Introduction
Microbial disease of the female genital tract is
common and of significant economic importance in humans. Microbial
infections of the genital tract often infect the endometrium of
humans to cause endometritis, uterine disease and infertility
(1,2). Under normal pathophysiological
conditions, a number of the mechanisms underlying the recognition
of microbial pathogens by the innate immune system in vertebrates
have been identified during the past 15 years (3,4).
These mechanisms of innate immunity are not only important for
classic immune cells, including neutrophils and macrophages, but
are also evident in the endometrial and ovarian cells of mammals.
At the cellular level, human chronic endometritis (CE) is
characterized by inflammation with the elaboration of cytokines,
chemokines and prostaglandins (2).
Although the multiple signaling mechanisms that control the
inflammatory response in the endometrium have been extensively
studied, the molecular mechanisms that mediate the development of
human CE are not completely understood. Thus, there is significant
interest in identifying novel mechanisms for use in therapeutic
interventions for treatment of human CE.
The initial immune defense of the endometrium
against microbes is now considered to be highly dependent on
pattern recognition receptors (5,6).
Immune cells possess pattern recognition receptors, such as the
Toll-like receptors 1–10 (TLR1-10) and several nucleotide-binding
domain-like receptors (NLRs), which bind molecules specific to
microbial organisms often known as pathogen-associated molecular
patterns or microbial-associated molecular patterns (7). Briefly, TLR1, TLR2 and TLR6 recognize
bacterial lipids, including lipoteichoic acid from Gram-positive
bacteria and TLR5 binds flagellin. Nucleic acids, often from
viruses, are recognized by TLR3, TLR7, TLR8 and TLR9, although TLR9
also recognizes bacterial DNA. The NLRs are also intracytoplasmic
receptors that recognize bacterial peptidoglycans and components of
viruses. Notably, TLR4, in complex with cluster of differentiation
14 and MD-2, binds lipopolysaccharide (LPS) on immune cells
(8,9). Activation of pattern recognition
receptors initiates the production of pro-inflammatory cytokines,
chemokines and prostaglandins, often via the mitogen-activated
protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways,
leading to the recruitment of neutrophils and monocytes to the site
of infection (7,9). Although the endometrial and systemic
innate immune responses have been investigated in humans with
endometritis, there are no studies associating TLR4 with induced
infectious endometritis in human susceptible to persistent
endometritis. The present study featured the following objectives:
i) To determine whether TLR4 is altered in the endometrial cells of
the uterus collected from human patients with CE and normal
endometrial (NE) tissue; and ii) to explore the possible mechanisms
that would be involved in any such effects that are observed.
Materials and methods
Study population and sample
collection
Between January 2012 and December 2013, a total of
25 patients with CE underwent in-office diagnostic hysteroscopy and
endometrial biopsy at the Nanjing Government Hospital (Nanjing,
China). The diagnostic standard was as follows: Normal menstrual
cycle, normal basis endocrine, no evident organ lesions found in
the uterus and ovaries by ultrasound, no use of relevant sex
hormone drugs for three months and tubal patency. A group
comprising 15 patients with NE tissue was enrolled in parallel, and
all the patients with CE and NE were included for further analyses.
All the cases were diagnosed using clinical and pathological
evidence. The study conformed to the principles outlined in the
Declaration of Helsinki. All the retrospective reviews of the
clinical data involving human samples were approved by the Human
Research Ethics Committee of the Nanjing Government Hospital, and
written informed consent was obtained from each patient.
Reagents
Antibodies against the following proteins were
purchased from Santa Cruz Biotechology, Inc (Santa Cruz, CA, USA):
TLR4 (1:800), total-P65 (T-P65) (1:800), inhibitor (I)κBα (1:500),
phosphorylated-P65 (P-P65) (1:300) and GAPDH (1:4000). The
antibodies against myeloid differentiation factor-88 (MyD88)
(1:1000), tumor necrosis factor (TNF) receptor-associated factor 6
(TRAF6) (1:800) and transforming growth factor-β-activated kinase 1
(TAK1) (1:800) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
For RT-qPCR (10),
total RNA was extracted from the frozen human tissue using
TRIzol® (Invitrogen Life Technologies, Carlsbad, CA,
USA) and reverse-transcribed into cDNA using oligo (dT) primers
with a Transcriptor First Strand cDNA Synthesis kit. PCR
amplifications were quantified using the SYBR Green PCR Master Mix
(Applied Biosystems, Inc., Foster City, CA, USA) and normalized to
GAPDH gene expression. The primers for the RT-qPCR are shown
in Table I.
