Introduction
Tumor vessels lay the foundation for tumor growth
and metastasis; therefore, the antitumor treatment focusing on
tumor angiogenesis has become a focus in clinical tumor treatment
(1,2). It was previously reported that, in
addition to the significant neovascularization, the tumor blood
vessels are characterized by abnormal vascular structure, including
multiple branches, structural disorder, overlapping of endothelial
cells and lack of pericytes (3).
Therefore, the treatment targeted at tumor vessels is aimed at
reducing their number and also improving their abnormal structure.
Vascular endothelial growth factor (VEGF) induces the proliferation
and migration of endothelial cells and increases microvascular
permeability (4–6). It was previously demonstrated that
the expression level of VEGF in tumor tissues is significantly
increased and promotes tumor angiogenesis (7,8).
Avastin is a novel anti-VEGF humanized monoclonal antibody and is
able to combine with all VEGF isomers with high affinity,
indirectly inhibiting VEGF from binding to its receptor, which is
the mechanism through which Avastin exerts its biological effects
(9,10).
In 2001, Jain proposed the theory of vascular
normalization and considered that vascular normalization
concurrently with the administration of agents targeting tumor
blood vessels may conduce to the antitumor effects of treatment
(11). Avastin was shown to
inhibit tumor angiogenesis in vivo and in vitro and
is currently the main drug used for targeting the blood vessels of
malignant tumors (12). However,
the number of studies on whether Avastin facilitates the
normalization of the tumor vasculature is limited. Therefore, in
this study, A549 lung adenocarcinoma cells were used to construct a
nude mouse model to analyze the antitumor effect of Avastin and its
effect on tumor vessel number and structure, in order to add to the
theoretical basis for antitumor treatment with Avastin.
Materials and methods
Model construction and treatment
A total of 30 BALB/c nude mice were purchased from
Shanghai Laboratory Animal Center of the Chinese Academy of
Sciences, Shanghai, China. The mice were aged 6–8 weeks and weighed
19–22 g. The animals were maintained under specific pathogen-free
conditions. This study was performed in strict accordance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of the First Affiliated Hospital of Zhengzhou
University.
A549 cells were cultured in RPMI-1640 culture medium
supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories, Logan, UT, USA) until the exponential growth phase.
The culture medium was discarded and the flask was washed with
twice with phosphate-buffered saline (PBS), followed by the
addition of 0.25% pancreatic enzymes (Gibco, Grand Island, NY, USA)
to digest the cells. Subsequently, RPMI-1640 with 10% FBS was added
to stop the digestion and the cells were centrifuged at 1,500 × g
for 3 min. The cell sediment was rinsed twice with PBS and
resuspended in PBS, with the cell concentration regulated to
5×107/ml. The cells (5×106/100 μl) were
inoculated in a fat pad near the axilla in the left rib of each
nude mouse. After 1 week, when the tumors had grown to 100–150
mm3, the tumor-bearing nude mice were randomly divided
into three groups (n=10 per group) as follows: The control group
(each mouse was intraperitoneally injected with 100 μl PBS every
other day, for a total of eight times); the Avastin I group
[Avastin (Roche, Basel, Switzerland) was intraperitoneally injected
at a dose of 3 mg/kg every other day, for a total of eight times];
and the Avastin II group (Avastin was intraperitoneally injected at
a dose of 6 mg/kg every other day, for a total of eight times).
Starting from the first day of treatment, the tumor size was
measured daily.
Survival rate analysis
After the mice model was constructed successfully,
when the tumors had grown to 100–150 mm3, the
tumor-bearing nude mice were randomly divided into three groups
(n=10 per group) as follows: The control group (each mouse was
intraperitoneally injected with 100 μl PBS every other day, for a
total of 8 times); the Avastin I group [Avastin (Roche, Basel,
Switzerland) was intraperitoneally injected at a dose of 3 mg/kg
every other day, for a total of 8 times]; and the Avastin II group
(Avastin was intraperitoneally injected at a dose of 6 mg/kg every
other day, for a total of 8 times). Starting from the first day of
treatment, the survival rate of the mice was observed during
treatment for 25 days.
