Introduction
Ventilator-associated pneumonia (VAP) is one of the
most common hospital-acquired pneumonias occurring in intubated
patients and remains a leading cause of morbidity and mortality of
patients in intensive care units (ICUs). Ventilator dependence and
long hospital stays increase the costs of hospitalization. A number
of risk factors exist for VAP, with bacterial colonization of the
gastric content with subsequent gastroesophageal reflux (GER) and
aspiration into the airways being an important risk factor
(1). There is currently no
particular method of preventing VAP; however, there are several
promising combined nursing strategies that are effective in
preventing VAP, including education programs, oral care and the
continuous control of endotracheal cuff pressure and
microaspiration of gastric contents. Dodek et al
demonstrated that appropriate positioning to reduce reflux,
improving oral hygiene, may reduce the incidence of VAP (2). In the present study, evidence for a
retrograde route of VAP transmission from the stomach to the
oropharynx to the lower respiratory tract was examined. Genome
macrorestriction-pulsed-field gel electrophoresis (GM-PFGE) was
performed to accomplish this and to subsequently assess the effects
of a combined intervention strategy, comparing the mean risk of VAP
between the control and intervention groups.
Materials and methods
Diagnosis of VAP
At present, the definition of VAP is controversial
as it is difficult to distinguish the condition from other usual
pulmonary infections. According to the definition by the American
Thoracic Society and the Infectious Diseases Society of America,
VAP is considered to be pneumonia in patients that have received
mechanical ventilation for ≥48 h, characterized by the presence of
a new or progressive infiltrate and signs of systemic infection
(including temperature, blood cell count, changes in sputum
characteristics and detection of the causative agent) (3). The condition can be divided into
late-onset VAP (LOP) and early-onset VAP (EOP), depending on
whether time spent on the ventilator was >5 or <5 days,
respectively. In the present study, the clinical diagnosis criteria
for VAP (positive quantitative endotracheal aspirate cultures and
Clinical Pulmonary Infection Score >6) were based on the
Hospital-Acquired Pneumonia Diagnosis and Treatment Guidelines by
The Chinese Medical Association Branch of Respiratory Diseases
(4).
A diagnosis of VAP (excluding certain associated
lung diseases, including tuberculosis, lung cancer and atelectasis)
was made if the condition of the patient met the following
criteria: i) Lung infection following mechanical ventilation for 48
h; ii) presence of infiltrates or new inflammatory pulmonary
lesions following mechanical ventilation; and iii) lung
consolidation and/or moist lung rales, as well as one of the
following: a) Blood cells >1.0×1010/l or
<4×109/l, with or without nuclear transfer; b) fever
(body temperature >37.5°C) with a large number of purulent
respiratory secretions; or c) new pathogenic bacteria isolated from
bronchial secretions.
Genotyping by GM-PFGE
Respiratory secretions from four patients with VAP
[randomly selected from the ICU of the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China)] were collected using
protected specimen brushes once every other day for quantitative
bacterial culture. The bacterial stains that antimicrobial
susceptibility testing showed to be undifferentiated were cultured
by centrifugation in lysogeny broth (Beijing Solarbio Science &
Technology Co., Ltd, Beijing, China) at 37°C and 4.48 × g to a
concentration of 109/ml after 18 h. The cells were
suspended in 100 μl buffer (0.1 mol/l EDTA, pH 8.0; 0.01 mol/l
Tris-Cl, pH 7.6; and 1 mol/l NaCl), mixed with 100 μl low-melting
point agarose (Sangon Biotech Co., Ltd., Shanghai, China) and
molded into plugs at 4°C. Following congealing of the agar gel, the
cells were lysed with lysis buffer at 37°C for 2 h and washed with
Sodium Chloride-Tris-EDTA (STE) buffer (0.1 mol/l NaCl;10 mmol/l
Tris-Cl, pH 8.0; and 1 mmol/l EDTA, PH 8.0), followed by
enzymolysis with proteinase K (1 mg/ml; Sangon Biotech Co., Ltd.)
for 16–20 h. The lysed cells were subsequently treated with 2
mmol/l phenylmethanesulfonyl fluoride (Beijing Cowin Bioscience
Co., Ltd., Beijing, China) for 45 min; this treatment was repeated
once more, prior to washing three times with STE buffer and mixing
at room temperature with a buffer containing 50 units SpeI
restriction enzyme (Beijing Cowin Bioscience Co., Ltd.). After 18 h
at 37°C, electrophoresis was performed using GenePath (Bio-Rad,
Hercules, CA, USA) at a field strength of 6 V/cm, at 14°C for 20 h.
