Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of mortality from cancer among
females, accounting for 23% of total cancer cases and 14% of cancer
mortalities (1,2). Despite the development of surgical
techniques and meticulously designed chemotherapy regimens, relapse
remains almost inevitable in patients with advanced cases of the
disease. Although there are a number of chemical therapeutic drugs
for the treatment of breast cancer that are able to kill or inhibit
the growth of tumors, they are usually associated with a number of
side-effects (3,4). Therefore, further investigation into
the molecular pathogenesis of breast cancer and the identification
of novel and effective biomarkers are urgently required.
MicroRNAs (miRNAs) are a class of small, non-coding
RNAs, which are capable of regulating the expression of genes at
the post-transcriptional level (5,6).
Mechanistically, miRNA functions by binding to the 3′ untranslated
region (UTR) of target mRNA, blocking translation and/or causing
mRNA degradation (7). Previous
investigations have demonstrated that miRNAs play a diverse role in
tumorigenesis and may function as oncogenes, tumor suppressors and
modulators of tumor proliferation, apoptosis and drug resistance
(8–10). Among numerous miRNAs, miR-185
stands out as an important molecule. Analyses of ovarian cancer,
pediatric renal tumor and prostate cancer cases have revealed a
decreased expression of miR-185, which may be involved in tumor
initiation and progression (11,12).
However, the biological function and underlying molecular
mechanisms of miR-185 in breast cancer have not been fully
elucidated. Therefore, in the current study, the association
between miR-185 and breast cancer was investigated.
Materials and methods
Cell culture and tissue samples
Two human breast cancer cell lines (MCF7 and SKBR3)
and a normal human mammary epithelial cell line (MCF10A) were
obtained from the Chinese Academy of Sciences (Shanghai, China).
All the cell lines used were cultured in RPMI 1640 medium (Gibco
Life Technologies, Beijing, China) supplemented with 10% fetal calf
serum, 100 IU/ml penicillin and 100 mg/ml streptomycin (Gibco Life
Technologies). In addition, human breast cancer tissues and distant
normal tissues were collected during routine therapeutic surgery at
the Department of Breast Surgery, the First Affiliated Hospital of
Zhejiang University School of Medicine (Hangzhou, China). Written
informed consent was obtained from all participants involved in
this study. The study was performed in accordance with the
Declaration of Helsinki and was approved by the Institutional
Review Board of Zhejiang University (Hangzhou, China).
RNA extraction and quantitative
analysis
Cells were seeded into 12-well plates and total RNA
was isolated using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer’s instructions.
RNA was reverse transcribed and amplified using a real
time-polymerase chain reaction (PCR) miRNA detection kit (Ambion
Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. PCR was performed using an ABI 7500
Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) with
the following conditions: One cycle of 95°C for 10 min; and 40
cycles of 95°C for 15 sec and 60°C for 1 min. The U6 small nuclear
RNA was used as the control. The mRNA expression of c-Met was
measured by quantitative PCR (qPCR), with GAPDH used as the
control. The primer sequences were as follows: Forward,
5′-CAGATGTGTGGTCCTTTG-3′, and reverse,
5′-ATTCGGGTTGTAGGAGTCT-3′.
MTT assay
Cell proliferation was determined using an MTT
assay. The cells were seeded into 96-well plates at a density of
3×104 cells/well. Next, 10 ml MTT (5 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) was added and the plates were
incubated in the dark at 37°C for 2 h. The absorbance was
determined using a Model 680 Microplate Reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 490
nm.
Apoptosis analysis
At 48 h post-transfection with the miR-185
mimics/inhibitor or control, the cells were washed with
phosphate-buffered saline (PBS), detached with trypsin and
harvested. Subsequently, the cells (1×106) were
centrifuged at 700 × g for 5 min and the supernatant solutions were
discarded. The cells were washed twice with PBS, 70% alcohol was
added and the mixture was centrifuged at 700 × g for 5 min.
Apoptotic cells were evaluated using the Annexin V-FITC/PI Cell
Apoptosis Detection kit (BD Pharmingen, San Diego, CA, USA),
following the manufacturer’s instructions.