 | Table IPrimers for the reverse transcription
quantitative polymerase chain reaction. |
Table I
Primers for the reverse transcription
quantitative polymerase chain reaction.
| Name | Forward | Reverse |
|---|
| Interleukin-1β |
CCGTGGACCTTCCAGGATGA |
GGGAACGTCACACACCAGCA |
| Tumor necrosis
factor-α |
ACTGAACTTCGGGGTGATCGGT |
TGGTTTGCTACGACGTGGGCTA |
| Interleukin-10 |
TGAATTCCCTGGGTGAGAAG |
CTCTTCACCTGCTCCACTGC |
| GAPDH |
ACTCCACTCACGGCAAATTC |
TCTCCATGGTGGTGAAGACA |
Western blotting
Total proteins extracted from the left ventricle
tissue and cultured cardiomyocytes were first lysed in
radioimmunoprecipitation assay lysis buffer and the protein
concentrations were measured using the Pierce®
Bicinchoninic Protein Assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). The protein extract (50 μg) was run on 8–12%
SDS-PAGE gels (Invitrogen Life Technologies) and transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). The membranes were blocked in Tris-buffered saline with Tween
20 (TBST) containing 5% skimmed milk powder for 1 h at room
temperature and incubated with various primary antibodies overnight
at 4°C. Following incubation with secondary antibodies for 1 h at
room temperature, membranes were washed with TBST four times, as
previously described (11).
Specific proteins were detected using an enhanced chemiluminescence
reagent (GE Healthcare, Piscataway, NJ, USA) and captured on
Hyperfilm (GE Healthcare). The results were analyzed through the
Quantity One software (Bio-Rad, Hercules, CA, USA) for the
