Introduction
The rapid development in the field of tissue
engineering has resulted in the existence of alternatives for the
treatment of large segmental bone defects. In general,
tissue-engineered bone is composed of seed cells, a scaffold and
growth factors. At present, mesenchymal stem cells are
preferentially used as seed cells due to their wide range of
sources, the low degree of damage they inflict on the body and
their capacity for multi-directional differentiation into
osteoblasts, neurocytes, chondrocytes and epithelial cells through
induction with certain conditioned media (1,2). The
scaffold determines the structure and mechanical properties of the
tissue engineered-bone. Previous studies have revealed that
β-tricalcium phosphate (β-TCP), a common scaffold material with
effective biocompatibility, histocompatibility and mechanical
properties, could supply the seed cells with an appropriate
environment for in vitro culture (3–5).
Intensive study in the field of bone tissue
engineering has focused on investigating growth factors, since they
potentially promote the proliferation and differentiation of seed
cells, as well as creeping substitution and the formation of new
bones. It has been indicated that there is a high concentration of
numerous growth factors in platelet-rich plasma (PRP), including
platelet-derived growth factor (PDGF), transforming growth factor
(TGF)-β1, TGF-β2, insulin-like growth factor (IGF), vascular
endothelial growth factor (VEGF), epidermal growth factor (EGF) and
endothelial cell growth factor (6). These growth factors promote the
proliferation, migration and differentiation of seed cells, as well
as facilitating collagen protein synthesis and vascularization
(7,8). Furthermore, previous studies
investigating the potential application of PRP in bone tissue
engineering have revealed that PRP may enhance osteogenesis and
accelerate bone defect healing to various degrees (9–11).
However, others studies investigating PRP have disputed these
applications (12,13). These studies argue that the
composition of PRP is so complicated that it is difficult to
clearly distinguish the single effect of each growth factor. In
2006, van den Dolder et al (14) quantitatively analyzed the
concentrations of different growth factors, including TGF-β1,
TGF-β2, PDGF-AA, PDGF-AB and PDGF-BB, in rat, goat and human PRP.
They also investigated the effects of PRP on cell growth and
differentiation by culturing rat bone marrow cells in PRP-coated
wells for 16 days in osteogenic media. Although the results
demonstrated that all three types of PRP stimulated initial cell
growth due to the presence of osteoinductive growth factors, the
data could not be generalized due to large interspecies variations.
In the study by Tajima et al (15) bone marrow stromal cells (BMSCs)
were suspended in PRP or platelet-poor plasma (PPP), which were
subsequently introduced into porous β-TCP blocks and implanted into
subcutaneous sites in rats. The results revealed that the implants
prepared using PPP had a greater osteoinductive capability compared
with those prepared with PRP.
A literature review revealed that PRP has not been
commonly studied in vivo for the construction of
tissue-engineered bone, particularly for the treatment of bone
defects in medium or large animal models (16,17).
In the present study, the osteogenic characteristics of a scaffold
combining β-TCP and PRP were investigated in vivo by
establishing a proximal tibial bone defect model in beagle dogs,
with mesenchymal stem cells used as seed cells. The present study
provides a useful foundation for the further study of bone tissue
engineering in medium or large animals.
Materials and methods
Isolation, cultivation, purification and
proliferation of beagle BMSCs
The procedures carried out in the present study were
approved by the Ethics Committee of Xiangya Hospital Affiliated to
Central South University (Changsha, China). An adult beagle dog (12
months old and weighing 10 kg) was obtained from the Animal
Department of Xiangya Medical College (Changsha, China). The dog
was intravenously anesthetized with 3% pentobarbital (1.0 ml/kg;
Propbs Chemical & Pharmaceutical Co. Ltd., Beijing, China) and
3 ml bone marrow was extracted using sterile techniques. The marrow
was washed and diluted in phosphate-buffered saline (PBS) solution
and poured slowly over Percoll (1.079 g/ml; GE, Shanghai, China)
with a volume of 1:1. The mixture was centrifuged at 223.6 × g for
15 min, following which the liquid formed four layers. The second
layer, which was white and membrane-like, was carefully drawn,
washed in PBS solution and centrifuged at 55.9 × g for 5 min.
Following removal of the supernatant, Dulbecco’s modified Eagle’s
medium - Low Glucose (DMEM-LG; Gibco®; Invitrogen Life
Technologies, Carlsbad, CA, USA) combined with 10% fetal calf serum
(Shanghai DingGuo Biotech Co. Ltd., Shanghai, China), containing
100 μg/ml penicillin and 100 μg/ml streptomycin, was added to
resuspend the cells. The cells were seeded in a 25-cm2
culture flask at a density of 2×105 cells/cm2
and cultured in a CO2 incubator (Queue®,
Asheville, NC, USA) following the addition of 4 ml DMEM-LG with
fetal calf serum. The media was changed after the first 48 h and
every three days thereafter. Finally, 0.25% trypsin (Sigma-Aldrich,
St. Louis, MO, USA) digestion was performed when cell fusion was
observed in 80–90% of the anchorage-dependent cells. Serial
subculture was performed at a proportion of 1:3.
Preparation of PRP
Venous blood (10 ml) was collected from the hind
limb of the beagle and placed in a 15-ml centrifuge tube containing
1 mg sodium citrate. PRP was extracted using the two-step
centrifugation method. Firstly, the blood sample was centrifuged at
125.775 × g for 10 min. The upper plasma layer and the erythrocytes
within 2 mm of the interface were transferred to another centrifuge
tube. The sample was then centrifuged at 724.464 × g for 10 min and
the upper plasma layer that contained a small amount of suspending
platelets was removed. The remaining plasma (~1 ml) was PRP. PRP
was prepared using this method and stored at −70°C until
required.