The pulse times were between 5 and 35 sec. The gel was then stained
with ethidium bromide (0.5 μg/ml; HaoSen Co., Jiangsu, China) for 1
h and washed for 1 h with ddH2O. Finally, the gel was
observed under a 302 nm ultraviolet light and images were captured.
Fragment patterns were compared according to the criteria set out
by Tenover et al (5).
Study population
Inclusion and exclusion criteria
The inclusion criteria for the patients observed in
the present study were as follows: i) >60 years old and ii)
receiving mechanical ventilation. The exclusion criteria comprised:
i) a critical condition that could cause mortality within 48 h; ii)
mechanical ventilation for <48 h; and iii) pulmonary
infection.
Intervention group
The 124 intubated patients (intervention group) were
treated at the ICU of the First Affiliated Hospital of Zhengzhou
University between January 2011 and May 2013. The intervention
treatment included the administration of a gastric motility
stimulant and the adoption of a semi-reclining position. Mosapride
citrate tablets (5 mg/tablet; Lunan Company, Shandong, China) at a
dosage of 5 mg/administration, three times a day, were selected as
the gastric motility stimulant. The stimulant was used continuously
until the study end-point or unless severe diarrhea (loose bowel
movements three times in one day) occurred.
The semi-reclining position was used as a treatment
if there were no tolerance issues or anti-semi-reclining indicators
(including use of a vascular active drug, refractory shock
following hypervolemic therapy inefficiency, or neurosurgery or
abdominal surgery within the previous seven days). The beds of able
patients were positioned at an angle of 30–45° to maintain these
patients in a semi-reclining repose; for those patients that
exhibited tolerance issues, a semi-reclining position was
maintained during nasogastric feeding (including gastrointestinal
injections), as well as for 2 h after feeding.
Control group
A total of 112 intubated patients received
traditional nursing in the ICU of the First Affiliated Hospital of
Zhengzhou University between January 2009 and December 2010. The
patients were not administered any gastric motility stimulants
during the treatment period.
All other procedures in the treatment of the two
groups of patients were consistent and included the following: i)
Monitoring of intra-cuff pressure every 4 h and maintenance at
>20 mmHg, as well as the continuous aspiration of subglottic
secretion, sputum smear, Gram staining and aerobic cultivation
twice per week; ii) administration of sucralfate tablets; and iii)
no use of H2 receptor antagonists and antacids unless
bleeding of the stress ulcers occurred. In addition, Acute
Physiology and Chronic Health Evaluation II (APACHE II) score of
all patients were evaluated. APACHE II was an indicator of illness
severity, which was determined using the worst value obtained
during the initial 24 h following ICU admission, as well as on the
day of VAP diagnosis (6,7).
Written informed consent was obtained from all
patients prior to their involvement in the study. The study was
approved by the Life Sciences Institutional Review Board of
Zhengzhou University.
Observation indices
A number of observations were recorded, including
baseline indices (characteristics of the patients prior to
treatment), length of hospital stay and number of days spent on the
ventilator. In addition, GER was monitored in all the patients. The
observation was concluded when one of the following occurred:
Mortality, extubation or diagnosis of VAP with a one month
follow-up to confirm whether the cause of the patient’s mortality
was associated with VAP. The incidence rate of VAP referred to the
number of VAP episodes per 1,000 ventilator-days. Mortality rates
for the patients with and without VAP were calculated and compared
between the two groups of patients.
Statistical analysis
All statistical data were analyzed using SPSS 12.0
software (SPSS, Inc, Chicago, IL, USA). Rate data were calculated
using a χ2 test. Significant differences between the two
groups with one variant were determined using the Student’ t-test.
A two-tailed value of P<0.05 was considered to indicate a
statistically significant difference.