semi-quantitation of the mean gray value of each blot. The specific
protein expression levels were normalized to the GAPDH present on
the same nitrocellulose membrane. All the presented results are
representative of at least three independent experiments.
Statistical analysis
The data are presented as the mean ± standard
deviation. For two-group comparisons, Gaussian samples were
compared using the two-tailed Student’s t-test, while non-Gaussian
samples were compared using the non-parametric Mann-Whitney U test.
Statistical analyses were performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
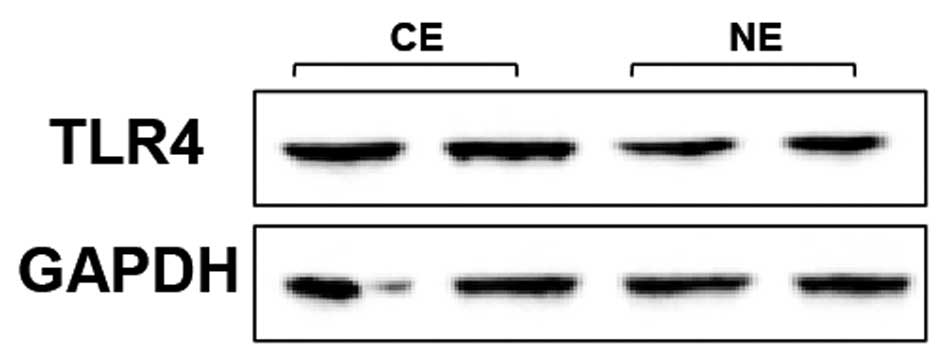
TLR4 expression is increased in human
CE
To investigate the potential role of TLR4 in the
development of human CE, whether expression levels of TLR4 were
altered in the pathological endometrium was first analyzed. The
RT-qPCR and western blot analysis assays showed that the mRNA and
protein expression levels of TLR4 were significantly
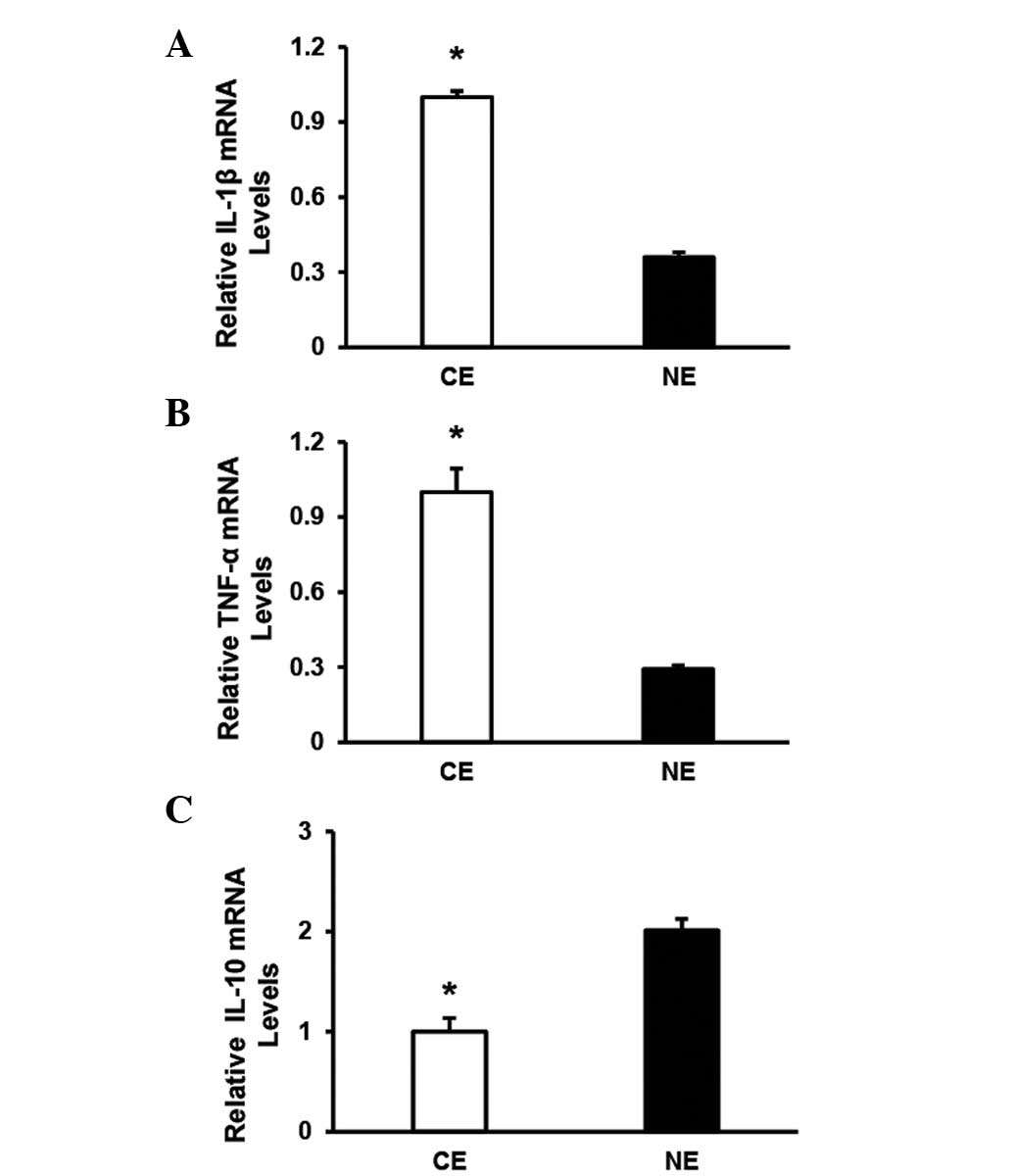
increased in human CE (n=7) compared with NE (n=5) (Fig. 1). In addition, the upregulation of
TLR4 in human CE was correlated with the induction of a
series of inflammatory markers at the mRNA level, including
interleukin (IL)-1β and TNF-α (Fig. 2A and B). However, the levels of
IL-10 were markedly decreased in human CE compared with NE
(Fig. 2C). Collectively, the
altered pattern of TLR4 expression suggests that TLR4
may be involved in the development of the inflammatory response in
human CE.
