletion of lung CD11c+ dendritic cells during OVA
challenge abolished the characteristic features of asthma,
including eosinophilic inflammation, goblet cell hyperplasia, and
bronchial hyperreactivity (10).
In the absence of CD11c(+) cells, endogenous or adoptively
transferred CD4(+) Th2 cells did not produce interleukin (IL)-4,
IL-5, and IL-13 in response to OVA aerosol, which were restored by
adoptive transfer of CD11c(+) DCs (10). Similarly, They have also
demonstrate that inflammatory DCs are necessary and sufficient for
induction of Th2 immunity in a house dust mite sensitized and
challenged asthmatic mice (11).
Collectively, these date suggested that DCs play an important role
in the stimulation and polarization of naive T cells towards a Th2
phenotype in the face of allergen exposure. Since NGF can amplify
Th2 response as mention above, the present study hypothesized that
NGF may be involved in regulating lung DCs maturation and
differentiation during the pathogenesis of asthma.
Materials and methods
Experimental animals and treatment
Female BALB/c mice (age, 6–7 weeks; weight, 20–22 g)
were obtained from the Experimental Animal Center of Central South
University (Changsha, China). The mice were housed in a quiet,
antigen-free environment, with standardized temperature and
humidity and free access to food and water. The experimental
protocols were approved by the Institutional Animal Care and Use
Committee of Xiangya School of Medicine, Central South University,
and were in accordance with the guidelines provided by the National
Institutes of Health (Bethesda, MD, USA).
Mice were allowed to acclimatize for one week prior
to the start of the experiment, and were then randomly divided into
four groups as follows (n=8 for each subgroup): control, asthma,
control immunoglobulin G (IgG) and anti-NGF. The mice were treated
as previously described (12).
Briefly, on days one and eight, all the groups, with the exception
of the control group, were sensitized via an intraperitoneal (i.p.)
injection of 20 μg OVA (Sigma-Aldrich, St. Louis, MO, USA) and 1 mg
aluminum hydroxide hydrate (Sigma-Aldrich) in 500 μl saline. On the
same days, the mice in the control group were sham-sensitized with
saline. On day 21, all the groups, with the exception of the
control group, were challenged once daily for five days with 1% OVA
(1% w/v in saline buffer) for 30 min using a nebulizer
(Boehringer-Ingelheim, Ingelheim, Germany), while the control mice
were exposed to aerosolized sterile saline only. At 30 min prior to
the inhalation treatment, mice in the anti-NGF group and control
IgG group were injected (i.p.) with an anti-NGF antibody (1:2,000;
Millipore Corporation, Billerica, MA, USA) or rabbit IgG (1:2,000;
Millipore Corporation) in phosphate-buffered saline (PBS; 4 ml/kg),
respectively (13). The control
and asthma group received vehicle only.
Analysis of airway responsiveness
At 24 h following the last OVA challenge, mice were
anesthetized and airway responsiveness to methacholine was measured
using whole-body plethysmography (PLY 3211; Buxco Electronics,
Troy, NY, USA), as previously described (14). Mice were treated for 2 min with
aerosolized saline or increasing doses of methacholine generated by
an ultrasonic nebulizer, following which airway resistance (RL) was
measured. RL was determined by dividing the driving pressure by the
rate of air flow. The results for each methacholine concentration
were expressed as the percentage of the baseline RL values
following 0.9% NaCl exposure. Following the measurement of airway
responsiveness, blood samples were collected by cardiac puncture
and the mice were sacrificed by exsanguination.
Lung histology
Samples of the right middle lung lobe were fixed in
4% paraformaldehyde and embedded in paraffin. Tissue sections (4
μm) were stained with hematoxylin and eosin, and morphological
changes in the lungs were observed using a light microscope.
Peribronchial inflammation of four mice from each group were
analyzed and scored as follows: 0, normal; 1, few cells were
observed; 2, a ring of inflammatory cells one cell layer deep; 3, a
ring of inflammatory cells 2–4 cell layers deep; and 4, a ring of
inflammatory cells >4 cell layers deep (15).
ELISA assay
NGF, interferon (IFN)-γ and interleukin (IL)-4
levels in the serum were quantified using ELISA kits, in accordance
with the manufacturer’s instructions (NGF: KA0400; Abnova, Taipei,
Taiwan; IFN-γ: DuoSet ELISA DY485; R&D Systems, Minneapolis,
MN, USA; IL-4: DuoSet ELISA DY404; R&D Systems). Absorbance was
measured at 450 nm using a plate reader.
Western blot analysis
NGF protein expression levels in the lungs were
determined using western blot analysis. Total protein was extracted
and 30 μg protein was separated using 8% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. The proteins were
electrotransferred to a polyvinylidene fluoride membrane (Millipore
Corporation). Non-specific binding was inhibited by incubating the
membranes with 0.05 g/ml skim milk powder at room temperature
(20°C) for 2 h, following which the membranes were incubated at 4°C
overnight with an anti-NGF antibody (1:500; Abcam, Cambridge, MA,
USA). Subsequently, the membranes were incubated with a goat
anti-rabbit IgG horseradish peroxidase-conjugated antibody
(1:2,000; Sigma-Aldrich). Reactions were visualized using enhanced
chemiluminescence reagents (Pierce Biotechnology, Inc., Rockford,
IL, USA) and quantified using Glyko Bandscan 5.0 software (Glyko,
Novato, CA, USA). Results were expressed as the mean band density
normalized against β-actin, which was used as an internal
control.