Results
GM-PFGE fingerprinting
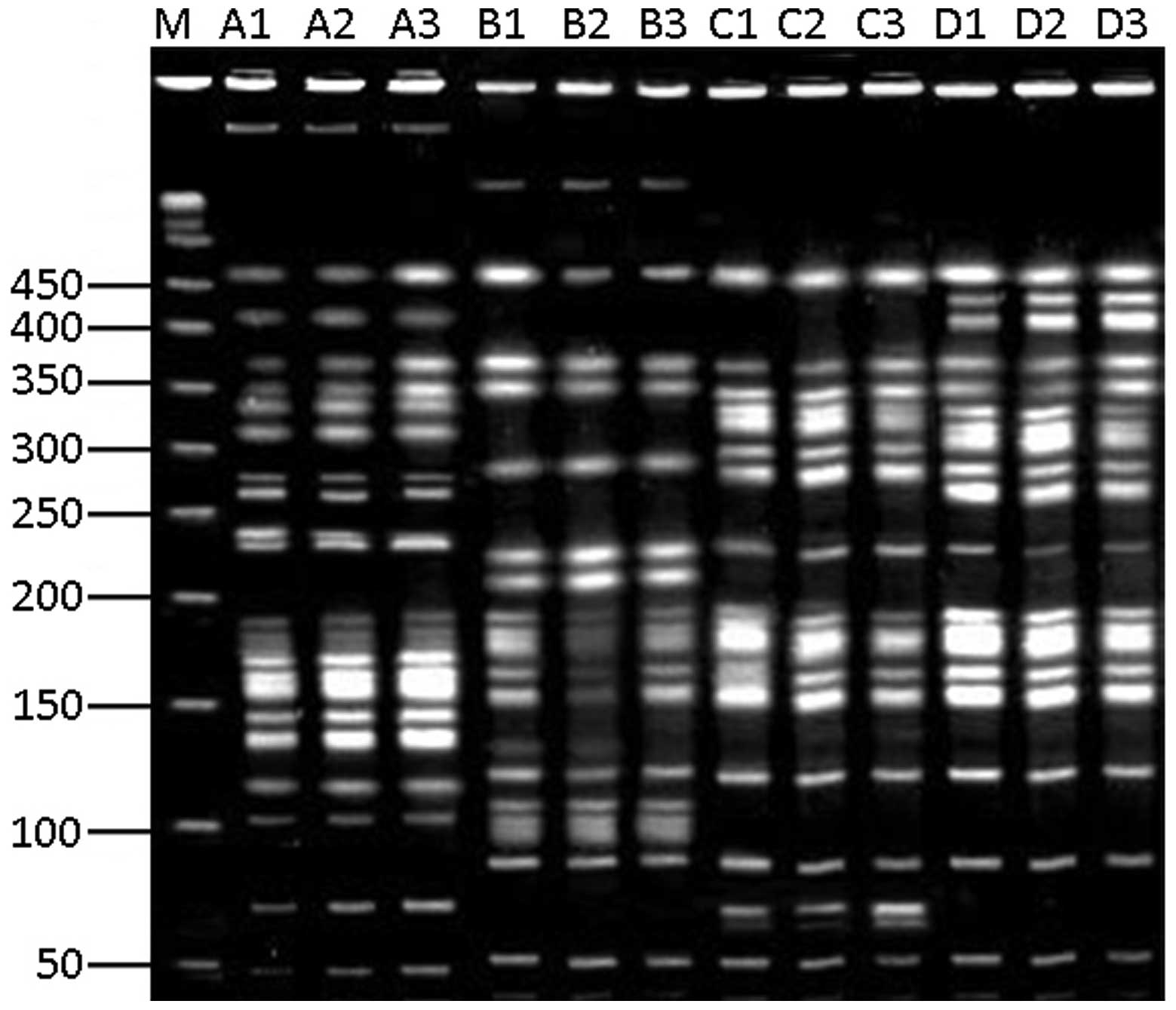
The primary pathogen, Pseudomonas aeruginosa,
was selected for genotyping using GM-PFGE. GM-PFGE fingerprinting
indicated that the P. aeruginosa from the gastric juice,
subglottic secretion drainage and drainage of the lower respiratory
tract were similar in each patient with VAP (Fig. 1). However, while the strains were
consistent across locations in a single patient, the strains
observed across the four patients with VAP differed.
