Introduction
Sjögren’s syndrome is a common chronic autoimmune
disease characterized by lymphocytic infiltration of glands and
ocular and oral dryness, which primarily affects the salivary and
lacrimal glands. This syndrome may occur as a primary Sjögren’s
syndrome (pSS), or in association with other systemic autoimmune
diseases, such as rheumatoid arthritis and systemic lupus
erythematosus (1). Sjögren’s
syndrome may manifest within a wide spectrum of diseases, ranging
from a limited, organ-specific autoimmune exocrinopathy to a
systemic disease with widespread autoimmune manifestations and
pronounced immunological features (2). pSS is characterized by polyclonal B
cell activation, leading to chronic hypergammaglobulinemia,
increased levels of β2-microglobulinemia and the
concomitant presence of a variety of autoantibodies (3). Multiple factors, including viral
infection, hormonal balance and genetic background, are involved in
the pathogenesis of pSS. The presence of T and B cells, macrophages
and dendritic cells varies according to the severity of the lesion
(4). The influence of abnormal
cytokine production in this disease has attracted considerable
attention (5).
Progranulin (PGRN) is an autocrine growth factor
with multiple physiological and pathological functions. PGRN can
bind to tumor necrosis factor receptors and is therapeutic against
inflammatory arthritis in mice (6). Therefore, PGRN is a potential target
for the treatment of autoimmune diseases. However, the changes in
PGRN expression in pSS patients remain unclear. In the present
study, the serum levels of PGRN in the peripheral blood of pSS
patients and healthy controls were examined to investigate the
possible role of PGRN in the pathogenesis and development of
pSS.
Materials and methods
Patients
In total, 26 newly diagnosed pSS patients were
recruited for the study. All patients met the criteria revised by
American College of Rheumatology in 1997 for the classification of
pSS (7). None of the patients had
been treated with immunosuppressive drugs prior to specimen
collection. The patients received symptomatic and supportive
treatment, as well as immunosuppressive therapy, within a period of
21 consecutive days. Peripheral blood samples were collected from
the patients. The control group included 26 healthy volunteers,
matching the gender and ages of the pSS patients (female, 25; male,
1; age range, 24–65 years; median age, 44.8±10.96 years). All the
subjects signed informed consent forms prior to entering the study.
Ethical approval for the research was obtained from the Medical
Ethical Committee of Qilu Hospital, Shandong University (Jinan,
China).
ELISA
Coagulated blood (5 ml) was collected from each
patient and control subject prior to and following the
administration of prednisone. The blood was centrifuged (5000 × g
for 10 min at 4°C) and the serum specimens were stored at −80°C.
The serum levels of PGRN and the inflammatory factor, interleukin-6
(IL-6), were measured using a commercial ELISA kit (Yonghui
Company, Beijing, China), according to the manufacturer’s
instructions.
Quantitative polymerase chain reaction
(qPCR)
Peripheral blood mononuclear cells were separated
using red blood cell lysis buffer (Pharmacia Diagnostics, Uppsala,
Sweden) and the total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. An Eppendorf Biophotometer (Brinkmann
Instruments, Westbury, NY, USA) was used to determine the RNA
concentration, and the concentration was adjusted to 1 μg/ml for
reverse transcription. The RNA was reverse-transcribed to form cDNA
using a ReverTra Ace qPCR RT kit (Toyobo Corporation, Osaka,
Japan). qPCR was performed using the Light Cycler TaqMan
Master kit (Toyobo Corporation), according to the manufacturer’s
instructions, on a Bio-Rad IQ5 detection system (Bio-Rad
Laboratories, Hercules, CA, USA). Fluorescence qPCR was performed
using SYBR Green (Toyobo Corporation). Each sample was determined
in triplicate, and the qPCR products were run on agarose gels to
confirm the expected size of the samples. Melting-curve analysis
was also performed to ensure the specificity of the products. The
relative mRNA expression levels of IL-6 were determined using the
comparative Ct method, using arithmetic formulae from the relative
expression software tool (Bio-Rad Laboratories). The relative
expression of PGRN was calculated using the ΔΔCt method.
The expression of mRNA was normalized against the expression of the
GAPDH gene.
Immunoblot analysis
Total proteins were harvested from the blood
collected from the patients and control group. The proteins were
separated using 10% SDS/PAGE, and subjected to immunoblot analyses.