DC isolation and flow cytometric
analysis
Single-cell suspensions were prepared from the
lungs, as previously described (16). Lung lavage was initially performed
using ice-cold PBS with 5 mM EDTA. The lungs were then perfused
with 10 ml PBS, containing 10 U/ml heparin, via the right ventricle
of the heart until the lungs turned white. The lungs were removed,
cut into small fragments and digested with 250 U/ml collagenase D
(Roche Diagnostics, Mannheim, Germany). EDTA (final concentration,
10 mM) was added after 5 min to stop the collagenase activity,
following which a 100-μm cell strainer (BD Biosciences, Franklin
Lakes, NJ, USA) was used to filter the disassociated lungs.
Finally, hypotonic lysis was performed to remove the erythrocytes,
resulting in a single cell suspension. The CD11c+ cells
were positively selected using an anti-mouse CD11c magnetic
microbeads kit (CD11c N418, catalog no. 130-052-001; Miltenyi
Biotec, Bergisch Gladbach, Germany) and autoMACS (Miltenyi Biotec),
in accordance with the manufacturer’s instructions (17). Lung DCs were stained using
fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD80
(11-0801; eBiosciences, Hatfield, UK), allophycocyanin-conjugated
anti-mouse CD86 (17-0862; eBiosciences), phycoerythrin-conjugated
anti-mouse major histocompatibility complex (MHC) class II
(11-5322; eBiosciences) and FITC-conjugated anti-mouse CD103
(11-1031; eBiosciences) antibodies, followed by staining with the
appropriate fluorochrome-conjugated isotype control Ig. Flow
cytometry was performed using a FACS Calibur flow cytometer
(Becton-Dickinson, Mountain View, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
and statistical analysis was performed using SPSS 19.0 software
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
followed by the Student-Newman-Keuls test was used for multiple
comparisons, while the correlation coefficient was calculated using
Spearman’s correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
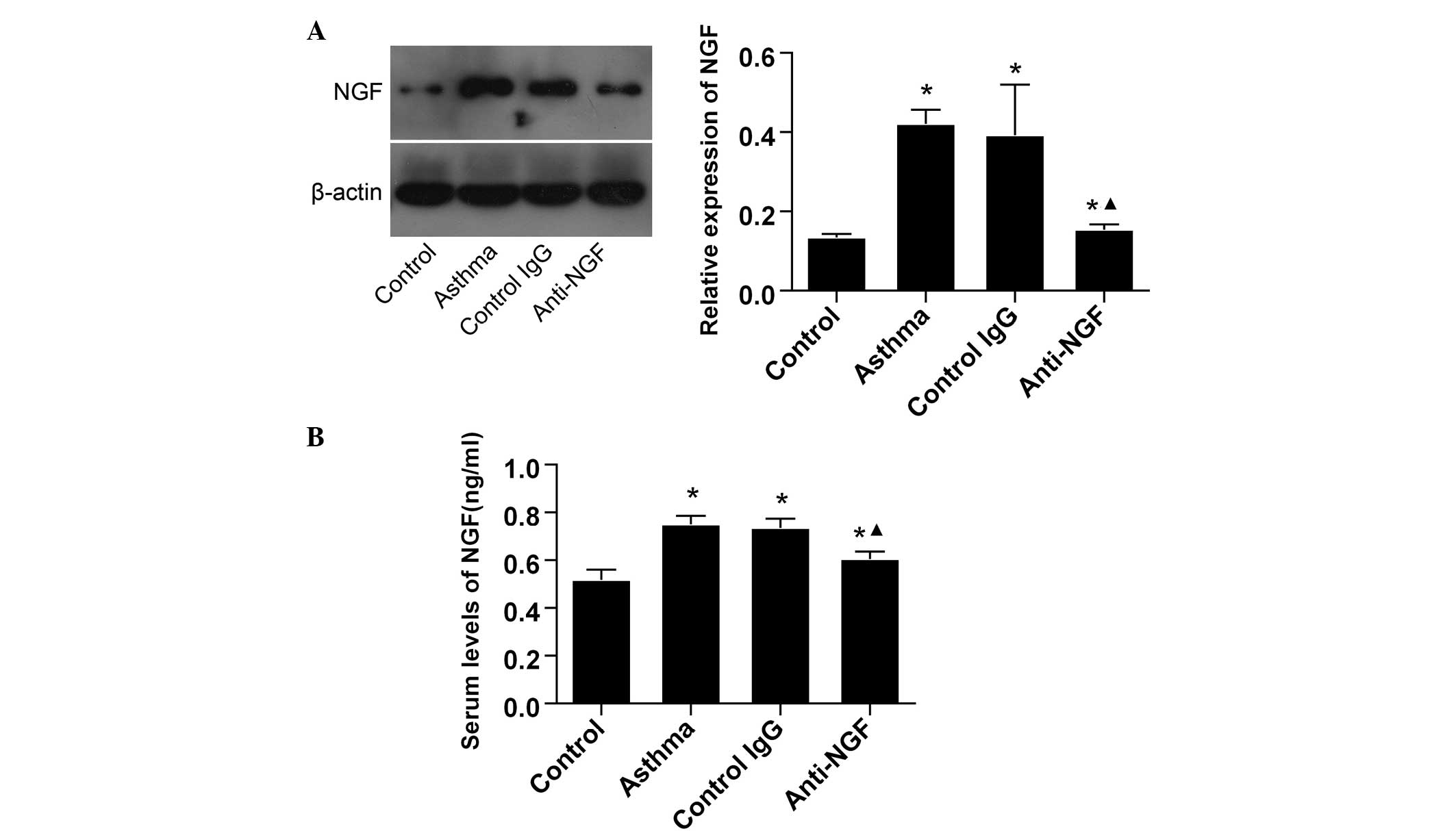
NGF is upregulated in the lungs and serum
of asthmatic mice
Expression levels of NGF were analyzed in the lung