ELISA
After 7 days of treatment, the mice in the three
groups were euthanized by cervical dislocation, and 0.1-g tumor
tissue samples were placed in radioimmunoprecipitation assay buffer
for 10 min to prepare the tissue lysate. Subsequently, a protease
inhibitor was added. The liquid was placed on ice for 30 min and
then centrifuged at 15,000 × g for 10 min. The supernatant fluid
was collected and the VEGF level in the tumor tissues was
determined with the VEGF ELISA kit (R&D Systems Inc.,
Minneapolis, MN, USA) according to the manufacturer’s
instructions.
Immunofluorescence method
Tumor tissues were embedded in optimal cutting
temperature compound to prepare frozen sections, which were fixed
by paraformaldehyde and sealed with 10% goat serum. Rat anti-mouse
CD31 (0610017-C) and rabbit anti-mouse α-smooth muscle actin (SMA;
CSB-E09343h) antibodies (Abcam, Cambridge, UK) were proportionally
diluted and dripped onto the tissue sections, coated and incubated
overnight 4°C. On the following day, the sections were rinsed with
PBS three times. Fluorescein isothiocyanate (FITC) goat anti-rat
(BA1101) and phycoerythrin goat anti-rabbit fluorescent secondary
antibodies (BA1034; Boster Biological Technology, Ltd., Wuhan,
China) were proportionally diluted and dripped onto the tissue
slides, incubated at 37°C for 1 h and rinsed with PBS three times.
Subsequently, DAPI was used for nuclear staining, the slides were
covered with cover slips and observed under a fluorescence
microscope (Olympus, Tokyo, Japan) at ×400 magnification. A total
of 10 visual fields were randomly selected among the tumor sections