Western blotting
Cultured cells were lysed using
radioimmunoprecipitation assay buffer, and tissue samples were
lysed using T-PER Tissue Protein Extraction Reagent (Sigma) in the
presence of a protease inhibitor cocktail (Pierce Biotechnology,
Inc., Rockford, IL, USA). The tissue and cell lysates were
subjected to sodium dodecyl sulfate polyacrylamide gel
electrophoresis. For immunoblotting, the membranes were blocked
with 5% non-fat milk in Tris-buffered saline, and incubated with a
mouse anti-human c-Met monoclonal antibody (Abcam, Cambridge, MA,
USA), followed by a horseradish peroxidase-conjugated secondary
antibody (Abcam). The signals were detected using Immobilon
(Millipore, Billerica, MA, USA) and the immunoreactive bands were
identified using an enhanced chemiluminescence kit (Sigma) for
western blotting detection and a ChemiGenius bioimaging system
(Syngene, Frederick, MD, USA). GAPDH levels were measured as a
loading control.
Plasmid construction and luciferase
activity assay
In order to perform the fluorescent reporter assay,
the following primers were used to amplify the 3′-UTR of the c-Met
gene: Forward, 5′-GATCCTGCTAGTACTATGTCAAAGCAACAGTC-3′, and reverse,
5′-AATTCTCAGGCAGTGAAAAAACCATTGGAC-3′. Subsequently, a plasmid
containing the 3′-UTR of c-Met and a fluorescent reporter was
constructed. MCF7 cells were seeded into 48-well plates and
cotransfected with mimic control, miR-185 mimics or miR-185
inhibitor. The enhanced green fluorescent protein (EGFP) activity
was normalized against the red fluorescent protein activity. After
72 h, the fluorescence intensity was determined using a
fluorescence spectrophotometer (Hitachi, Ltd., Tokyo, Japan). The
primers were designed by Primer Premier 5.0 (Premier Biosoft, Palo
Alto, CA, USA).
Statistical analysis
All the data are presented as the mean ± standard
error of mean and were analyzed using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). For comparisons between two groups, the
statistical significance was determined using the Student’s t-test.
Comparisons among groups were performed using analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
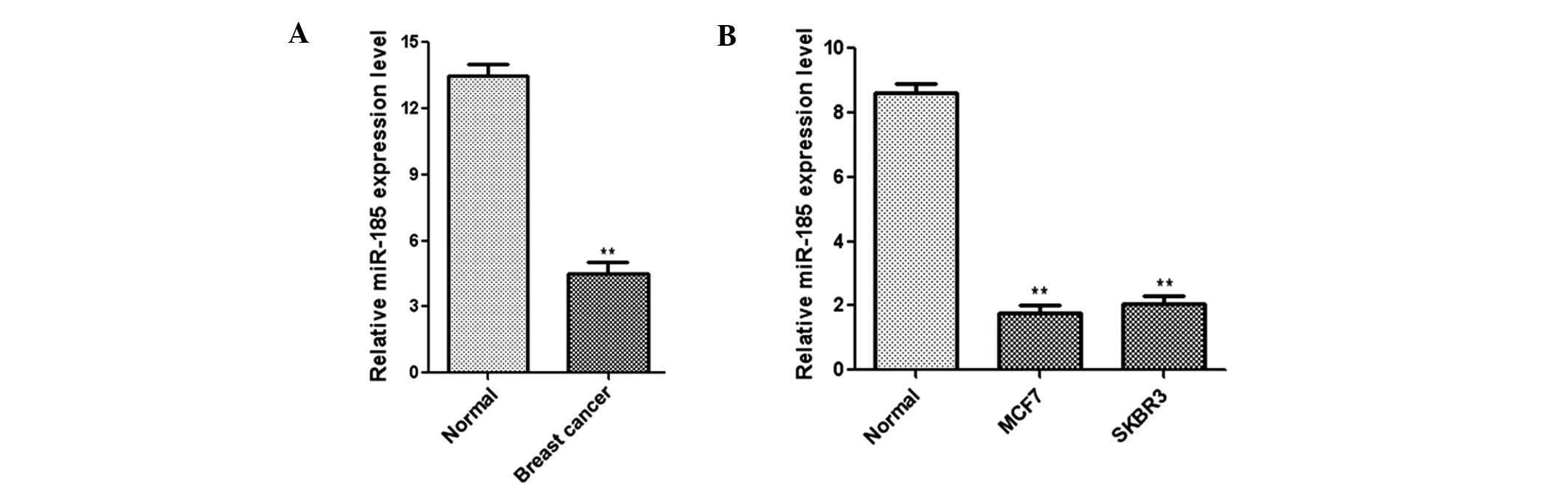
miR-185 is downregulated in breast cancer
tissue
To investigate the clinical relevance of miR-185 in
human breast cancer, miR-185 expression was analyzed in 24 paired
breast cancer and adjacent non-tumor tissues. qPCR analysis
indicated that the expression level of miR-185 was clearly
downregulated in the cancer tissues when compared with the
corresponding non-tumor samples (Fig.