tissues and serum to determine whether NGF production increased in
asthmatic mice. Using western blot analysis and ELISA, NGF
expression levels were shown to increase in the lungs and serum of
asthmatic mice when compared with the control mice. As
hypothesized, pretreatment with an anti-NGF monoclonal antibody
decreased the NGF expression levels in the lung tissues and serum
of asthmatic mice when compared with the mice in the control IgG
and control groups (Fig. 1).
NGF neutralization attenuates airway
hyperreactivity (AHR), airway inflammation and the Th2 response in
asthmatic mice
As shown in Fig.
2A, at methacholine concentrations of ≥2.5 mg/ml, RL was found
to significantly increase with increasing methacholine
concentrations in the asthma group, as compared with the control
group (P<0.05). Treatment with control IgG did not exhibit a
marked effect on methacholine-induced hyperreactivity, with the RL
in the control IgG-treated mice not significantly different when
compared with the asthmatic mice. By contrast, treatment with
anti-NGF antibodies significantly reduced the RL when compared with
the asthma group; however, a number of RL values in the anti-NGF
group mice remained significantly higher compared with those in the
control group. These results revealed that anti-NGF treatment
inhibits allergen-induced AHR in mice.
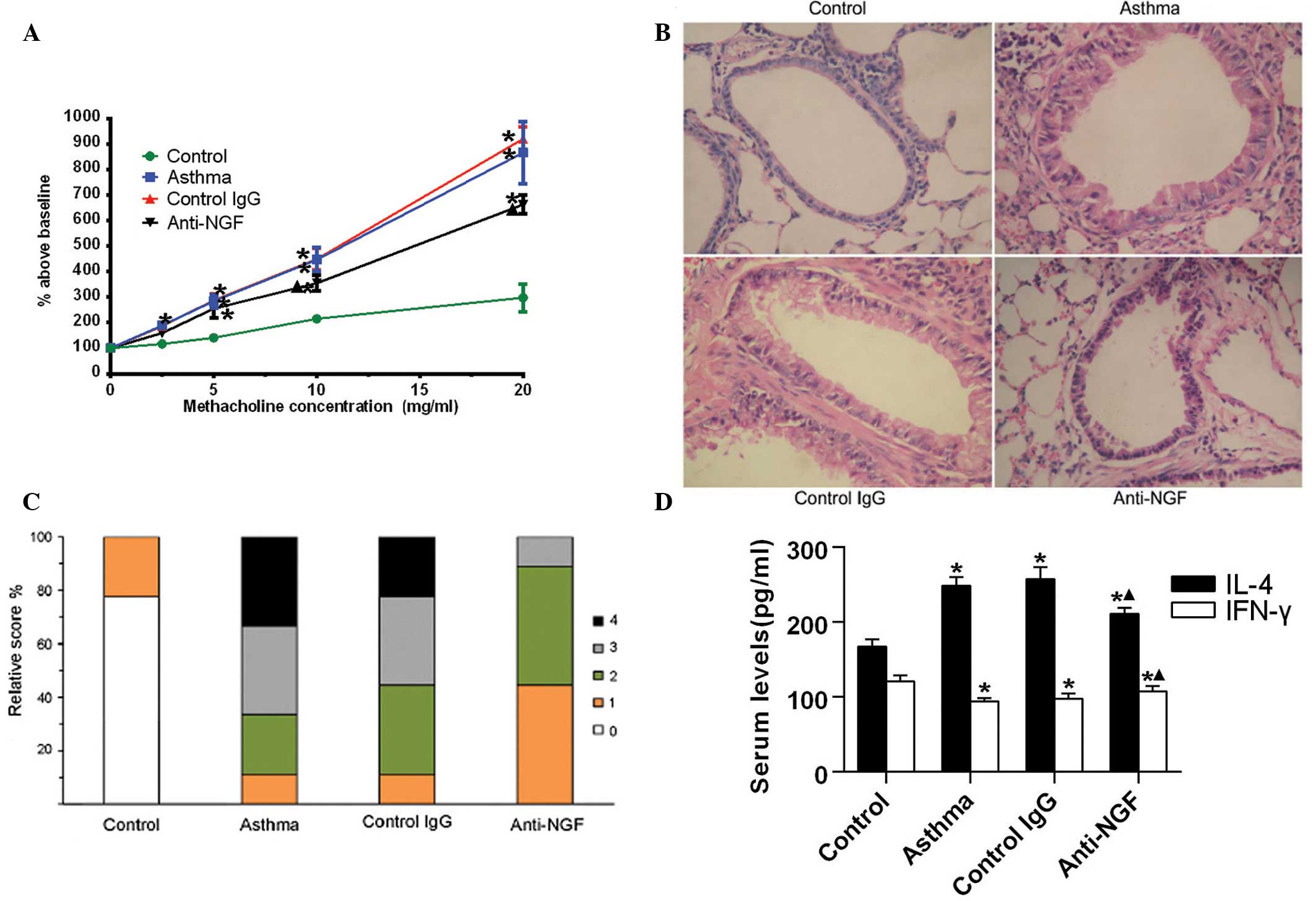 | Figure 2NGF neutralization attenuates airway
hyperreactivity, airway inflammation and the T helper 2 response in
asthmatic mice. Mice were sensitized via an intraperitoneal
injection of 20 μg ovalbumin (OVA) and 1 mg aluminum hydroxide
hydrate in 500 μl saline on days one and eight. From day 21, the
mice were challenged with 5% OVA for five consecutive days. At 24 h
following the last OVA challenge, mice were anesthetized and the
airway responsiveness to methacholine was analyzed. (A) Changes in
mice airway resistance (RL) in response to methacholine exposure.
Data are expressed as a percentage above baseline (normal
saline-induced RL) and presented as the mean ± standard deviation
(n=7). (B) Representative photomicrographs of hematoxylin and