from each group to calculate the vessel number. The proportion of
normal blood vessels was calculated as follows: 40 blood vessels
were randomly selected (magnification, ×400), the blood vessels
stained red by CD31 were counted and the proportion of FITC
α-SMA-positive vessels was calculated.
Statistical analysis
All the data were analyzed with SPSS 17.0
statistical software (SPSS Inc., Chicago, IL, USA) and measured
data were expressed as means ± standard deviation. Multi-group
comparisons were conducted with one-factor analysis of variance and
inter-group comparison was performed with the least significant
difference method. Comparisons of survival rates were performed
with the Kaplan-Meier method and the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
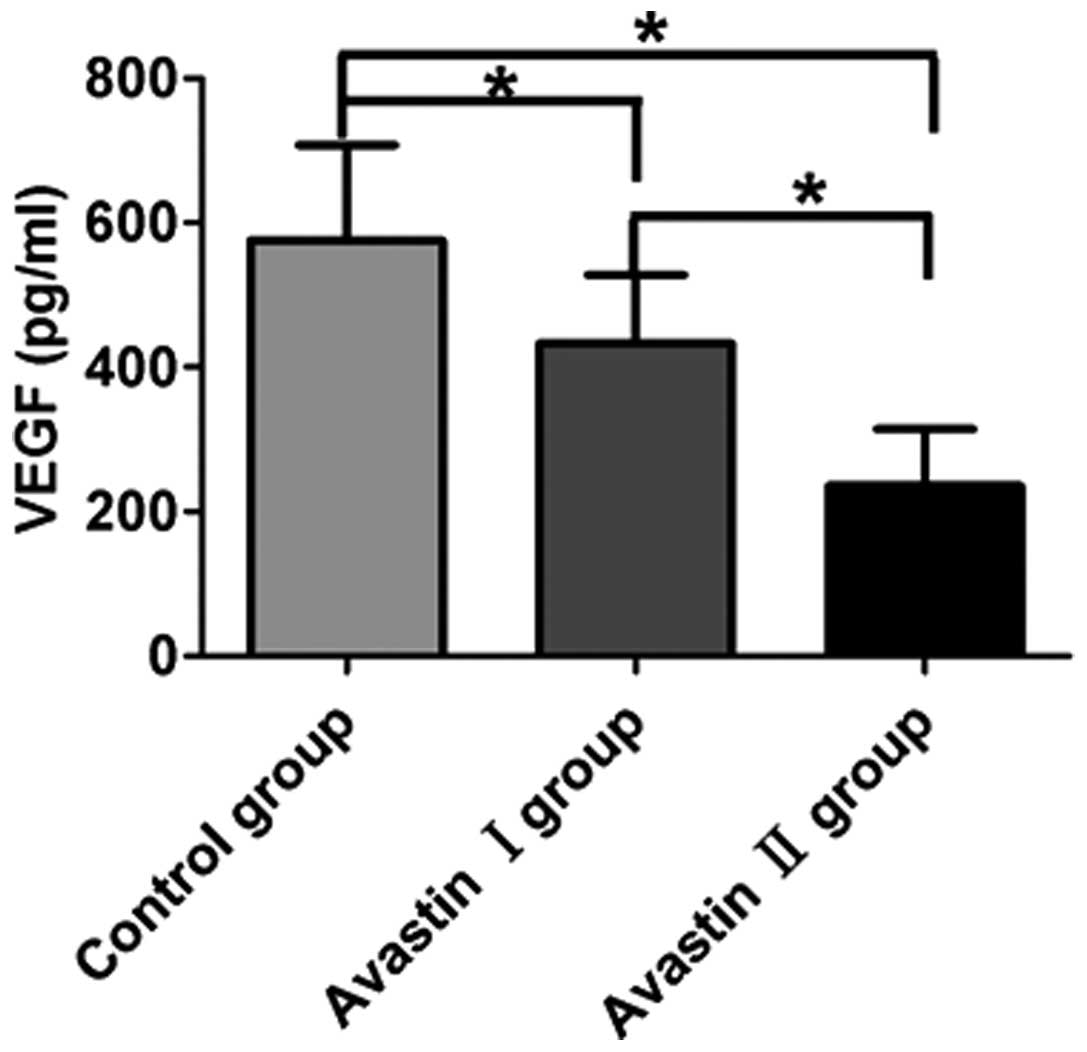
Avastin inhibits the expression of VEGF
in tumor tissues
Compared with the control group (575.72±49.75
pg/ml), the Avastin I (433.32±49.75 pg/ml) and Avastin II
(235.75±40.17 pg/ml) groups exhibited significantly downregulated
VEGF protein levels in the mouse tumor tissues and the differences
were statistically significant (P<0.05). Following treatment,
the Avastin II group exhibited a more prominent downregulation of
VEGF expression compared with the Avastin I group and the
difference was statistically significant (P<0.05) (Fig. 1).
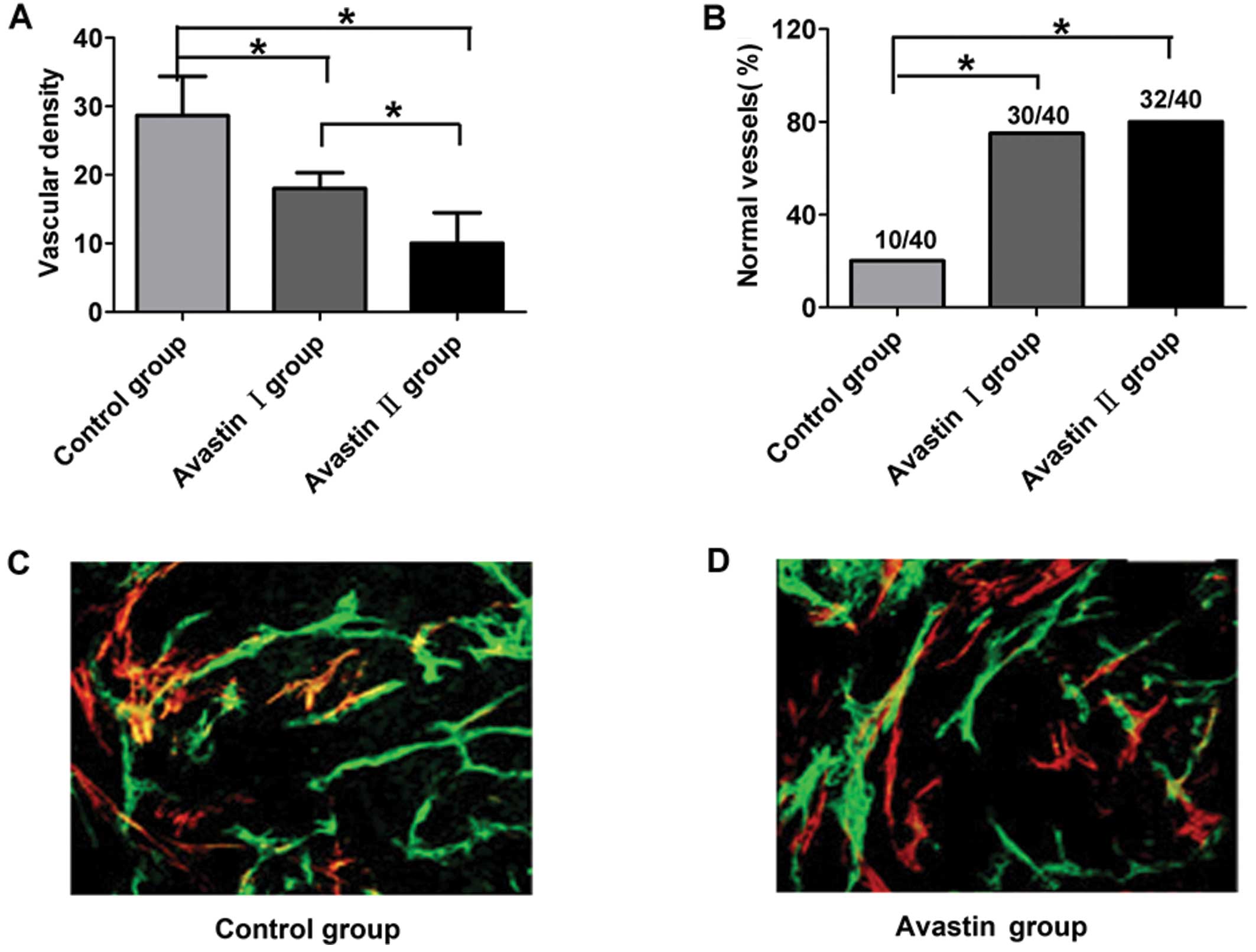
Avastin controls the tumor blood vessel
number and promotes vascular pericyte coverage
After 7 days of treatment, the Avastin I and II
groups exhibited markedly decreased vascular density compared with