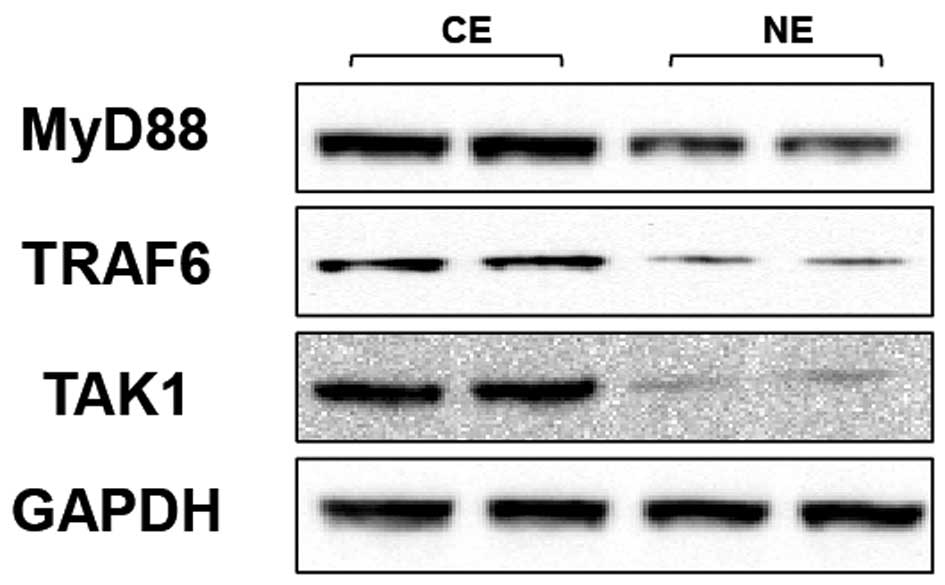
TLR4 regulates MyD88 signaling in human
CE
To dissect the possible molecular mechanisms through
which the regulation of TLR4 affects the inflammatory response in
human CE, the expressions/activities of TLR4 signaling molecules
(the adapter protein MyD88 and the accessory molecules TRAF6 and
TAK1) were investigated. The levels of MyD88, TRAF6 and TAK1 were
clearer in human CE than NE, as shown by western blot analysis
(Fig. 3). These results indicate
that MyD88-TRAF6-TAK1 signaling is critical for the effect of TLR4
on human CE.
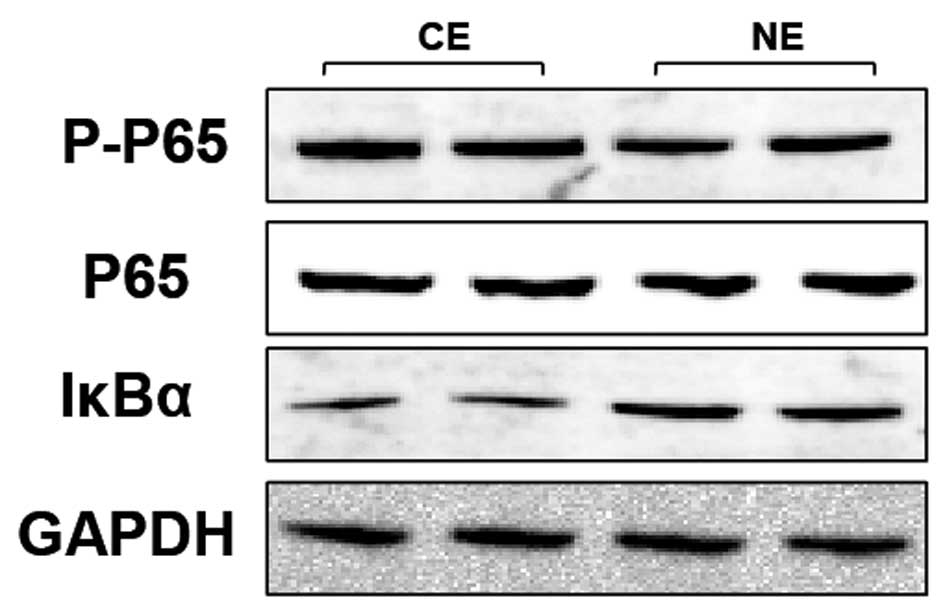
TLR4-mediated inflammation is largely
dependent on the promotion of NF-κB signaling in human CE
Increasing evidence demonstrated that the NF-κB
pathway is known to have an important role in inflammation, and the
triggering of the TLR pathway leads to the activation of NF-κB and
subsequent regulation of immune and inflammatory genes (12). The activation of the NF-κB pathway
in the indicated groups was therefore investigated. By western blot
analysis, expression levels of the T-P65, an important component
that usually forms the most common heterodimers of NF-κB, was shown
to not be different between the human CE and NE groups (Fig. 4). However, P-P65, comprising the
functionally active form of the transcription factor in the
nucleus, and IκBα, which binds with NF-κB to inhibit its
activation, were also detected (Fig.