Preparation of the β-TCP and PRP
composite (β-TCP/PRP)
The β-TCP samples (cylindrical; diameter, 1.0 cm;
height, 4.0 cm) were cut into 0.5-cm slices using a clean bench
(GS-15R; Haier, Qingdao, China). The slices were repeatedly washed
with saline and sterilized at 130°C following drying. The slices
were immersed in PRP until the TCP material was completely
saturated by the plasma. The activating agent (10% calcium chloride
solution containing 100 μg/ml bovine thrombin) was added at a ratio
of 1:1, and the mixture was reacted in a 37°C water bath to form
the β-TCP/PRP gel composite.
Preparation of the scaffold with the
seeding of BMSCs onto the composites
Third-generation BMSCs were collected and the cell
suspension density was adjusted to 1.0×105/l. The cell
suspension was implanted onto the β-TCP and β-TCP/PRP composites
using a micropipette (50 μl for each bracket). The materials were
stored for 4 h in the CO2 incubator (37°C and 5%
CO2 saturated humidity) to allow further adhesion of the
cells. A total of 2 ml high-glucose DMEM (Gibco; Invitrogen Life
Technologies) containing 10% fetal calf serum, dexamethasone
(1×10−7 M), β-glycerophosphate (10 mM) and ascorbic acid
(500 mg/l) was added per well. Finally, the composite was placed in
the CO2 culture incubator for a further week.
Establishment of the bone defect in the
upper segment of the tibia in beagles
A total of 27 beagle dogs were intravenously
anesthetized with 3% pentobarbital (1.0 ml/kg; Propbs Chemical
& Pharmaceutical Co. Ltd.). Bilateral incisions were made at
the medial lower extremities and the medial tibiae were accessed
following periosteal stripping. Cavity-shaped bone defects (<10
mm in diameter) were established on both sides at 2 cm below the
medial tibial plateau.
The dogs were classified into three groups (n=9 per
group) and implanted with different types of scaffolds. The β-TCP
scaffold with seeded BMSCs was implanted into Group I animals and
the β-TCP/PRP scaffold with seeded BMSCs was implanted into Group
II animals. Autogenic ilium was used for Group III animals.
Following the implantation of the scaffolds, the periosteum in the
surgical area was removed and the subcutaneous tissue and skin were
tightly sutured. The dogs were administered penicillin to prevent
postoperative infection.
Evaluation methods
The beagles in each group were examined using X-ray
radiographs at three time-points: 4, 8 and 12 weeks after
implantation (with three radiographs at each time-point). At each
time-point, three dogs were sacrificed from each group by an
intravenous injection of 3% pentobarbital (1.0 ml/kg), followed by
an intravenous injection of air into the hind limb. Subsequently,
immunocytochemical staining of osteocalcin (OCN), hematoxylin and
eosin (H&E) staining, reverse transcription-polymerase chain
reaction (RT-PCR) analysis and biomechanical tests were performed
in order to examine callus formation, the regeneration of bone
defects and the reaction of the surrounding tissue.
Immunocytochemical staining of
OCN
Sections (3–5 μm) were dewaxed and hydrated, washed
with PBS solution and incubated with 3% H2O2
for 5–10 min to eliminate the endogenous peroxidase activity. The
sections were subsequently washed in distilled water and immersed
in PBS solution for 5 min. Mouse anti-dog OCN polyclonal-antibody
(dilution 1:100; Abcam, Cambridge, MA, USA) was added and the
sections were incubated overnight at 4°C. The sections were then
washed in PBS solution (three times, 5 min each), and biotinylated
anti-mouse immunoglobulin G secondary antibody (Zhongshan Jinqiao
Co. Ltd., Beijing, China) was added followed by incubation at 37°C
for 10–15 min. Following washing with PBS solution (three times, 5
min each), horseradish peroxidase-labeled streptavidin (Zhongshan
Jinqiao Co. Ltd.) was added and the sections were incubated at 37°C
for 10–15 min. The sections were then washed in PBS solution (three
times, 5 min each) and 3,3′-diaminobenzidine tetrahydrochloride
chromogenic agent (Zhongshan Jinqiao Co. Ltd.) was added for color
development. Following washing in distilled water, the sections
were counterstained with hematoxylin and mounted. OCN was observed
using an optical microscope (CX21; Olympus, Tokyo, Japan).
H&E staining
Following dewaxing and hydration, the sections were
soaked in hematoxylin (Weigert iron hematoxylin stain; Shanghai
Harling Biotechnology Co. Ltd, Shanghai, China) for 5 min, washed
with distilled water and differentiated with 1% hydrochloric acid
alcohol for 30 sec. Subsequent to washing again in distilled water,
the sections were immersed in eosin for 2 min, washed with
distilled water and dehydrated by a graded ethanol series. Finally,
the sections were cleaned with xylol, mounted in neutral resin and
observed using optical microscopy.
RT-PCR
The sequences of the primers used were as follows:
OCN forward, GAATCCCGCAAAGGTGGCTGA CCACATTGGCTT and reverse,
AAGCCAATGTGGTCA GCCACCTTTGCGGGATTC (185 bp); alkaline phosphatase
(ALP) forward, GAGTGACACGGACAAGAAGCC CCAACCAGGACCACTGTGCCTCA and
reverse, TGA GGCACAGTGGTCCTGGTTGGGGCTTCTTGTCCGTGT CACTC (292 bp);
collagen type I α1 (CollA1) forward,
TCCAGGGTTCCAACGAGAAGACCTCCCGTTTGCC and reverse,
GGCAAACGGGAGGTCTTCTCGTTGGAA CCCTGGA (145 bp); and GAPDH forward,
AAGGTCGGA GTCAACGGATTTGGCATCAGCAGAAGGAGCAG and reverse,
CTGCTCCTTCTGCTGATGCCAAATCCGTTG ACTCCGACCTT (372 bp). The sequences
were designed using Primer Premier software (Premier Biosoft, Palo
Alto CA, USA). The total RNA was extracted from each sample using a
TRIzol® kit (Molecular Research Centre, Inc.,
Cincinnati, OH, USA). The cDNA was subsequently synthesized using a
RT kit (R0901-100ML; Sigma-Aldrich). Firstly, the total RNA (2.0
μl), Oligo(dT)18 primer (1.0 μl) and DNase/RNase-free
ddH2O (9.0 μl) were added to a 0.2-ml RNase-free
Eppendorf tube. Following mixing and mild centrifugation (13.975 ×
g for 90 sec at 22–24°C), the tube was reacted at 70°C for 5 min.