1A). In addition, the normal human mammary epithelial cell line
(MCF10A) and breast cancer cell lines (MCF7 and SKBR3) were
analyzed with qPCR. A significant downregulation in the expression
level of miR-185 was observed in the breast cancer cell lines when
compared with the normal cell line (Fig. 1B). These results indicated that
expression levels of miR-185 are decreased significantly in breast
cancer tissues and cell lines.
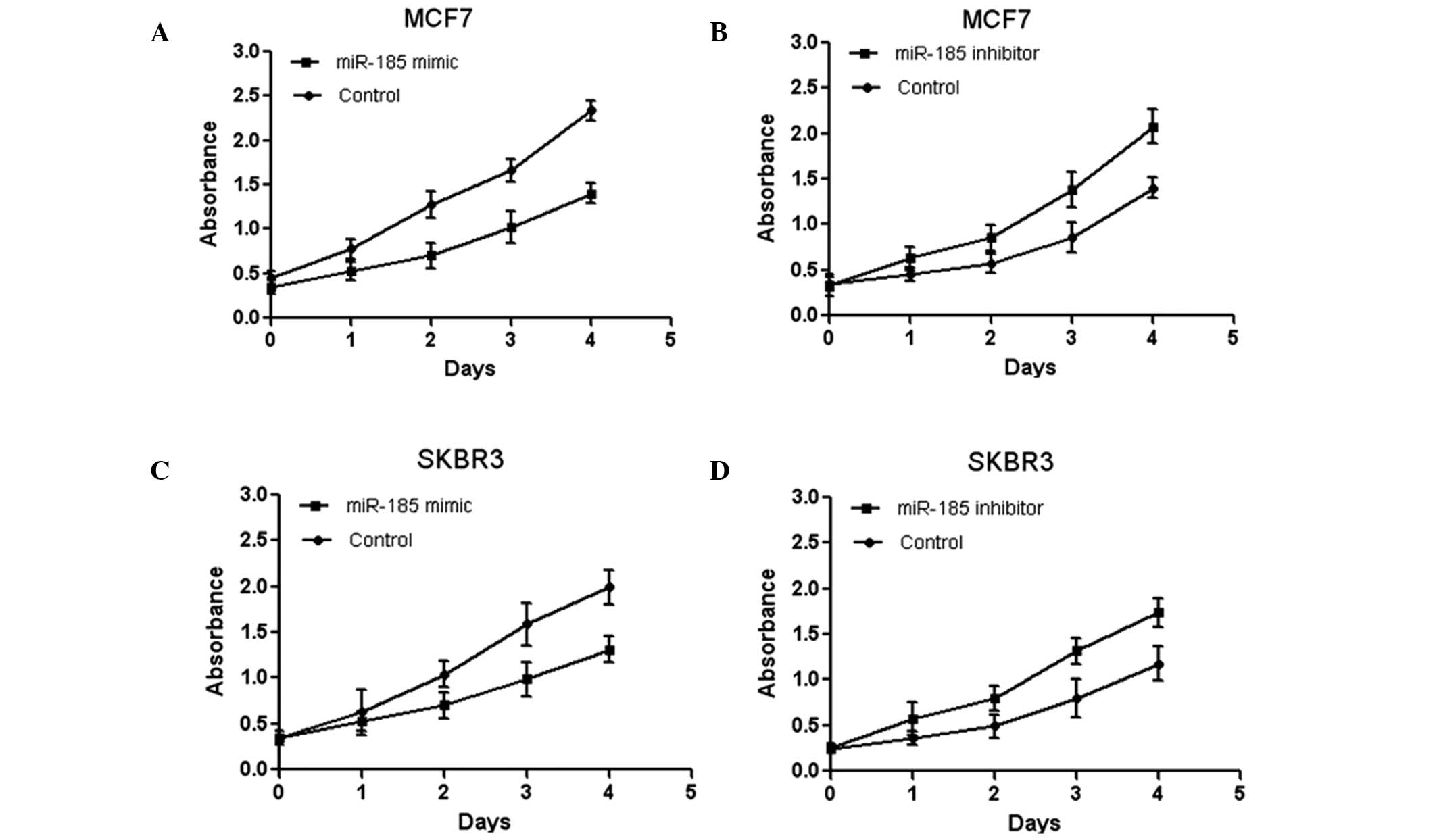
Effects of miR-185 on breast cancer cell
proliferation
To investigate the effect of miR-185 on breast
cancer cell proliferation, miR-185 mimics was transfected into the
human breast cancer cell lines, MCF7 and SKBR3, and the
proliferation was assessed by an MTT assay. The data indicated that
overexpression of miR-185 significantly inhibited MCF7 cell
proliferation (Fig. 2A). In
addition, the MCF7 cells were transfected with an miR-185 inhibitor
and were found to exhibit increased proliferation, as demonstrated
by an MTT assay (Fig. 2B). Similar
results were observed for the SKBR3 cells (Fig. 2C and D). Collectively, these
results demonstrated that miR-185 inhibited breast cancer cell
proliferation in vitro.