the control group, while the vascular density of the Avastin II
group decreased more significantly (P<0.05) (Fig. 2A). Compared with the control group,
the proportion of normal vascular structures in the mouse tumors
from the Avastin I and II groups was distinctly increased
(P<0.05). There was no statistical significance in the
proportion of normal vascular structures between the Avastin I and
II groups (P>0.05) (Fig.
2B–D).
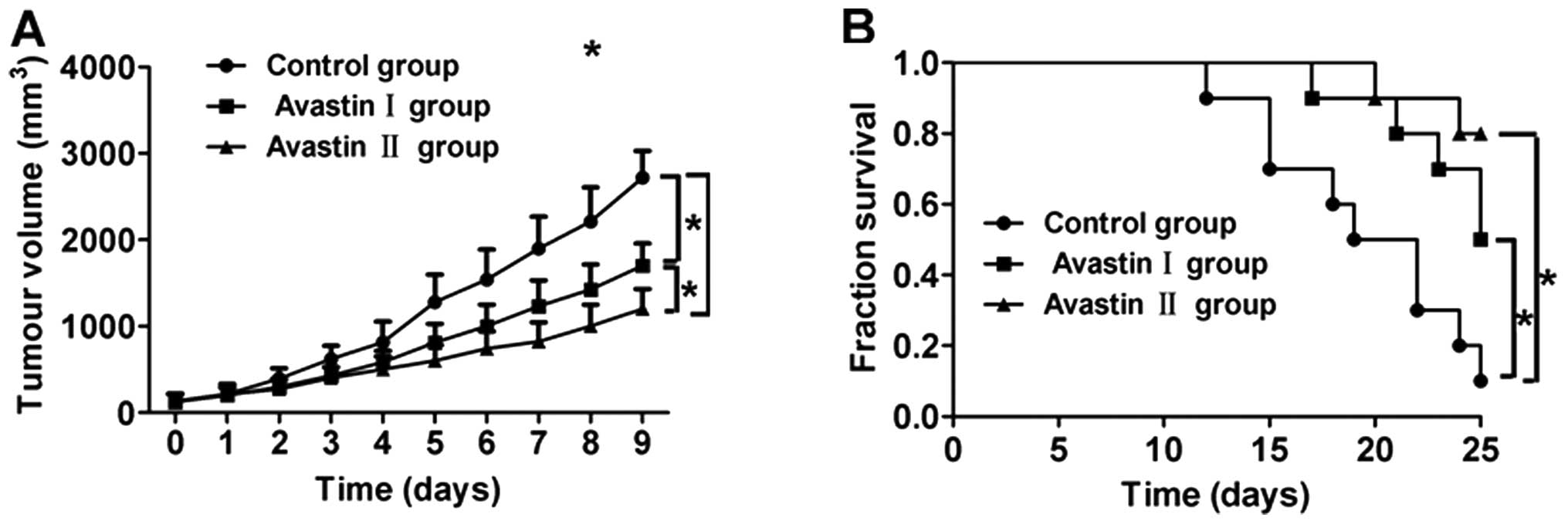
Avastin inhibits tumor growth and
increases the survival rate of tumor-bearing mice
Compared with the control group, the tumor growth in
mice from the Avastin I and II groups slowed down significantly
after treatment, while the growth rate of the tumors in the Avastin
II group was significantly lower compared with that in the Avastin
I group, with a statistically significant difference (P<0.05)
(Fig. 3A). Furthermore, the
survival rates of the mice in the Avastin I and II groups were
significantly higher compared with that of control group
(P<0.05) (Fig. 3A). There was
no statistically significant difference in survival rate between
the Avastin I and II groups (P>0.05) (Fig. 3B).
Discussion
Folkman (13)
reported that when a solid tumor grows to 1–2 mm, tumor growth
depends on the oxygen and nutrients supplied by newly formed blood
vessels, whereas the time to tumor metastasis is also associated
with tumor angiogenesis. Therefore, tumor blood vessels are
essential for tumor growth, infiltration and metastasis and
antitumor treatment targeting tumor vessels has become a focus of
investigation. Several antiangiogenic drugs are currently used in