eosin-stained lung sections from the mice (magnification, ×100).
(C) Peribronchial inflammation scores of the mice; peribronchial
inflammation of four mice from each group were analyzed and scored
as follows: 0, normal; 1, few cells observed; 2, a ring of
inflammatory cells one cell layer deep; 3, a ring of inflammatory
cells 2–4 cells deep; and 4, a ring of inflammatory cells >4
cells deep. (D) Serum expression levels of IL-4 and IFN-γ in the
mice were detected using an ELISA. Data are presented as the mean ±
standard deviation (n=7). *P<0.05, vs. control group;
▲P<0.05, vs. asthma and control IgG groups. NGF,
nerve growth factor; IgG, immunoglobulin G; IFN, interferon; IL,
interleukin. |
Histological examination of the lung tissue sections
revealed that while the peribronchiolar infiltration of
inflammatory cells was almost absent in the control mice, numerous
inflammatory infiltrates were observed around the bronchial walls
of the mice in the asthma and control IgG groups. By contrast, the
content of inflammatory infiltrates and bronchial damage were
reduced in the lungs of mice in the anti-NGF group (Fig. 2B). Quantitative analysis confirmed
that the degree of peribronchial inflammation in the anti-NGF group
decreased significantly compared with the asthma and control IgG
groups (P<0.05; Fig. 2C). These
observations indicated that pretreatment with anti-NGF antibodies
significantly reduced the airway inflammatory response in
sensitized mice when administered prior to the allergen
challenge.
In addition, the serum expression levels of IL-4, as
detected by ELISA, in the asthma and control-IgG groups were
significantly higher when compared with the control group. However,
the levels of IFN-γ were lower compared with those in the control
group. By contrast, levels of IL-4 were reduced significantly, but
levels of IFN-γ increased markedly, in the mice of the anti-NGF
group when compared with those in the asthma and control-IgG groups
(Fig. 2D). Therefore, these
results indicated that NGF neutralization attenuates the Th2
response and strengthens the Th1 response in asthmatic mice.