The 5× RT buffer (4.0 μl), 10 mM deoxynucleotide triphosphates
(dNTPs; 2.0 μl) and RNasin® (1.0 μl) were added to the
tube and mild centrifugation was carried out following mixing. The
solution was reacted at 37°C for 5 min, and 1.00 μl M-MLV reverse
transcriptase (M1302-40KU; Sigma-Aldrich) was added. The mixture
was reacted at 42°C for 60 min and then at 70°C for 10 min. The
synthesized cDNA was stored at −20°C until required. PCR was
subsequently performed. Firstly, 10× PCR buffer (5.0 μl), 10 mM
dNTPs (2.0 μl), MgCl2 (3.0 μl), 10 pmol/μl target gene
forward primer (1.6 μl), 10 pmol/μl target gene reverse primer (1.6
μl), 10 pmol/μl β-actin gene forward primer (1.6 μl), 10 pmol/μl
β-actin gene reverse primer (1.6 μl), cDNA (3.0 μl), 1.0 U/μl
Taq DNA polymerase (2.6 μl) and DNase/RNase-free
ddH2O (28.0 μl) were added to a 0.2-ml PCR tube. The
mixture was subsequently transferred to an Eppendorf tube and
placed into the TripleMaster PCR system (Eppendorf, Hamburg,
Germany) for DNA synthesis according to the designed primers (OCN,
ALP and CollA1). The PCR products were visualized on an ethidium
bromide-stained agarose gel.
Biomechanical tests
The tibiae of three dogs from Groups I, II and III
were obtained 12 weeks after surgery. The meniscus and attached
ligaments and muscle tissues were carefully dissected. The ends of
the tibiae were closed with denture powder and polished with fine
sandpaper to create a smooth surface. The tibiae were placed
vertically in a universal material tester (Instron 8032;
TestResources, Shakopee, MN, USA) for compression analyses with a
loading speed of 5 mm/min. The maximum load (the maximum external
force that the tibia could bear) and the maximum compressive
strength (maximum load/contact area) were recorded during the
experiment.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using SPSS 12.0 software (SPSS,
Inc., Chicago, IL, USA). A two-sample Student’s t-test was used to
evaluate the differences between groups. Differences were
considered statistically significant with a two-tailed value of
P<0.05.
Results
Observation of the implanted scaffolds in
the beagle dogs in each group
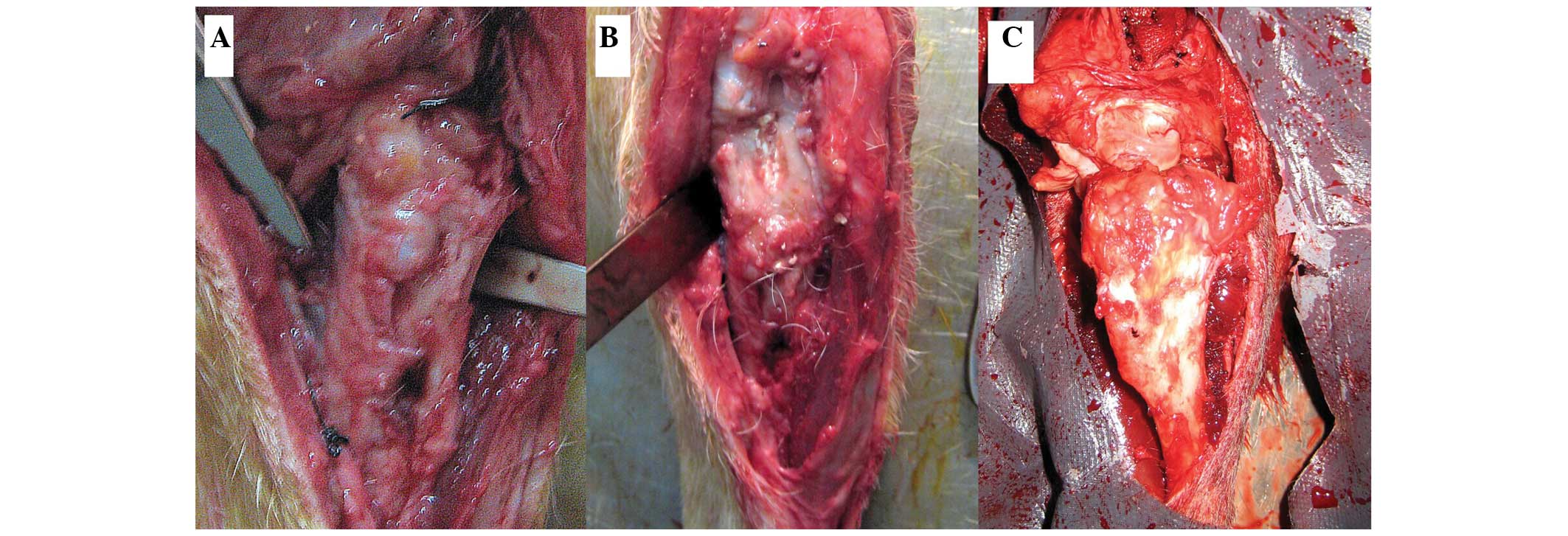
All wounds healed well following implantation of the
scaffolds into the tibial defects of the dogs in each group. No
systemic or local inflammation or toxicity were observed in any
group. No significant inflammation or rejection was detected for
any of the implanted scaffolds, as shown in Fig. 1.