The primary mouse anti-human PGRN monoclonal antibody (clone
296628) was purchased from R&D Systems (Minneapolis, MN, USA),
while the primary mouse anti-GAPDH monoclonal and secondary
horseradish peroxidase-conjugated goat anti-mouse antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Bound antibodies were quantified using an enhanced
chemiluminescence system (Pierce Biotechnology, Inc., Rockford, IL,
USA). The experiments were performed three times.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The data are presented as
the median ± interquartile range and were analyzed with the
Mann-Whitney U test. Comparisons among the pre-treated,
post-treated and control groups were performed with an independent
sample non-parametric test. In addition, correlations between PGRN
and IL-6 levels were assessed using Spearman’s rank correlation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
mRNA and protein expression levels of
PGRN are increased in pSS patients
In this study, 26 newly diagnosed pSS patients were
enrolled (Table I). The control
group included 26 healthy volunteers, matching the gender and ages
of the pSS patients. Among the pSS patients (Table I), 25 were female and one was male,
with an age range of 24–65 years (median age, 44.8±10.96 years).
The course of the disease from the initial appearance of symptoms
to the enrollment in the study varied between 2 and 98 months
(median disease course, 20.4±22.0 months).
 | Table IClinical parameters of the pSS
patients. |
Table I
Clinical parameters of the pSS
patients.
| Patients | Gender | Age (years) | Disease course
(months) | ESR (mm/h) | RF | anti-SSA Ab |
|---|
| 1 | F | 48 | 71 | 115/76 | + | + |
| 2 | F | 35 | 2 | 9/15 | + | + |
| 3 | F | 32 | 26 | 67/87 | − | − |
| 4 | F | 46 | 22 | 31/15 | + | + |
| 5 | M | 51 | 98 | 57/39 | + | + |
| 6 | F | 33 | 19 | 22/16 | + | + |
| 7 | F | 43 | 33 | 11/7 | + | + |
| 8 | F | 51 | 7 | 52/46 | + | + |
| 9 | F | 52 | 8 | 63/31 | − | − |
| 10 | F | 58 | 29 | 15/9 | + | + |
| 11 | F | 63 | 39 | 19/25 | − | + |
| 12 | F | 48 | 15 | 24/11 | + | + |
| 13 | F | 65 | 28 | 46/25 | + | + |
| 14 | F | 56 | 8 | 75/49 | − | + |
| 15 | F | 34 | 6 | 16/8 | + | + |
| 16 | F | 26 | 2 | 69/49 | + | + |
| 17 | F | 39 | 3 | 29/12 | − | − |
| 18 | F | 46 | 9 | 78/48 | + | + |
| 19 | F | 35 | 10 | 33/27 | − | − |
| 20 | F | 45 | 2 | 23/16 | + | + |
| 21 | F | 24 | 4 | 56/20 | + | + |
| 22 | F | 36 | 6 | 39/25 | − | − |
| 23 | F | 45 | 26 | 69/33 | + | + |
| 24 | F | 42 | 22 | 85/30 | + | + |