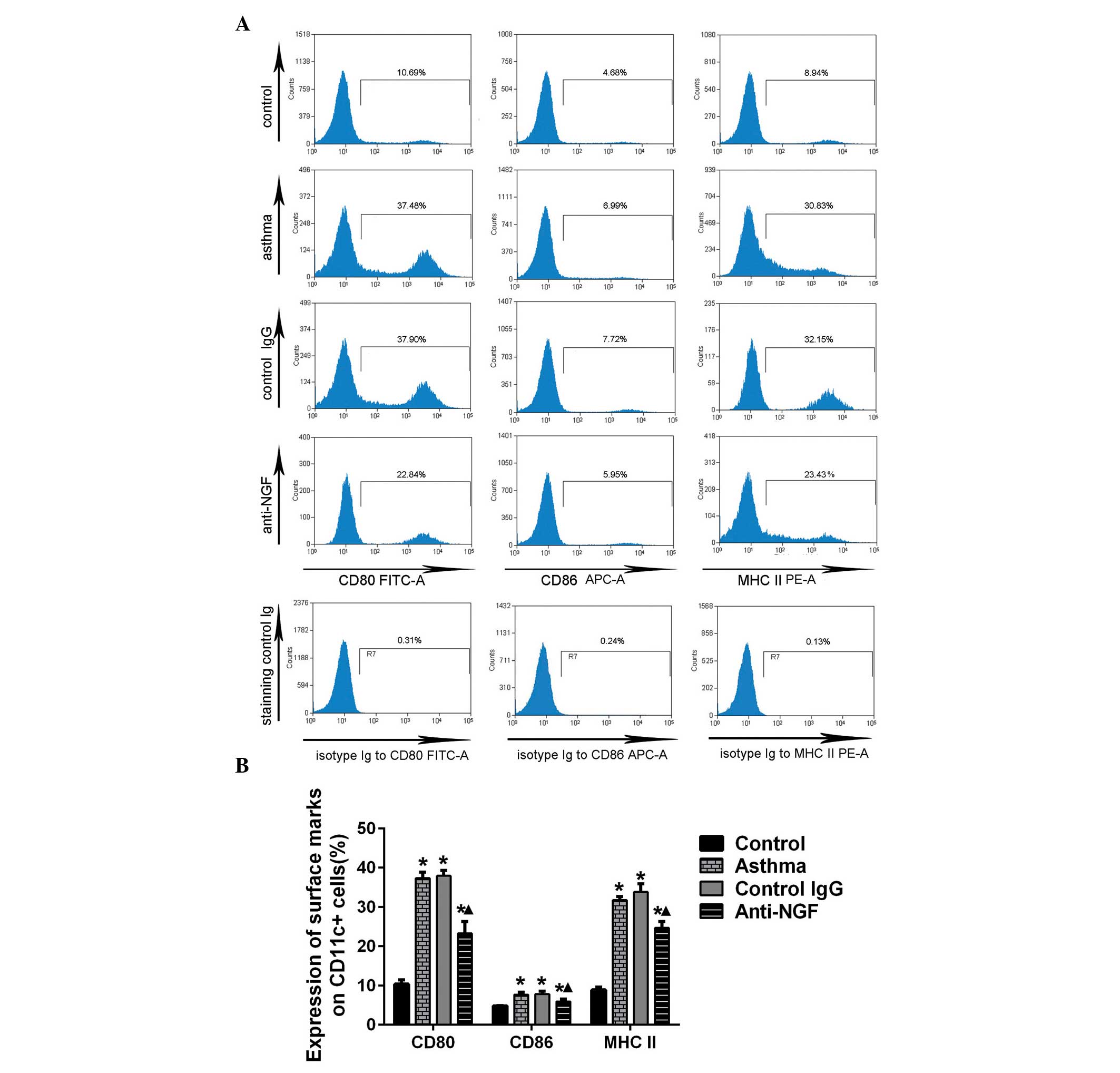
NGF neutralization inhibits the
maturation of lung DCs in asthmatic mice
CD80, CD86 and MHC class II are surface markers that
are highly expressed in mature DCs. The expression levels of CD80,
CD86 and MHC class II on the lung DCs were found to be upregulated
in the mice in the asthma and IgG control groups, as compared with
the control group. A marked downregulation in the expression levels
of CD80, CD86 and MHC class II was observed in the lung DCs of the
mice in the anti-NGF group when compared with the mice in the
asthma and control IgG groups (Fig.
3).
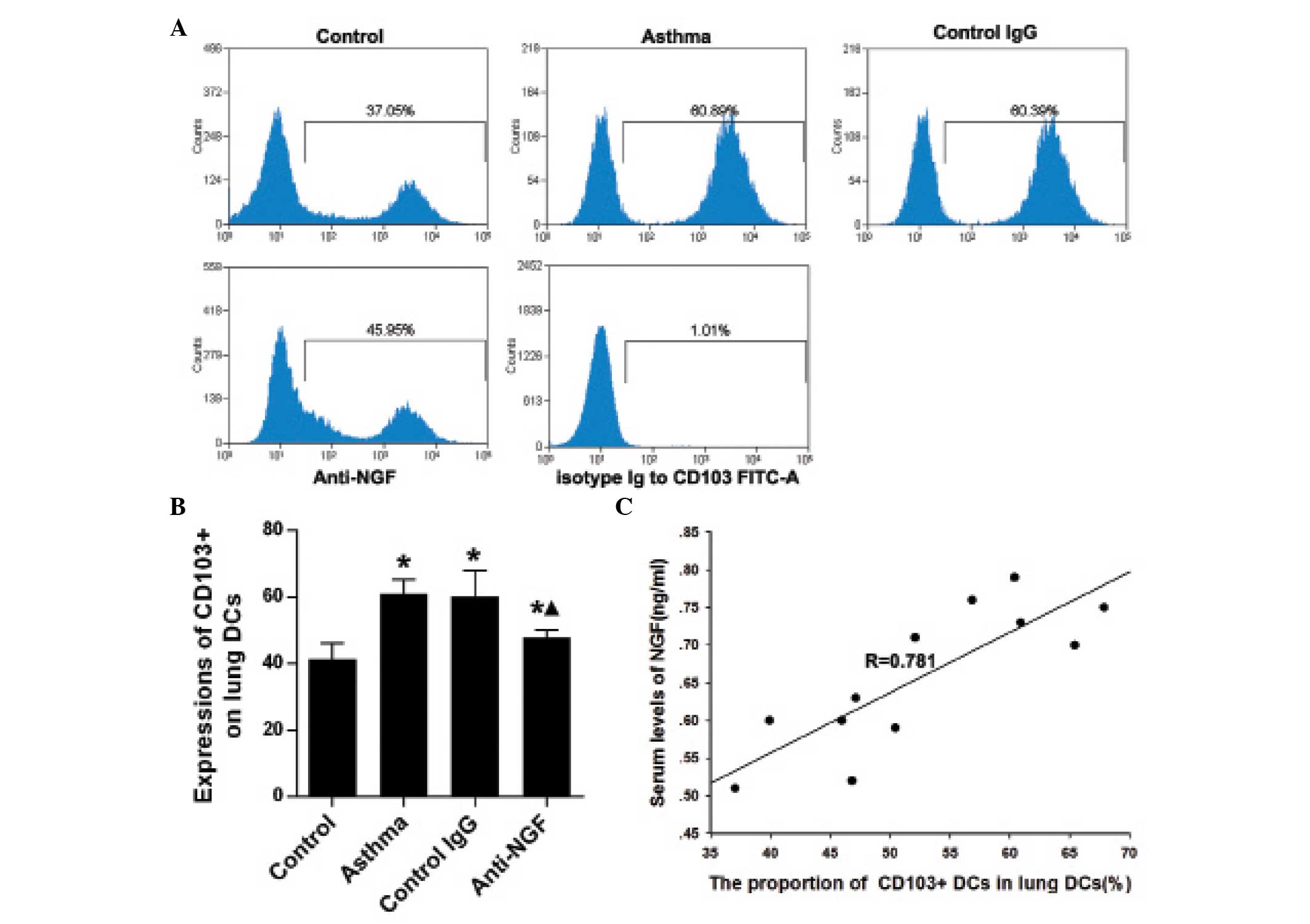
NGF neutralization reduces the percentage
of CD103+ lung DCs in asthmatic mice
Compared with the control mice, the percentage of
CD103+ lung DCs was significantly increased in the mice
in the asthma and control IgG groups (P<0.05), as demonstrated
by flow cytometric analysis. Anti-NGF treatment decreased the
percentage of lung DCs with a CD103+ phenotype in
asthmatic mice (Fig. 4A and B).
Furthermore, as shown in Fig. 4C,
the ratio of lung CD103+ DCs to lung DCs exhibited a
positive correlation with the expression levels of NGF in the
plasma of the mice. These results indicated that elevated levels of
NGF may contribute to the increased number of lung
CD103+ DCs in asthmatic mice.
Discussion
Asthma is an airway inflammatory disease with an
underlying Th2 cell-mediated inflammatory response in the airways
(18). Increased levels of NGF
expression are observed in patients with asthma following bronchial
provocation with an allergen, and are associated with the severity
of the inflammatory process and disease (19–21).
Furthermore, a previous study demonstrated that NGF may exacerbate
allergic lung inflammation in an animal model of asthma (22). However, the underlying mechanisms
are yet to be fully elucidated. The observations of the present
study provide new evidence indicating that NGF exacerbates lung
inflammation by promoting lung DC maturation and polarization
towards a Th2-stimulating phenotype in a mouse model of asthma.
In accordance with previous studies, the present
study found that OVA-sensitized and challenged mice developed