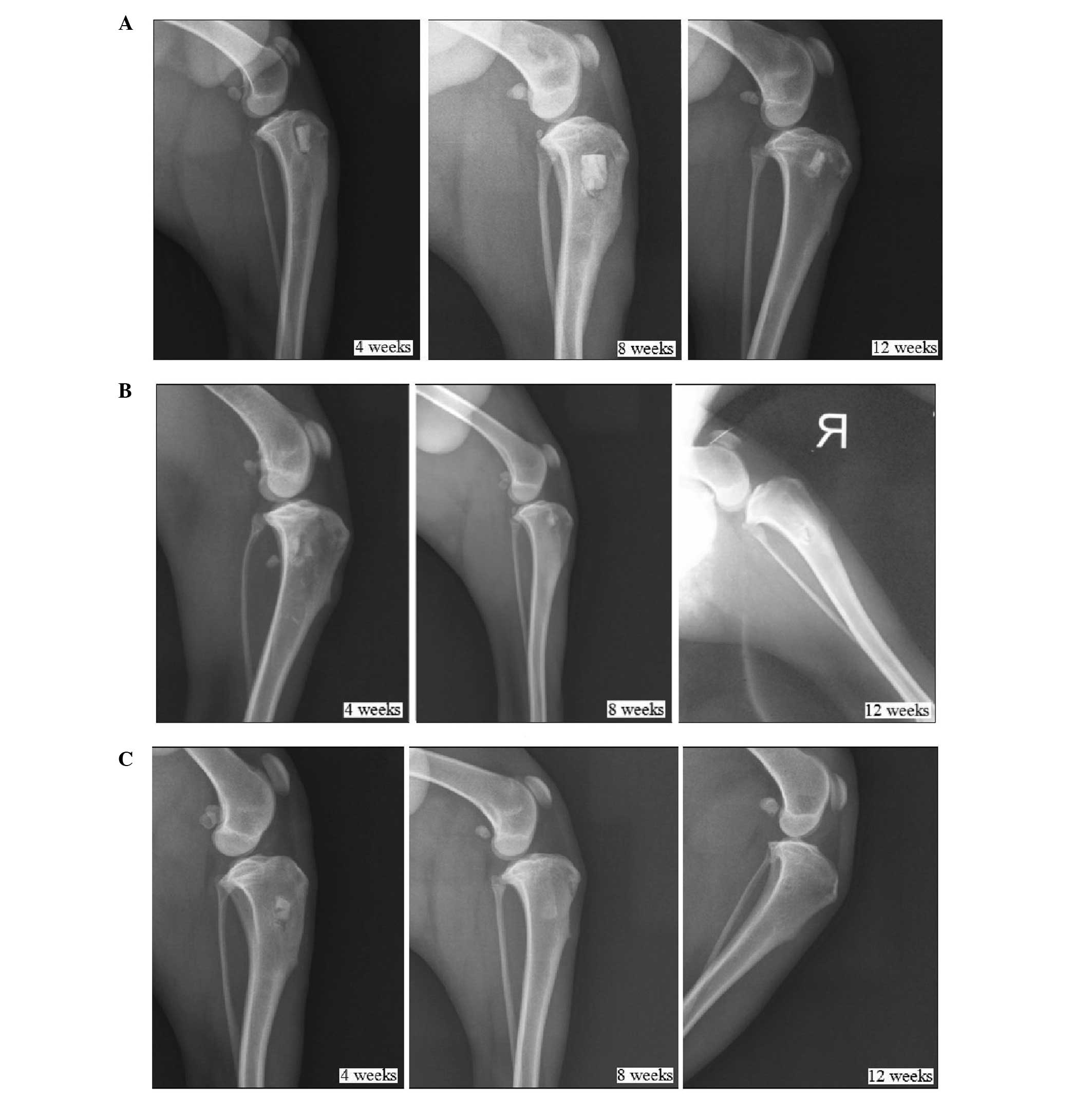
Postoperative X-ray examination
No material absorption or bone healing was observed
in Group I (implantation of β-TCP with seeding of BMSCs) until 12
weeks after implantation. The scaffold-bone interface remained
clear. The shadow around the scaffold suggested the occurrence of
bone resorption, as shown in Fig.
2A. In Group II (implantation of β-TCP/PRP with seeding of
BMSCs), the scaffold-bone interface was indistinct, which was
indicative of a relatively fast fusion. Bone healing was generally
complete 12 weeks after the implantation. However, as shown in
Fig. 2B, a small amount of
high-density shadow remained, which may have been the remaining
TCP. The most rapid bone healing was observed in the animals in
Group III (implantation of autogenic ilium). Indistinct fusion of
the scaffold-bone interface was detected just 4 weeks after the
implantation, and complete bone healing was observed at 12 weeks
after the implantation (Fig.
2C).
Immunohistochemical staining of OCN and
H&E staining
The results obtained from the immunohistochemical
staining of OCN and H&E staining were similar among the three
groups. New cartilage formation was observed in each group, with
higher expression in the samples from Groups II and III compared
with those from Group I (Fig.
3).