| 25 | F | 55 | 20 | 39/26 | − | − |
| 26 | F | 58 | 16 | 37/21 | + | + |
To determine the mRNA expression level of PGRN,
peripheral blood mononuclear cells from the healthy controls and
pSS patients were separated prior to and following treatment with
prednisone. The total mRNA was isolated and the mRNA expression
level of PGRN was investigated with qPCR. Using IQ5 software, the
data are presented as the fold change in the gene expression
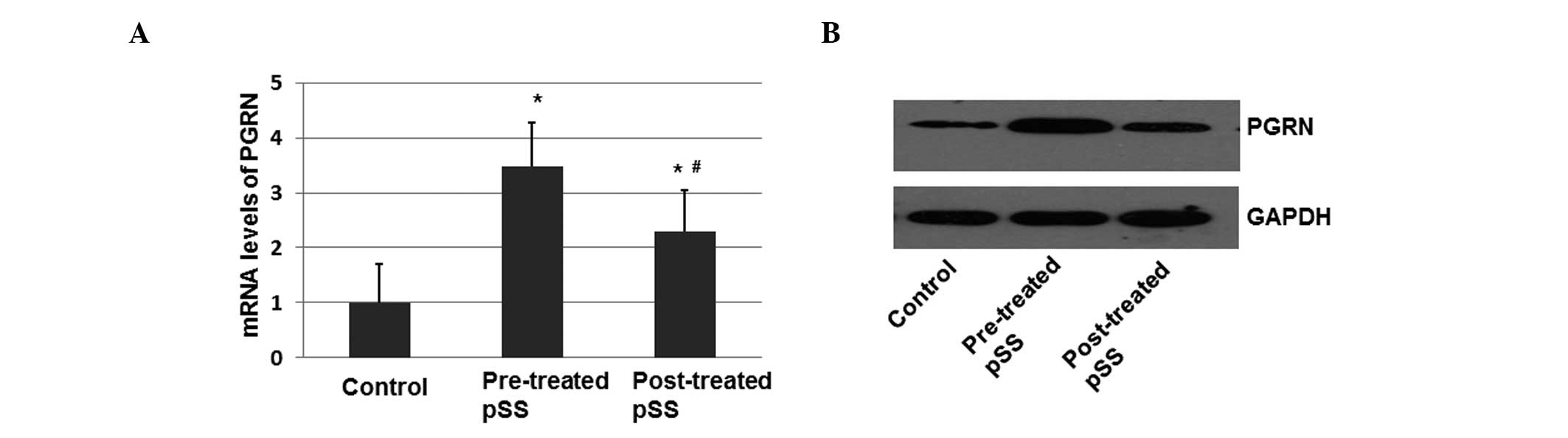
normalized against GAPDH. As shown in Fig. 1A, there was a 3.45-fold increase in
the relative mRNA expression of PGRN in the pSS patients prior to
treatment with prednisone (10 mg) when compared with the healthy
controls (P<0.05; Fig. 1A).
Following treatment with prednisone, the mRNA expression level of
PGRN showed only a 1.6-fold increase when compared with the healthy
controls (P<0.05). The difference in the expression levels
before and after treatment with prednisone was statistically
significant (P<0.05).
In order to determine the protein expression levels
of PGRN, peripheral blood mononuclear cells were separated and the
total proteins were isolated and determined by immunoblotting. The
protein expression level of PGRN was increased in the pSS patients
prior to treatment when compared with the healthy controls
(P<0.05; Fig. 1B). Following
treatment, the protein expression levels of PGRN decreased
(Fig. 1B). These results indicated
that PGRN expression may be positively associated with the
development of pSS.
Serum PGRN levels are increased in pSS
patients
ELISA was performed to investigate the serum levels
of PGRN (Table II), and IL-6
served as the cytokine control. As demonstrated in Table II, the levels of PGRN in pSS
patients were significantly upregulated when compared with the
healthy controls (P<0.05; Table
II). In addition, the difference between the PGRN levels prior
to and following prednisone treatment was statistically significant
(P<0.05). Following treatment, the serum levels of PGRN were
significantly downregulated, but remained higher than the healthy
control levels (P<0.05; Table
II). The IL-6 levels were higher in pSS patients prior to
treatment when compared with the healthy control (P<0.05) and
post-treatment patient groups (P<0.05; Table II). Therefore, the PGRN level in
the patients was altered based on the development of pSS.
 | Table IIComparison of serum levels of PGRN
and IL-6 by ELISA. |
Table II
Comparison of serum levels of PGRN
and IL-6 by ELISA.