Basic participant information
All patients in the intervention and control groups
were selected strictly in accordance with the inclusion and
exclusion criteria. The age, gender and basic data of the patients
were normalized between the two groups. Furthermore, all patients
were treated in the same hospital and the number of patients who
were from medical or surgical departments was also normalized
across the groups (Table I).
 | Table IBasic participant information. |
Table I
Basic participant information.
| Group | |
|---|
|
| |
|---|
| Characteristic | Control | Intervention | P-value |
|---|
| n | 112 | 124 | - |
| Age in years, mean ±
SD | 70.63±6.31 | 71.06±5.74 | 0.851 |
| Gender, n male/n
female | 67/57 | 59/53 | 0.764 |
| APACHE II score, mean
± SD | 22.84±5.75 | 24.84±4.96 | 0.672 |
Etiology of VAP
The most commonly detected bacteria were P.
aeruginosa (29.42%), Acinetobacter baumannii (10.85%),
Staphylococcus aureus (7.48%) and Stenotrophomonas
maltophilia (7.32%). The number of patients with VAP infected
with two or more bacteria was 55.81%. The primary pathogens
identified in patients with EOP were Gram-positive bacteria
(59.27%), including S. aureus, while Gram-negative bacteria
(including P. aeruginosa and A. baumannii) were most
frequently identified in patients with LOP (70.8%).
Effect of intervention
Table II shows
that the combined nursing strategy described in the present study
was able to decrease the number of days spent on a ventilator, the
incidence rate of VAP and the mortality rate of the intubated
patients (all P<0.05); however, no significant difference in the
ratio of EOP/LOP was found between the two groups. The mortality
rate from VAP was not reduced by these nursing measures once it had
occurred.
 | Table IIComparison of several indexes between
the control and intervention groups. |
Table II
Comparison of several indexes between
the control and intervention groups.
| Group | |
|---|
|
| |
|---|
| Indexes | Control | Intervention | P-value |
|---|
| VAP, n (EOP/LOP) | 84 (26/58) | 43 (11/23) | 0.882 |
| Ventilator-days, mean
± SD | 12.34±4.98 | 7.37±5.32 | <0.050 |
| Incidence of
VAPa, ‰ | 40.81 | 21.25 | <0.050 |
| Mortality rate,
% |
| Intubated
patients | 41.94 | 29.46 | <0.050 |
| Patients with
VAP | 35.71 | 47.06 | 0.252 |
Discussion
PFGE has been used as the gold standard for the
genotyping of bacteria, which can be a powerful tool for the study
of nosocomial infections. There is debate as to whether a
retrograde route of transmission from the stomach to oropharynx to
lower respiratory tract contributes to VAP. In the present study,
GM-PFGE fingerprinting results demonstrated that this route does
exist in patients with VAP. This result was consistent with that of
a previous study (8) and may be
the reason why the interventions in the present study were
effective in the prevention of VAP. Grap et al (9) observed that an early, single
application of chlorhexidine significantly reduced the occurrence
of VAP in trauma patients. The results of the present study
indicate that this may have been due to the chlorhexidine blocking
the infection route from the stomach to respiratory tract, at least
to a certain extent.
In the present study, the results of GM-PFGE
genotyping indicated that the P. aeruginosa populations at
different drainage locations in each patient were consistent. A
previous animal study involving New Zealand white rabbits indicated
that bacteria from the gastrointestinal tracts of the rabbits were
not the main sources of EOP, but may have contributed to the
development of LOP (10).
Consequently, the prevention of VAP through inhibition of the
gastroesophageal reflux (GER) may be effective.
Numerous studies have been performed to investigate
the prevention of VAP, and several strategies have been proven to
be effective preventative measures, including educational
intervention (11,12), sufficient and professional oral
care (13–15) and the use of specific equipment,
such as heat and moisture exchange filters (16) or silver-coated endotracheal tubes
(17). None of these measures,
however, should be used as an isolated intervention.
The combined strategy used in the present study,
which focused predominantly on body position and the inhibition of
the occurrence of GER, revealed that a semi-reclining position and
the use of mosapride (an inhibitor of GER) were able to decrease
the incidence rate of VAP from 40.81 to 21.25% (P<0.05). The
number of days spent on a ventilator was reduced by almost five
days in the intervention group (P<0.05), and the mortality rate
of intubated patients decreased from 41.94 to 29.46% (P<0.05).
However, the interventions had no effect on the mortality rate
following the occurrence of VAP, demonstrating that the
effectiveness of these interventions lies entirely in preventing
VAP onset. These results were consistent with those of a previously
published study (18). An animal
model of mechanical ventilation using healthy New Zealand white
rabbits demonstrated that drugs promoting gastrointestinal motility
(mosapride citrate) were useful in reducing the incidence rate of
VAP caused by bacteria from the digestive tract (10).
As the development of VAP is a complicated process,
its overall prevention requires numerous stages, courses and
methods. Further studies are required to investigate the infection
route from the stomach to the respiratory tract in patients with
VAP, and more effective and personalized strategies for reducing
episodes of VAP should be utilized.