clinical practice and it was demonstrated that they are able to
significantly inhibit tumor angiogenesis and, thus, control tumor
growth (14,15). Of note, in addition to the
prominent angiogenesis, the tumor vasculature is characterized by
severe structural and functional abnormalities. The abnormal vessel
structure hinders the delivery of antitumor drugs to the tumor
tissues, compromising their antitumor effect. Accordingly,
treatments targeting tumor vessels must be aimed at inhibiting
tumor angiogenesis and also at normalizing the existing vessels, in
order to promote antitumor drug delivery to solid tumors via
vascular system.
VEGF plays an important role in tumor angiogenesis
and blocking of VEGF signaling may effectively inhibit tumor
angiogenesis (16). Avastin is a
novel anti-VEGF humanized monoclonal antibody, which is able to
significantly inhibit tumor angiogenesis in vivo and tumor
growth (17). Our results
demonstrated that Avastin at a concentration of 3 mg/kg
significantly downregulated VEGF levels in tumor tissues and
inhibited angiogenesis in A549 lung cancer tissues. Jain (11) observed an abnormal lack of pericyte
coverage in the tumor vasculature and this observation may
indirectly reflect the structural changes of the tumor vessels
(18,19). In our experiment, we demonstrated
that after 7 days of treatment with Avastin, the of tumor vessel
pericyte coverage was significantly increased compared with the
control, suggesting that Avastin promotes the normalization of the
tumor vasculature. Our result were consistent with those previously
reported (20).