4). The results show that the expression of P-P65 (Fig. 4) was significantly higher in the
human CE group compared with the NE group, while the expression
pattern of IκBα was reversed among the two groups (Fig. 4). The protein expression of IκBα
was highly increased in the human CE group compared with the NE
group. Taken together, these data indicate that the expression
levels of the NF-κB pathway is significantly correlated with TLR4
activation in human CE.
Discussion
The results of the present study provide evidence of
the critical role of TLR4 in human chronic endometritis. The level
of TLR4 was observed to be significantly upregulated in human CE.
In addition, it was found that the expression of MyD88, TRAF6 and
TAK1 molecules are involved in the mechanism of TLR4 in human
endometrial endothelial cell responses to bacterial infection.
Furthermore, it was identified that TLR4 exerted a pro-inflammatory
effect by the activation of NF-κB, thus facilitating its
transcriptional activity. These results suggest that the
TLR4-dependent NF-κB activation pathway contributes to the
inflammatory response in human CE.
Bacterial infection of the female genital tract can
cause pelvic inflammatory disease, infertility, endomyometritis,
septic pelvic thrombophlebitis and even pregnancy complications,
including preterm labor (8).
Notably, the endometrium is a unique mucosa, which is normally
sterile throughout pregnancy but becomes exposed to numerous
bacteria during the postpartum period (13). Indeed, specific Gram-negative
infections have been identified in the human endometrium (14). In previous years, increasing
attention has been directed to innate immunity, which is the
initial defense system against pathogens. In particular, TLRs,
which comprise a family of membrane proteins consisting of ≥10
members, are indicated to play significant roles in innate immunity
(9,15). The innate immune signaling also
contributes to tissue homeostasis, including the microflora,
proliferation and apoptosis of epithelial cells, and regeneration.
However, abnormal activation of innate immune signaling may also
cause sepsis, chronic inflammation, autoimmune diseases, tissue and
organ injuries, fibrosis and carcinogenesis that are unfavorable to
the host (16). Of note, a
previous study by Krikun et al (17) demonstrated that TLR4 mRNA
and protein expression were reported in primary cultures of
epithelial and stromal cells from the human endometrium. In
addition, TLR4 can recognize chlamydial LPS and cHSP60 (18). The present study tested the
hypothesis that TLR4-dependent signaling is essential for the
response to infections by the epithelial cells of the human
endometrium. TLR4 protein expression was detectable in all the
tissue lines by western blotting, but the expression of TLR4 in the
human CE endothelial cell was significantly higher than that of the
NE. These data revealed that high TLR4 expression level are
associated with human CE, which indicated the importance of TLR4 in
inflammatory response progression. Notably, the TLR4 signaling
pathway requires downstream adaptor molecules such as MyD88, that
interact directly with the Toll-interleukin receptor domain of TLRs
on the cell plasma membrane (19).
Following recognition of ligands by TLRs, MyD88 recruits IL-1
receptor-associated kinase, which stimulates TRAF6, TAK1 and
NF-κB-inducing kinase complex, leading to the activation of IκB
kinases, which stimulate IκBα phosphorylation and degradation,
resulting in NF-κB translocation to the nucleus, binding to target
DNA sequences and stimulation of gene expression (20). In the present study, it was
observed that MyD88 and the associated downstream molecules were
also significantly augmented, suggesting that the MyD88-mediated
signaling could be involved in stimulating an over-exuberant
inflammatory response, possibly influencing disease
progression.
To further understand the mechanism responsible for
TLR4/MyD88 signaling, the activation of downstream NF-κB pathways
was explored. NF-κB activation has been shown to play a critical
role in regulating the expression of groups of genes involved in
immune and inflammatory responses, cell death and survival, cell
growth and the cell cycle. NF-κB is a critical transcription factor
in TLR-mediated signaling pathways (21,22).