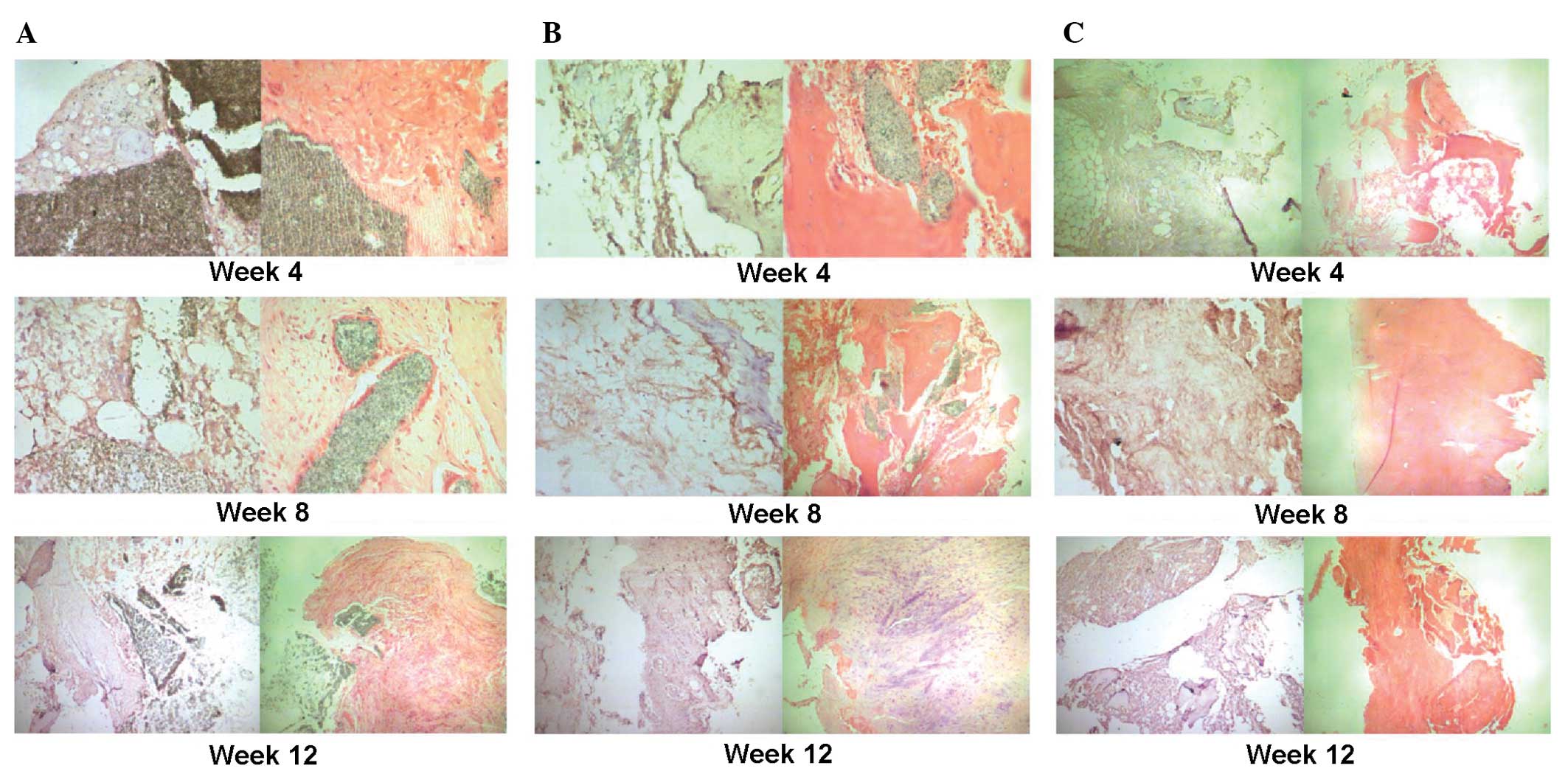 | Figure 3Immunohistochemical staining of
osteocalcin and hematoxylin and eosin staining at different
time-points (4, 8 and 12 weeks) following implantation of the
scaffolds. (A) Group I (pure β-TCP). At 4 weeks following
implantation, new formation of cartilage and bone was observed at
the edges of the scaffold (magnification, ×200). The expression of
newly formed cartilage and bone increased at 8 weeks following
implanation and a number of osteogenic cells arranged in a spiral
were observed (magnification, ×200). At 12 weeks following
implantation there was an even higher expression of newly formed
cartilage and bone at the edges of the scaffold cells and the
osteogenic cells were arranged in a spiral; however, the majority
of the β-TCP remained detectable (magnification, ×100). (B) Group
II (β-TCP/PRP). At 4 weeks following implantation, new formation of
cartilage and bone was observed at the edges of the scaffold and
the scaffold began to degrade (magnification, ×200). The expression
of newly formed cartilage and bone increased at 8 weeks following
implanation and the scaffold was further degraded and absorbed
(magnification, ×100). This continued at 12 weeks following
impantation where the formation of capillary vessels was also
detected (magnification, ×200). (C) Group III (autogenic ilium). At
4 weeks following implantation, the new formation of cartilage,
bone and capillary vessels was observed. This increased at 8 and 12
weeks following implantation with much mature bone tissue observed
at 12 weeks following implantation (magnification, ×100). |
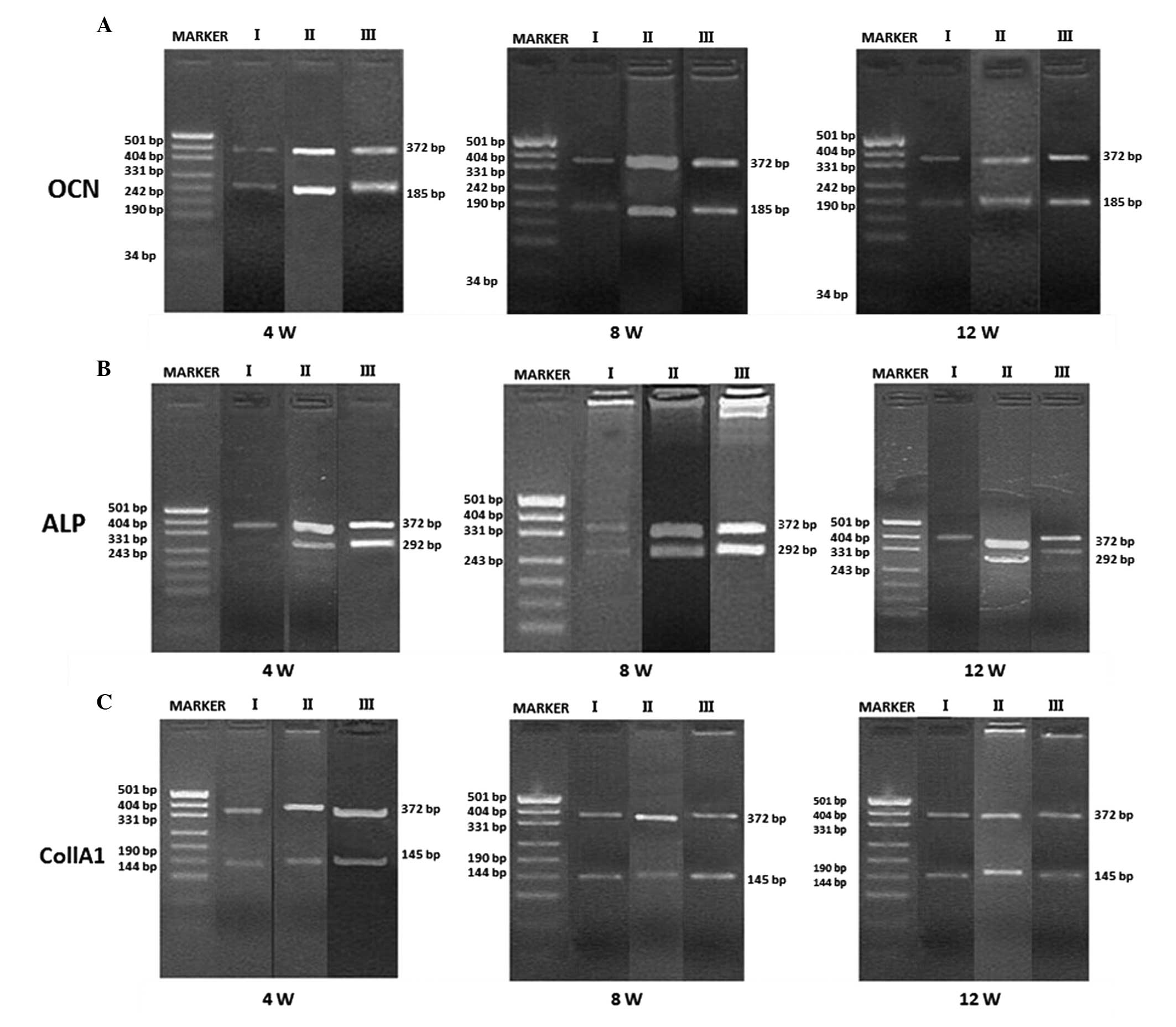
RT-PCR
Using GAPDH as an internal control (expressed as a
372-bp band), the expression levels of OCN, ALP and Col1A1 were
detected using RT-PCR at the three time-points (4, 8 and 12 weeks
after implantation) for the samples in each group. OCN, ALP, and
Col1A1 were weakly expressed in all groups 4 weeks after
implantation. At 8 and 12 weeks after implantation, the expression
levels of OCN, ALP, and Col1A1 in Groups II and III were higher
than those in Group I, but all were strongly positive (Fig. 4). According to the comparisons
between the target bands and the reference gray values, the
expression levels of OCN, ALP and Col1A1 were not statistically
different at 4 weeks after implantation. At 8 and 12 weeks
following implantation, the expression level of each mRNA in Groups
II and III was statistically higher than that of the same mRNA in
Group I (P<0.05). However, no significant difference was
identified between the expression levels of the mRNAs in Groups II
and III (P>0.05), as shown in Table
I.
 | Table ImRNA expression levels of OCN, ALP and
Col1A1 for Groups I, II and III at different time-points following
implantation of the scaffolds. |