| Groups | PGRN (pg/l) | IL-6 (pg/ml) |
|---|
| pSS |
| Pre-treatment | 14.57±7.93ab | 1.81±1.03ab |
|
Post-treatment | 10.39±7.47a | 1.05±079 |
| Healthy
control | 9.80±5.67 | 0.84±0.69 |
PGRN levels correlate with IL-6 in pSS
patients
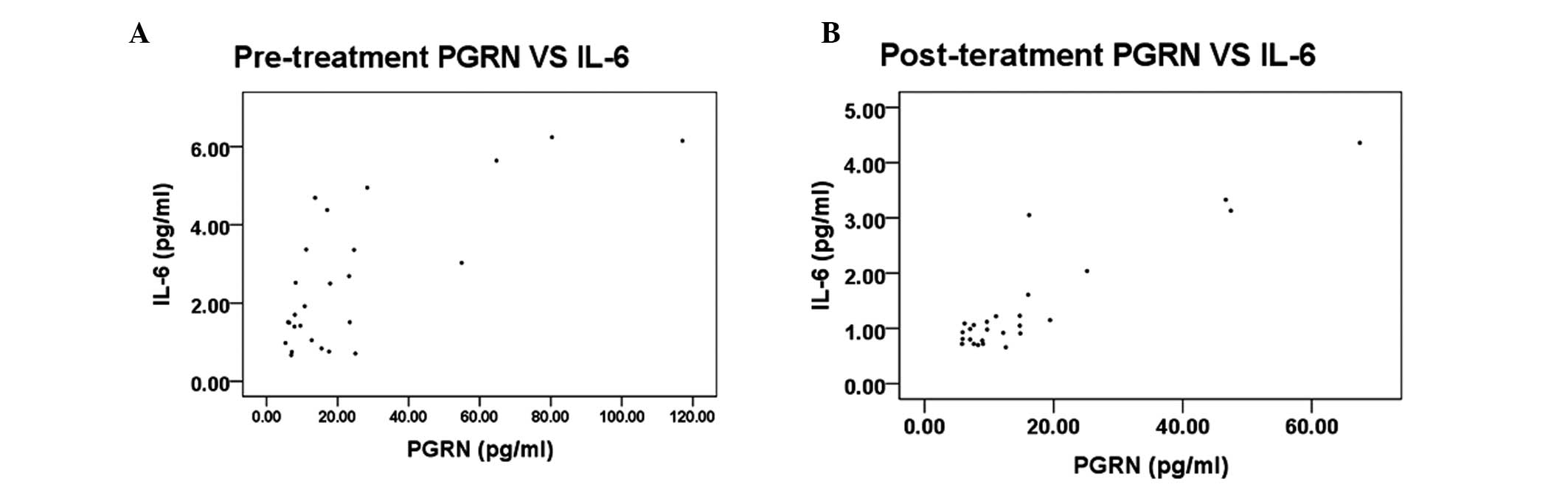
To examine the association between the serum levels
of PGRN and the pSS-related inflammatory factor, IL-6, Spearman’s
rank correlation analysis was performed in pSS patients prior to
and following treatment. The results demonstrated that the serum
level of PGRN in the pre-treatment group correlated with the level
of IL-6 (r=0.617, P=0.001; Fig.
2A). Similarly, the serum level of PGRN in the post-treatment
group correlated with the level of IL-6 (Fig. 2B).
Discussion
PGRN is an autocrine growth factor containing 7.5
repeats of a cysteine-rich motif in the order, P-G-F-B-A-C-D-E,
where P is the half motif (8).
PGRN is predominantly expressed in epithelial and immune cells,
neurons (9) and chondrocytes
(10), and high expression levels
of PGRN are found in a variety of human cancer types (11). Several studies have revealed that
PGRN plays an important role in a number of pathological processes,
including early embryonic development, wound healing and
inflammation (12–17). PGRN also functions as a regulator
of cartilage development and degradation (18). PGRN, binding directly to the tumor
necrosis factor receptor, is involved in a number of physiological
and pathological functions. Upregulation of PGRN has been reported
in chemotherapy-induced amenorrhea (19).
The present study demonstrated that the levels of
PGRN in the peripheral blood were upregulated in the pre-treated
and post-treated pSS patients when compared with the healthy
controls, indicating that PGRN may be involved in the development
of pSS. In the pre-treated pSS patients, the levels of IL-6 were
higher compared with the control and post-treated patient groups.
In addition, the IL-6 levels were shown to linearly correlate with
the levels of PGRN (P<0.05). IL-6 has been identified as an
important factor in the pathogenesis of pSS (20), and murine lupus models have
demonstrated the involvement of IL-6 in B-cell hyperactivation and
the onset of systemic lupus erythematosus (21,22).
In conclusion, the present study demonstrated that
PGRN is upregulated in pSS patients, indicating a possible role of
PGRN in the pathogenesis and development of pSS.
Acknowledgements
This study was supported by grants from the
Independent Innovation Foundation of Shandong University (no.
2012TS136), the ‘Eleventh Five-Year’ National Science and
Technology Support Program (no. 2008BA159802) and the Chinese
Medical Society Clinical Research Special Fund (no.
08010220100).
References
|
1
|
Ramos-Casals M, Brito-Zerón P and Font J:
The overlap of Sjögren’s syndrome with other systemic autoimmune
diseases. Semin Arthritis Rheum. 36:246–255. 2007.
|
|
2
|
Ramos-Casals M, Tzioufas AG and Font J:
Primary Sjögren’s syndrome: new clinical and therapeutic concepts.