Acknowledgements
This study was supported by the ‘Project of Medical
Science and Technology Research of Henan Province’ (no. 201203032)
and the ‘Project of Science and Technology Research of Zhengzhou
City’ (no. 0910SGYS33389-13).
References
|
1
|
Abdel-Gawad TA, El-Hodhod MA, Ibrahim HM
and Michael YW: Gastroesophageal reflux in mechanically ventilated
pediatric patients and its relation to ventilator-associated
pneumonia. Crit Care. 13:R1642009. View
Article : Google Scholar
|
|
2
|
Dodek P, Keenan S, Cook D, et al: Canadian
Critical Care Trials Group; Canadian Critical Care Society:
Evidence-based clinical practice guideline for the prevention of
ventilator-associated pneumonia. Ann Intern Med. 141:305–313. 2004.
View Article : Google Scholar
|
|
3
|
American Thoracic Society; Infectious
Diseases Society of America. Guidelines for the management of
adults with hospital-acquired, ventilator-associated, and
healthcare-associated pneumonia. Am J Respir Crit Care Med.
171:388–416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xue XY, Gao ZC, Zhu JH, Xu Y and Li X:
Clinical evaluation of chinese guidelines for community-acquired
pneumonia. Beijing Da Xue Xue Bao. 38:276–279. 2006.(In
Chinese).
|
|
5
|
Tenover FC, Arbeit RD, Goering RV, et al:
Interpreting chromosomal DNA restriction patterns produced by
pulsed-field gel electrophoresis: criteria for bacterial strain
typing. J Clin Microbiol. 33:2233–2239. 1995.PubMed/NCBI
|
|
6
|
Gursel G and Demirtas S: Value of APACHE
II, SOFA and CPIS scores in predicting prognosis in patients with
ventilator-associated pneumonia. Respiration. 73:503–508. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elwakil MA: APACHE II scoring and
antibiotics significance against VAP associated risks. Pak J Biol
Sci. 14:1036–1037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schleder BJ: Taking charge of
hospital-acquired pneumonia. Nurse Pract. 29:50–53. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grap MJ, Munro CL, Hamilton VA, Elswick RK
Jr, Sessler CN and Ward KR: Early, single chlorhexidine application
reduces ventilator-associated pneumonia in trauma patients. Heart
Lung. 40:e115–e122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Q, He LX, Hu BJ, et al: The role of
gastro-intestinal tract microorganisms in the development of
ventilator-associated pneumonia: an experimental study. Zhonghua
Jie He He Hu Xi Za Zhi. 31:509–512. 2008.PubMed/NCBI
|
|
11
|
Kellie SP, Scott MJ, Cavallazzi R, et al:
Procedural and educational interventions to reduce
ventilator-associated pneumonia rate and central line-associated
blood stream infection rate. J Intensive Care Med. 29:165–174.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jansson M, Kääriäinen M and Kyngäs H:
Effectiveness of educational programmes in preventing
ventilator-associated pneumonia: a systematic review. J Hosp
Infect. 84:206–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Tawfiq JA, Amalraj A and Memish ZA:
Reduction and surveillance of device-associated infections in adult
intensive care units at a Saudi Arabian hospital, 2004–2011. Int J
Infect Dis. 17:e1207–e1211. 2013.PubMed/NCBI
|
|
14
|
Scannapieco FA and Binkley CJ: Modest
reduction in risk for ventilator-associated pneumonia in critically
ill patients receiving mechanical ventilation following topical
oral chlorhexidine. J Evid Based Dent Pract. 12:15–17. 2012.
View Article : Google Scholar
|
|
15
|
Zurmehly J: Oral care education in the
prevention of ventilator-associated pneumonia: quality patient
outcomes in the intensive care unit. J Contin Educ Nurs. 44:67–75.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Auxiliadora-Martins M, Menegueti MG,
Nicolini EA, et al: Effect of heat and moisture exchangers on the
prevention of ventilator-associated pneumonia in critically ill
patients. Braz J Med Biol Res. 45:1295–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kane T and Claman F: Silver tube coatings
in pneumonia prevention. Nurs Times. 108:21–23. 2012.PubMed/NCBI
|
|
18
|
Li Bassi G and Torres A:
Ventilator-associated pneumonia: role of positioning. Curr Opin
Crit Care. 17:57–63. 2011.PubMed/NCBI
|