Table I
mRNA expression levels of OCN, ALP and
Col1A1 for Groups I, II and III at different time-points following
implantation of the scaffolds.
| Group | Time-point
(weeks) | No. samples | OCN/GAPDH | Col1A1/GAPDH | ALP/GAPDH |
|---|
| I | 4 | 3 | 0.82±0.09 | 0.74±0.10 | 1.11±0.77 |
| 8 | 3 | 0.85±0.12 | 0.93±0.10 | 0.99±0.11 |
| 12 | 3 | 0.89±0.07 | 0.89±0.07 | 0.80±0.42 |
| II | 4 | 3 | 0.86±0.11 | 0.76±0.10 | 1.14±0.10 |
| 8 | 3 | 1.01±0.13 | 1.15±0.08 | 1.12±0.10 |
| 12 | 3 | 1.12±0.11 | 1.13±0.10 | 0.96±0.35 |
| III | 4 | 3 | 0.85±0.10 | 0.77±0.07 | 1.20±0.09 |
| 8 | 3 | 1.08±0.09 | 1.19±0.10 | 1.15±0.10 |
| 12 | 3 | 1.12±0.11 | 1.11±0.11 | 0.95±0.59 |
Biomechanical tests
Table II shows the
maximum load and compressive strength of the tibia segments in each
group, as measured 12 weeks after implantation. The maximum load
and compressive strength for the samples in Groups II and III were
significantly than those for the samples in Group I (P<0.05).
However, no statistical differences were identified in these values
between Groups II and III (P>0.05).
 | Table IIMaximum load and compressive strength
of the tibia segments for Groups I, II and III at 12 weeks
following implantation of the scaffolds. |
Table II
Maximum load and compressive strength
of the tibia segments for Groups I, II and III at 12 weeks
following implantation of the scaffolds.
| Group | No. samples | Maximum load (N) | Maximum compressive
strength (MPa) |
|---|
| I | 6 | 3637.5±129.6 | 17.5±0.6 |
| II | 6 | 3917.7±96.8 | 20.2±0.8 |
| III | 6 | 3946.7±106.2 | 19.6±0.8 |
Discussion
Seed cells, scaffolds and growth factors play
important roles in bone tissue engineering. Numerous studies have
focused on the functional mechanism of PRP in bone tissue
engineering. However, few studies have explored the osteogenic
characteristics of PRP in medium and large animal models for the
treatment of bone defects (18–20).
In the present study, a β-TCP and PRP gel composite was introduced
as the scaffold to carry BMSCs, making it simultaneously
osteoconductive and osteoninductive. The scaffolds were implanted
into the tibial bone defects of beagle dogs following one week of
in vitro directional cell osteogenic induction. Compared
with the autogenic ilium and pure β-TCP scaffolds with BMSC
seeding, the β-TCP/PRP scaffold exhibited no significant
inflammation or rejection, with excellent histocompatibility. As
observed from the X-ray radiograph images captured at 8 and 12
weeks after the implantation, the osteogenic effects of the
β-TCP/PRP scaffolds with BMSC seeding were superior to those of the
pure β-TCP scaffolds, and were similar to those of the autogenic
ilium. General bone healing was observed 12 weeks after the
implantation of the β-TCP/PRP scaffolds, indicating their effective
osteogenic capability.