Ann Rheum Dis. 64:347–354. 2005.
|
|
3
|
Fox RI, Stern M and Michelson P: Update in
Sjögren syndrome. Curr Opin Rheumatol. 12:391–398. 2000.
|
|
4
|
Tang W, Lu Y, Tian QY, et al: The growth
factor progranulin binds to TNF receptors and is therapeutic
against inflammatory arthritis in mice. Science. 332:478–484. 2011.
View Article : Google Scholar
|
|
5
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hrabal R, Chen Z, James S, Bennett HP and
Ni F: The hairpin stack fold, a novel protein architecture for a
new family of protein growth factors. Nat Struct Biol. 3:747–752.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bateman A and Bennett HP: The granulin
gene family: from cancer to dementia. Bioessays. 31:1245–1254.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng JQ, Guo FJ, Jiang BC, et al: Granulin
epithelin precursor: a bone morphogenic protein 2-inducible growth
factor that activates Erk1/2 signaling and JunB transcription
factor in chondrogenesis. FASEB J. 24:1879–1892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daniel R, He Z, Carmichael KP, Halper J
and Bateman A: Cellular localization of gene expression for
progranulin. J Histochem Cytochem. 48:999–1009. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Z, Ong CH, Halper J and Bateman A:
Progranulin is a mediator of the wound response. Nat Med.
9:225–229. 2003. View
Article : Google Scholar
|
|
11
|
Kessenbrock K, Fröhlich L, Sixt M, et al:
Proteinase 3 and neutrophil elastase enhance inflammation in mice
by inactivating antiinflammatory progranulin. J Clin Invest.
118:2438–2447. 2008.PubMed/NCBI
|
|
12
|
Zhu J, Nathan C, Jin W, et al: Conversion
of proepithelin to epithelins: roles of SLPI and elastase in host
defense and wound repair. Cell. 111:867–878. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo F, Lai Y, Tian Q, Lin EA, Kong L and
Liu C: Granulin-epithelin precursor binds directly to ADAMTS-7 and
ADAMTS-12 and inhibits their degradation of cartilage oligomeric
matrix protein. Arthritis Rheum. 62:2023–2036. 2010.PubMed/NCBI
|
|
14
|
Tishler M, Yaron I, Shirazi I, Yossipov Y
and Yaron M: Increased salivary interleukin-6 levels in patients
with primary Sjögren’s syndrome. Rheumatol Int. 18:125–127.
1999.PubMed/NCBI
|
|
15
|
Lotz M, Jirik F, Kabouridis P, Tsoukas C,
Hirano T, Kishimoto T and Carson DA: B cell stimulating factor
2/interleukin 6 is a costimulant for human thymocytes and T
lymphocytes. J Exp Med. 167:1253–1258. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Naka T, Nishimoto N and Kishimoto T: The
paradigm of IL-6: from basic science to medicine. Arthritis Res.
4(Suppl 3): S233–S242. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki H, Yasukawa K, Saito T, Narazaki M,
Hasegawa A, Taga T and Kishimoto T: Serum soluble interleukin-6
receptor in MRL/lpr mice is elevated with age and mediates the
interleukin-6 signal. Eur J Immunol. 23:1078–1082. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang B, Matsuda T, Akira S, Nagata N,
Ikehara S, Hirano T and Kishimoto T: Age-associated increase in
interleukin 6 in MRL/lpr mice. Int Immunol. 3:273–278. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tackey E, Lipsky PE and Illei GG:
Rationale for interleukin-6 blockade in systemic lupus
erythematosus. Lupus. 13:339–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gröndal G, Gunnarsson I, Rönnelid J,
Rogberg S, Klareskog L and Lundberg I: Cytokine production, serum
levels and disease activity in systemic lupus erythematosus. Clin
Exp Rheumatol. 18:565–570. 2000.PubMed/NCBI
|
|
21
|
Eilertsen GØ, Nikolaisen C, Becker-Merok A
and Nossent JC: Interleukin-6 promotes arthritis and joint
deformation in patients with systemic lupus erythematosus. Lupus.
20:607–613. 2011.PubMed/NCBI
|
|
22
|
Mihara M, Nishimoto N and Ohsugi Y: The
therapy of autoimmune diseases by anti-interleukin-6 receptor
antibody. Expert Opin Biol Ther. 5:683–690. 2005. View Article : Google Scholar : PubMed/NCBI
|