In our study, Avastin inhibited tumor angiogenesis
and promoted the normalization of tumor vessels, significantly
inhibiting the growth of A549 lung tumors in treated mice in a
dose-dependent manner. The antitumor effects of Avastin are
mediated through the inhibition of tumor angiogenesis and also
through the normalization of the tumor vasculature, which increases
the amount of Avastin delivered to the tumor tissues to exert its
antiangiogenic effect. Avastin also significantly increased the
survival rate of A459 tumor-bearing mice.
In conclusion, Avastin significantly inhibits tumor
angiogenesis and promotes normalization of tumor vessels, thus
inhibiting tumor growth and increasing survival rate. However, the
mechanism underlying the vascular normalization achieved by Avastin
requires further investigation.
References
|
1
|
Shojaei F: Anti-angiogenesis therapy in
cancer: current challenges and future perspectives. Cancer Lett.
320:130–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tonra JR and Hicklin DJ: Targeting the
vascular endothelial growth factor pathway in the treatment of
human malignancy. Immunol Invest. 36:3–23. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morikawa S, Baluk P, Kaidoh T, et al:
Abnormalities in pericytes on blood vessels and endothelial sprouts
in tumors. Am J Pathol. 160:985–1000. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chatterjee S, Heukamp LC, Siobal M, et al:
Tumor VEGF: VEGFR2 autocrine feed-forward loop triggers
angiogenesis in lung cancer. J Clin Invest. 123:1732–1740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen P, Zhu J, Liu DY, et al:
Over-expression of survivin and VEGF in small-cell lung cancer may
predict the poorer prognosis. Med Oncol. 31:7752014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taira T and Yamamoto N: Anti-VEGF therapy
for lung cancer. Jpn J Clin Med. 70:2159–2164. 2012.(In
Japanese).
|
|
7
|
Szajewski M, Kruszewski WJ, Lakomy J, et
al: VEGF-C and VEGF-D overexpression is more common in left-sided
and well-differentiated colon adenocarcinoma. Oncol Rep.
31:125–130. 2014.PubMed/NCBI
|
|
8
|
Wang X, Chen X, Fang J and Yang C:
Overexpression of both VEGF-A and VEGF-C in gastric cancer
correlates with prognosis, and silencing of both is effective to
inhibit cancer growth. Int J Clin Exp Pathol. 6:586–597.
2013.PubMed/NCBI
|
|
9
|
Zhou M, Yu P, Qu X, Liu Y and Zhang J:
Phase III trials of standard chemotherapy with or without
bevacizumab for ovarian cancer: A meta-analysis. PLoS One.
8:e818582013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo X, Liu TS, Yu YY, et al: Efficacy and
safety of bevacizumab (BEV) plus chemotherapeutic agents in the
treatment of metastatic colorectal cancer, mCRC. Chin J Oncol.
35:604–607. 2013.(In Chinese).
|
|
11
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wedam SB, Low JA, Yang SX, et al:
Antiangiogenic and antitumor effects of bevacizumab in patients
with inflammatory and locally advanced breast cancer. J Clin Oncol.
24:769–777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keunen O, Johansson M, Oudin A, et al:
Anti-VEGF treatment reduces blood supply and increases tumor cell
invasion in glioblastoma. Proc Natl Acad Sci USA. 108:3749–3754.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu JY and Wakelee HA: Monoclonal
antibodies targeting vascular endothelial growth factor: current
status and future challenges in cancer therapy. BioDrugs.
23:289–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Twelves C, Chmielowska E, Havel L, et al:
Randomised phase II study of axitinib or bevacizumab combined with
paclitaxel/carboplatin as first-line therapy for patients with
advanced non-small-cell lung cancer. Ann Oncol. 25:132–138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Tomaso E, London N, Fuja D, et al:
PDGF-C induces maturation of blood vessels in a model of
glioblastoma and attenuates the response to anti-VEGF treatment.
PLoS One. 4:e51232009.PubMed/NCBI
|
|
20
|
Dings RP, Loren M, Heun H, et al:
Scheduling of radiation with angiogenesis inhibitors anginex and
Avastin improves therapeutic outcome via vessel normalization. Clin
Cancer Res. 13:3395–3402. 2007. View Article : Google Scholar : PubMed/NCBI
|