The most common method of promoting the
differentiation of BMSCs into osteoblasts is chemical drug
induction (21–23). In the present study, a specific
inducing media, which included dexamethasone, β-glycerophosphate
and ascorbic acid, was used to facilitate the in vitro
differentiation of BMSCs into osteoblasts. Dexamethasone promotes
osteoblast differentiation, is able to regulate the secretion of
IGF and facilitates extracellular matrix collagen synthesis.
β-glycerophosphate can supply phosphate ions to osteoblasts,
thereby promoting the deposition and calcification of physiological
calcium, which is required for the formation of mineralized
nodules. Ascorbic acid can adjust the activity of ALP and the
synthesis of non-collagen matrix proteins. PRP contains a high
concentration of growth factors, which promote seed cell
proliferation, movement, differentiation, collagen synthesis and
vascularization. Marx et al (24) proposed that the reason that PRP is
beneficial for bone defect healing may be attributed to the high
concentration of PDGF and TGF-β in PRP. Fennis et al
(25) found that the addition of
PRP to an autogenic bone graft had a positive effect on bone
healing in the early stages. Cell differentiation increased as the
direct effect of PRP declined and further proliferation and
differentiation promoted the healing of bone defects at a high
level. The high concentrations of PDGF and TGF-β work effectively
in the early stages of bone defect healing by increasing the number
of BMSCs in the trauma area along with other growth factors in the
PRP, such as basic fibroblast growth factor (24,25).
Vascularization of the implanted tissue-engineered bone usually
occurs two weeks after implantation (26). The growth factors in PRP, including
VEGF, IGF, EGF and PDGF, are able to promote revascularization of
the scaffold (6). Good
revascularization accelerates local blood supply in the bone defect
areas and further promotes osteogenesis(26).
β-TCP is a type of bioactive ceramic, which is
degraded in vivo by dissolving and by phagocytic cells.
Since its degradation products are non-toxic, non-teratogenic and
non-tumorigenic, β-TCP is considered a promising material for bone
tissue engineering (27). An ideal
scaffold requires a degradation time that is in accordance with
osteoblast growth and proliferation, as well as matrix secretion.
The present study demonstrated, using immunohistochemical staining
of OCN and H&E staining, that the calcium phosphate in the
β-TCP/PRP scaffold implanted into the beagles generally degraded 12
weeks after implantation, whereas the majority of the calcium
phosphate remained in Group I (implantation of β-TCP with BMSC
seeding). Furthermore, OCN expression was stronger in the β-TCP/PRP
group compared with that in the TCP group at all time-points, with
an increased formation of new cartilage. The results suggest that
the degradation rate of the β-TCP/PRP scaffold matched the
proliferation rate of the BMSCs and the synthesis and secretion of
the matrix. Therefore, the β-TCP/PRP scaffold is suitable to be
applied in bone tissue engineering to treat bone defects. The
tissue sections were prepared following the sacrifice of the dogs
at 4, 8 and 12 weeks after implantation. The statistical analysis
of the mRNA expression levels for OCN, ALP and Col1A1 further
indicated that the β-TCP/PRP scaffolds were similar to autogenous
iliac bone in the promotion of osteogenesis, but were significantly
more effective than the pure β-TCP scaffolds. These results
corresponded with those obtained from the X-ray radiographs and
immunohistochemical and H&E stains.
The mechanical properties of a scaffold are closely
associated with its degradation rate. The initial mechanical
strength should be able to resist physiological stress so that it
does not experience collapse during the growth of tissue cells.
Since mechanical strength may also influence the tension generated
by the intracellular skeleton, a resilient surface provides a good
environment for fiber configuration, as well as cell expansion and
differentiation. The β-TCP/PRP scaffold has superior mechanical
properties and degradation rates compared with cancellous bone. In
the present study, the tibial specimens collected 12 weeks after
implantation were placed in the Instron universal material tester
for vertical compression experiments. The results revealed that the
β-TCP/PRP scaffold had similar mechanical properties to iliac bone,
but superior mechanical properties to the pure β-TCP scaffold.
In the present study, the tibial bone defects in
beagles were successfully repaired when a β-TCP/PRP gel composite
was used as the scaffold carrying in vitro-cultured BMSCs.
The osteogenic effect of the of β-TCP/PRP scaffold was similar to
that of the autogenic ilium. As a novel application in bone tissue
engineering, PRP is likely to promote new bone formation through
the release of the growth factors it contains. Thus, β-TCP/PRP may
be an ideal scaffold to treat bone defects with tissue
engineering.
Acknowledgements
This study was financially supported by the
Fundamental Research Funds for the Central Universities of China.
The authors would like to thank The Central Laboratory of the
National Hepatobiliary and Enteric Surgery Research Center, The Key
Laboratory of Tumor Proteomics of the Chinese Ministry of Health
and The Institute of Powder Metallurgy of Central South University.
The authors are also grateful to Medjaden Bioscience Limited (Hong
Kong, China) for their assistance in the preparation of the
manuscript.
References
|
1
|
Gafni Y, Turgeman G, Liebergal M, et al:
Stem cells as vehicles for orthopedic gene therapy. Gene Ther.
11:417–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park KW, Eglitis MA and Mouradian MM:
Protection of nigral neurons by GDNF-engineered marrow cell
transplantation. Neurosci Res. 40:315–323. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiltfang J, Merten HA, Schlegel KA, et al:
Degradation characteristics of alpha and beta tri-calcium-phosphate
(TCP) in minipigs. J Biomed Mater Res. 63:115–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamada Y, Boo JS, Ozawa R, et al: Bone
regeneration following injection of mesenchymal stem cells and
fibrin glue with a biodegradable scaffold. J Craniomaxillofac Surg.