histopathological and biochemical features of asthma, including the
infiltration of inflammatory cells in the airway, increased
thickness of the basement membrane, increased airway responsiveness
to methacholine and increased expression of NGF in the lungs and
serum (22,23). In addition, anti-NGF treatment was
shown to markedly reduce the levels of IL-4 and significantly
increase the levels of IFN-γ in the serum of the mice. These
results are consistent with previous observations (6,7,22),
indicating the possible involvement of NGF in the development of
the Th2 immune response. However, the mechanisms underlying NGF
exacerbation of the Th2 inflammatory response in the airway remain
unclear.
A novel observation of the present study was that
NGF neutralization inhibits the maturation of lung DCs in asthmatic
mice. Recently, an in vivo study (7) demonstrated that anti-NGF treatment
significantly reduced allergic airway inflammation, upregulated the
expression levels of IFN-γ and IL-10 and increased the number of
Th1 and T regulatory cells; however, downregulated IL-4 and tumor
necrosis factor (TNF)-α expression and reduced the number of Th2
and Th17 cells in a mouse model of asthma. These results indicated
that NGF exacerbates allergic airway inflammation by modulating T
cell responses (7). By functioning
as a potent antigen-presenting cell (APC), DCs play an important
role in initiating and maintaining T cell responses. Following
allergen capture and processing, DCs mature, migrate to the T cell
area in the draining mediastinal lymph node (LN) and induce a T
cell response in the draining LN (24). During this process, DCs acquire a
mature phenotype, where the expression levels of costimulatory
molecules necessary for optimal naive T cell activation are
upregulated, and acquire the capacity to stimulate an effector
response (24). However, there is
limited knowledge and research with regard to the role of NGF in
the maturation and differentiation of DCs. In the present study,
the expression levels of CD80, CD86 and MHC II were shown to be
upregulated in the lung DCs of asthmatic mice when compared with
the DCs in the control mice that were administered a vehicle only.