The main pathway of TLR4-mediated signaling that leads to NF-κB
activation involves the adaptor molecule termed MyD88 signaling
complexes (23). In the present
study, it was found that the expression levels of P-P65 was
markedly upregulated in the human CE group compared with the
control group. Unlike the other components of the NF-κB cascade,
the decrease in expression of IκBα in the control group was
reversed. This result could be explained by TLR4 inducing the NF-κB
cascade through the phosphorylation and ubiquitination of IκBα to
release NF-κB for its translocation to the nucleus. A previous
study (24) confirmed that as
there was more IκB-α degradation, the expression of the IκB-α
protein would also decrease. Furthermore, consistent with the
upstream NF-κB components, the assessment of the protein levels of
the downstream pro-inflammatory cytokine markers (IL-1β and TNF-α)
exhibited the same trends in the human endometrial tissue. By
contrast, the anti-inflammatory cytokine IL-10 exhibited a
significant decline. Based on the present results, it may be
proposed that TLR4 has a pivotal role in the activities of the
NF-κB signaling pathway, which in turn regulates the expression of
genes involved in the inflammatory response in human CE.
In conclusion, the present study demonstrated that
elevated TLR4 expression levels could be associated with the
disease progression in patients with CE, which indicates that TLR4
may serve as a valuable marker in human CE. However, the possible
underlying mechanisms for its participation in human CE progression
is not entirely clear; therefore, other signaling pathways require
investigating in order to gain an improved understanding of the
molecular mechanism in this field.
References
|
1
|
Turner ML, Healey GD and Sheldon IM:
Immunity and inflammation in the uterus. Reprod Domest Anim.
47(Suppl 4): 402–409. 2012. View Article : Google Scholar
|
|
2
|
Sheldon IM and Bromfield JJ: Innate
immunity in the human endometrium and ovary. Am J Reprod Immunol.
66(Suppl 1): 63–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar
|
|
5
|
Wira CR, Fahey JV, Sentman CL, Pioli PA
and Shen L: Innate and adaptive immunity in female genital tract:
cellular responses and interactions. Immunol Rev. 206:306–335.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mor G and Cardenas I: The immune system in
pregnancy: a unique complexity. Am J Reprod Immunol. 63:425–433.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beutler BA: TLRs and innate immunity.
Blood. 113:1399–1407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krikun G, Trezza J, Shaw J, et al:
Lipopolysaccharide appears to activate human endometrial
endothelial cells through TLR-4-dependent and TLR-4-independent
mechanisms. Am J Reprod Immunol. 68:233–237. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Chen W, Zhu Y, et al: Caspase
recruitment domain 6 protects against cardiac hypertrophy in
response to pressure overload. Hypertension. 64:94–102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao C, Li L, Chen W, et al: Deficiency of
IKKɛ inhibits inflammation and induces cardiac protection in
high-fat diet-induced obesity in mice. Int J Mol Med. 34:244–252.
2014.
|
|
12
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheldon IM, Cronin J, Goetze L, Donofrio G
and Schuberth HJ: Defining postpartum uterine disease and the
mechanisms of infection and immunity in the female reproductive
tract in cattle. Biol Reprod. 81:1025–1032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pellati D, Mylonakis I, Bertoloni G, et
al: Genital tract infections and infertility. Eur J Obstet Gynecol
Reprod Biol. 140:3–11. 2008. View Article : Google Scholar
|
|
15
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar
|
|
16
|
Yang L and Seki E: Toll-like receptors in
liver fibrosis: cellular crosstalk and mechanisms. Front Physiol.
3:1382012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krikun G, Lockwood CJ, Abrahams VM, Mor G,
Paidas M and Guller S: Expression of Toll-like receptors in the
human decidua. Histol Histopathol. 22:847–854. 2007.PubMed/NCBI
|
|
18
|
Chow JC, Young DW, Golenbock DT, Christ WJ
and Gusovsky F: Toll-like receptor-4 mediates
lipopolysaccharide-induced signal transduction. J Biol Chem.
274:10689–10692. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll protein
signals activation of adaptive immunity. Nature. 388:394–397. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang G and Ghosh S: Toll-like
receptor-mediated NF-kappaB activation: a phylogenetically
conserved paradigm in innate immunity. J Clin Invest. 107:13–19.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li LC, Varghese Z, Moorhead JF, Lee CT,
Chen JB and Ruan XZ: Cross-talk between TLR4-MyD88-NF-κB and
SCAP-SREBP2 pathways mediates macrophage foam cell formation. Am J
Physiol Heart Circ Physiol. 304:H874–H884. 2013.PubMed/NCBI
|
|
22
|
Kawai T and Akira S: Signaling to
NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fitzgerald KA, Rowe DC, Barnes BJ, et al:
LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll
adapters TRAM and TRIF. J Exp Med. 198:1043–1055. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|