31:27–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao H and Kuboyama N: A biodegradable
porous composite scaffold of PGA/beta-TCP for bone tissue
engineering. Bone. 46:386–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anitua E: Plasma rich in growth factors:
preliminary results of use in the preparation of future sites for
implants. Int J Oral Maxillofac Implants. 14:529–535.
1999.PubMed/NCBI
|
|
7
|
Carlson NE and Roach RB Jr: Platelet-rich
plasma: clinical applications in dentistry. J Am Dent Assoc.
133:1383–1386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang S and Wang Z: Platelet-rich
plasma-derived growth factors promote osteogenic differentiation of
rat muscle satellite cells: in vitro and in vivo studies. Cell Biol
Int. 36:1195–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Formigli L, Benvenuti S, Mercatelli R, et
al: Dermal matrix scaffold engineered with adult mesenchymal stem
cells and platelet-rich plasma as a potential tool for tissue
repair and regeneration. J Tissue Eng Regen Med. 6:125–134. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schuckert KH, Jopp S and Teoh SH:
Mandibular defect reconstruction using three-dimensional
polycaprolactone scaffold in combination with platelet-rich plasma
and recombinant human bone morphogenetic protein-2: de novo
synthesis of bone in a single case. Tissue Eng Part A. 15:493–499.
2009. View Article : Google Scholar
|
|
11
|
Yoshimi R, Yamada Y, Ito K, et al:
Self-assembling peptide nanofiber scaffolds, platelet-rich plasma,
and mesenchymal stem cells for injectable bone regeneration with
tissue engineering. J Craniofac Surg. 20:1523–1530. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kon E, Filardo G, Delcogliano M, et al:
Platelet autologous growth factors decrease the osteochondral
regeneration capability of a collagen-hydroxyapatite scaffold in a
sheep model. BMC Musculoskelet Disord. 11:2202010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasten P, Vogel J, Luginbühl R, et al:
Influence of platelet-rich plasma on osteogenic differentiation of
mesenchymal stem cells and ectopic bone formation in calcium
phosphate ceramics. Cells Tissues Organs. 183:68–79. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van den Dolder J, Mooren R, Vloon AP,
Stoelinga PJ and Jansen JA: Platelet-rich plasma: quantification of
growth factor levels and the effect on growth and differentiation
of rat bone marrow cells. Tissue Eng. 12:3067–3073. 2006.PubMed/NCBI
|
|
15
|
Tajima N, Sotome S, Marukawa E, Omura K
and Shinomiya K: A three-dimensional cell-loading system using
autologous plasma loaded into a porous β-tricalcium-phosphate block
promotes bone formation at extraskeletal sites in rats. Materials
Science and Engineering C. 27:625–632. 2007.
|
|
16
|
Jiang ZQ, Liu HY, Zhang LP, Wu ZQ and
Shang DZ: Repair of calvarial defects in rabbits with platelet-rich
plasma as the scaffold for carrying bone marrow stromal cells. Oral
Surg Oral Med Oral Pathol Oral Radiol. 113:327–333. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shim JH, Moon TS, Yun MJ, et al:
Stimulation of healing within a rabbit calvarial defect by a
PCL/PLGA scaffold blended with TCP using solid freeform fabrication
technology. J Mater Sci Mater Med. 23:2993–3002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao W, Pang L, Jiang M, Lv R, Xiong Z and
Hu YY: Skeletal repair in rabbits using a novel biomimetic
composite based on adipose-derived stem cells encapsulated in
collagen I gel with PLGA-beta-TCP scaffold. J Orthop Res.
28:252–257. 2010.PubMed/NCBI
|
|
19
|
Lucarelli E, Fini M, Beccheroni A, et al:
Stromal stem cells and platelet-rich plasma improve bone allograft
integration. Clin Orthop Relat Res. 62–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kovács K, Velich N, Huszár T, Fenyves B,
Suba Z and Szabó G: Histomorphometric and densitometric evaluation
of the effects of platelet-rich plasma on the remodeling of
beta-tricalcium phosphate in beagle dogs. J Craniofac Surg.
16:150–154. 2005.PubMed/NCBI
|
|
21
|
Noël D, Gazit D, Bouquet C, et al:
Short-term BMP-2 expression is sufficient for in vivo osteochondral
differentiation of mesenchymal stem cells. Stem Cells. 22:74–85.
2004.PubMed/NCBI
|
|
22
|
Mauney JR, Volloch V and Kaplan DL:
Matrix-mediated retention of adipogenic differentiation potential
by human adult bone marrow-derived mesenchymal stem cells during ex
vivo expansion. Biomaterials. 26:6167–6175. 2005. View Article : Google Scholar
|
|
23
|
Friedman MS, Long MW and Hankenson KD:
Osteogenic differentiation of human mesenchymal stem cells is
regulated by bone morphogenetic protein-6. J Cell Biochem.
98:538–554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marx RE, Carlson ER, Eichstaedt RM,
Schimmele SR, Strauss JE and Georgeff KR: Platelet-rich plasma:
Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 85:638–646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fennis JP, Stoelinga PJ and Jansen JA:
Mandibular reconstruction: a histological and histomorphometric
study on the use of autogenous scaffolds, particulate
cortico-cancellous bone grafts and platelet rich plasma in goats.
Int J Oral Maxillofac Surg. 33:48–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arpornmaeklong P, Kochel M, Depprich R, et
al: Influence of platelet-rich plasma (PRP) on osteogenic
differentiation of rat bone marrow stromal cells. An in vitro
study. Int J Oral Maxillofac Surg. 33:60–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lohfeld S, Cahill S, Barron V, et al:
Fabrication, mechanical and in vivo performance of
polycaprolactone/tricalcium phosphate composite scaffolds. Acta
Biomater. 8:3446–3456. 2012. View Article : Google Scholar : PubMed/NCBI
|