Treatment with anti-NGF antibodies reduced OVA-stimulated CD80,
CD86 and MHC class II expression in the DCs. Therefore, these
results indicate that NGF may have an important role in promoting
the maturation of lung DCs in asthmatic mice. However, the present
study investigated the maturation of lung DCs in vivo only;
thus, further in vitro investigations are required.
Furthermore, results from previous in vitro experiments
investigating whether NGF promotes the maturation of DCs are
controversial (25,26). Noga et al (25) reported that following stimulation
with NGF, no significant upregulation of CD80 and CD86 was observed
in human monocyte-derived DCs (MoDCs). In addition, in allogeneic
leucocyte reactions, MoDCs stimulated with NGF were unable to
induce massive T-cell proliferation. By contrast, Jiang et
al (26) demonstrated that NGF
markedly promoted lipopolysaccharide (LPS)-induced expression of
CD80, CD86 and the proinflammatory cytokines, IL-1, IL-6 and TNF-α,
and the T cell-stimulating capacity of MoDCs, indicating that NGF
may promote LPS-induced DC maturation (26). In addition, Braun et al
(6) hypothesized that the Th2
response is not initiated under the influence of NGF, but instead
an existing Th2 immune response is augmented by NGF. Therefore,
further in vivo study is required to verify whether NGF may
promote lung DC maturation in the background of allergic asthma.
The results of the present study further the understanding of the
mechanisms underlying NGF augmenting the Th2 response in asthma.
IFN-γ is known to be a potent inducer of CD80, CD86 and MHC II on
DCs (27). However, decreased
expression levels of CD80, CD86 and MHC class II were observed in
the lung DCs of the mice in the anti-NGF group, as compared with
those in the asthma and control-IgG groups, despite increased serum
levels of IFN-γ. One possible explanation is that a variety of
factors determine the maturation of DCs, and IFN-γ is just one of
these factors. Anti-NGF treatment may result in the decrease of
other promoting factors or the increase of inhibiting factors in
asthmatic mice; thus, leading to this seemingly contradictory
phenomenon. However, further investigations are required to confirm
this hypothesis.
An additional important observation of the present
study was that NGF neutralization reduced the percentage of lung
DCs with a CD103+ phenotype in asthmatic mice. Nakano
et al (28) reported that
lung CD103+ DCs may prime Th2 differentiation to inhaled
allergens. Furthermore, mice lacking CD103+ DCs have
been shown to produce markedly reduced allergic responses to
clinically relevant allergens, including cockroach antigens and
house dust extracts. Therefore, these results indicate that lung
CD103+ DCs play a significant role in priming the Th2
response to inhaled antigens (28). In the present study, anti-NGF
treatment prior to an OVA challenge was found to significantly
reduce the number of lung CD103+ DCs in asthmatic mice,
indicating that an NGF-induced Th2 response may be associated with
an increased number of lung CD103+ DCs in asthmatic
mice.
In conclusion, the results of the present study
provide an explanation for the NGF promoting activation of lung
DCs, which undergo maturation and polarization towards a
Th2-stimulating phenotype, inducing a Th2 response in asthma.
Therefore, downregulating NGF levels may benefit patients with
allergic asthma. However, only limited conclusions can be derived
from the present results and further studies are required to
confirm these observations.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 81170023).
References
|
1
|
Global Strategy for Asthma Management and
Prevention, Global Initiative for Asthma (GINA). 2012, http://www.ginasthma.org/documents/5/documents_variants/37uri.
Accessed December 30, 2012
|
|
2
|
Bosnjak B, Stelzmueller B, Erb KJ and
Epstein MM: Treatment of allergic asthma: modulation of Th2 cells
and their responses. Respir Res. 12:1142011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nassenstein C, Schulte-Herbruggen O, Renz
H and Braun A: Nerve growth factor: the central hub in the
development of allergic asthma? Eur J Pharmacol. 533:195–206. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Päth G, Braun A, Meents N, Kerzel S,
Quarcoo D, Raap U, Hoyle GW, Nockher WA and Renz H: Augmentation of
allergic early-phase reaction by nerve growth factor. Am J Respir
Crit Care Med. 166:818–826. 2002.PubMed/NCBI
|
|
5
|
Chen YL, Huang HY, Lee CC and Chiang BL:
Small interfering RNA targeting nerve growth factor alleviates
allergic airway hyperresponsiveness. Mol Ther Nucleic Acids.
3:e1582014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braun A, Appel E, Baruch R, Herz U,
Botchkarev V, Paus R, Brodie C and Renz H: Role of nerve growth
factor in a mouse model of allergic airway inflammation and asthma.
Eur J Immunol. 28:3240–3251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi Y, Jin Y, Guo W, Chen L, Liu C and Lv
X: Blockage of nerve growth factor modulates T cell responses and
inhibits allergic inflammation in a mouse model of asthma. Inflamm
Res. 61:1369–1378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lambrecht BN and Hammad H: Biology of lung
dendritic cells at the origin of asthma. Immunity. 31:412–424.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willart MA and Hammad H: Alarming
dendritic cells for allergic sensitization. Allergol Int.
59:95–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Rijt LS, Jung S, Kleinjan A, Vos N,
Willart M, Duez C, Hoogsteden HC and Lambrecht BN: In vivo
depletion of lung CD11c+ dendritic cells during allergen challenge
abrogates the characteristic features of asthma. J Exp Med.
201:981–991. 2005.PubMed/NCBI
|
|
11
|
Hammad H and Lambrecht BN: Dendritic cells
and airway epithelial cells at the interface between innate and
adaptive immune responses. Allergy. 66:579–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seo JW, Cho SC, Park SJ, Lee EJ, Lee JH,
Han SS, Pyo BS, Park DH and Kim BH: 1′-Acetoxychavicol acetate
isolated from Alpinia galanga ameliorates ovalbumin-induced
asthma in mice. PLoS One. 8:e564472013.
|
|
13
|
Feng JT, Wu XM, Li XZ, Zou YQ, Qin L and
Hu CP: Transformation of adrenal medullary chromaffin cells
increases asthmatic susceptibility in pups from allergen-sensitized
rats. Respir Res. 13:992012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He R, Feng J, Xun Q, Qin Q and Hu C:
High-intensity training induces EIB in rats through neuron
transdifferentiation of adrenal medulla chromaffin cells. Am J
Physiol Lung Cell Mol Physiol. 304:L602–L612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Constabel H, Stankov MV, Hartwig C,
Tschernig T and Behrens GM: Impaired lung dendritic cell migration
and T cell stimulation induced by immunostimulatory
oligonucleotides contribute to reduced allergic airway
inflammation. J Immunol. 183:3443–3453. 2009. View Article : Google Scholar
|
|
16
|
Kushwah R and Hu J: Analysis of pulmonary
dendritic cell maturation and migration during allergic airway
inflammation. J Vis Exp. e40142012.PubMed/NCBI
|
|
17
|
Shao Z, Makinde TO, McGee HS, Wang X and
Agrawal DK: Fms-like tyrosine kinase 3 ligand regulates migratory
pattern and antigen uptake of lung dendritic cell subsets in a
murine model of allergic airway inflammation. J Immunol.
183:7531–7538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wegmann M: Th2 cells as targets for
therapeutic intervention in allergic bronchial asthma. Expert Rev
Mol Diagn. 9:85–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JS, Kang JY, Ha JH, Lee HY, Kim SJ,
Kim SC, Ahn JH, Kwon SS, Kim YK and Lee SY: Expression of nerve
growth factor and matrix metallopeptidase-9/tissue inhibitor of
metalloproteinase-1 in asthmatic patients. J Asthma. 50:712–717.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Renz H and Kiliç A: Neurotrophins in
chronic allergic airway inflammation and remodeling. Chem Immunol
Allergy. 98:100–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li QG, Wu XR, Li XZ, Yu J, Xia Y, Wang AP
and Wang J: Neural-endocrine mechanisms of respiratory syncytial
virus-associated asthma in a rat model. Genet Mol Res.
11:2780–2789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang YG, Tian WM, Zhang H, Li M and Shang
YX: Nerve growth factor exacerbates allergic lung inflammation and
airway remodeling in a rat model of chronic asthma. Exp Ther Med.
6:1251–1258. 2013.PubMed/NCBI
|
|
23
|
de Vries A, Engels F, Henricks PA,
Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC,
Nijkamp FP and Fischer A: Airway hyper-responsiveness in allergic
asthma in guinea-pigs is mediated by nerve growth factor via the
induction of substance P: a potential role for trkA. Clin Exp
Allergy. 36:1192–1200. 2006.PubMed/NCBI
|
|
24
|
von Garnier C and Nicod LP: Immunology
taught by lung dendritic cells. Swiss Med Wkly. 139:186–192.
2009.PubMed/NCBI
|
|
25
|
Noga O, Peiser M, Altenähr M, Knieling H,
Wanner R, Hanf G, Grosse R and Suttorp N: Differential activation
of dendritic cells by nerve growth factor and brain-derived
neurotrophic factor. Clin Exp Allergy. 37:1701–1708. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Y, Chen G, Zheng Y, Lu L, Wu C,
Zhang Y, Liu Q and Cao X: TLR4 signaling induces functional nerve
growth factor receptor p75NTR on mouse dendritic cells via p38MAPK
and NF-kappa B pathways. Mol Immunol. 45:1557–1566. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue M, Zhu L, Meng Y, Wang L, Sun H, Wang
F, Wang E and Shan F: Detailed modulation of phenotypes and
functions of bone marrow dendritic cells (BMDCs) by
interferon-gamma (IFN-γ). Int Immunopharmacol. 17:366–372.
2013.PubMed/NCBI
|
|
28
|
Nakano H, Free ME, Whitehead GS, Maruoka
S, Wilson RH, Nakano K and Cook DN: Pulmonary CD103(+) dendritic
cells prime Th2 responses to inhaled allergens. Mucosal Immunol.
5:53–65. 2012. View Article : Google Scholar : PubMed/NCBI
|