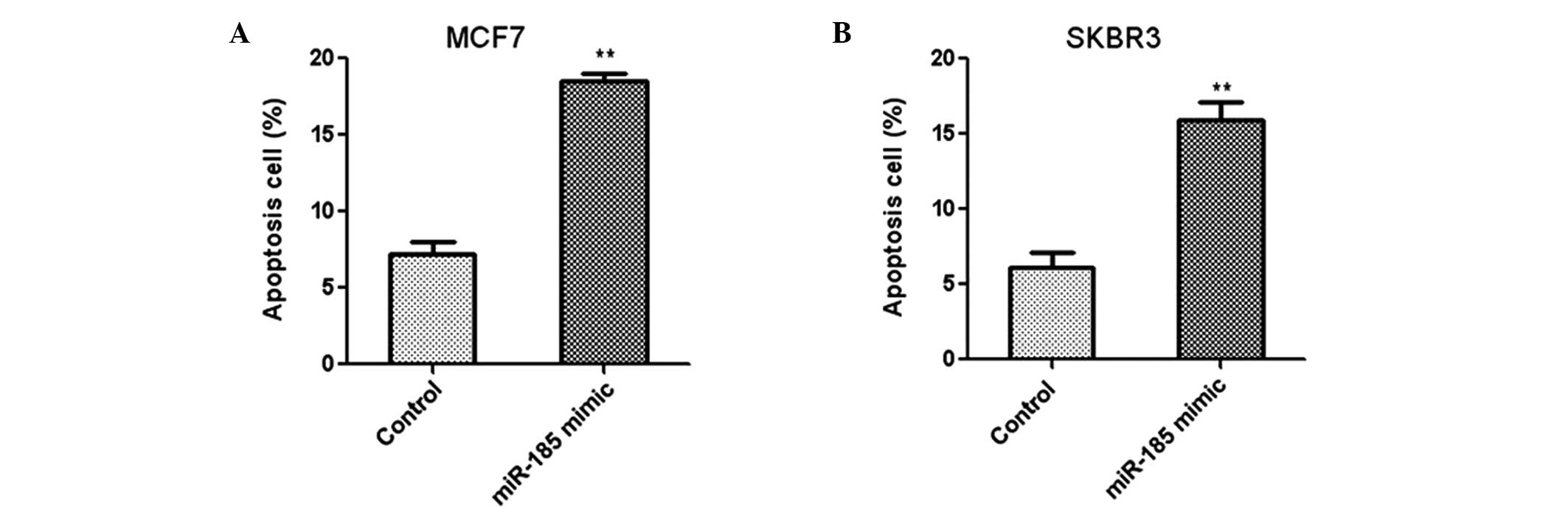
Overexpression of miR-185 promotes breast
cancer cell apoptosis
The effect of miR-185 on the apoptosis of human
breast cancer cells was investigated using flow cytometry. Two
breast cancer cells lines, MCF7 and SKBR3, were transfected with
miR-185 mimics and the apoptosis rate was analyzed using annexin
V/propidium iodide staining. The results indicated that
overexpression of miR-185 led to a significant increase in the
apoptosis rates of MCF7 (Fig. 3A)
and SKBR3 (Fig. 3B) cells.
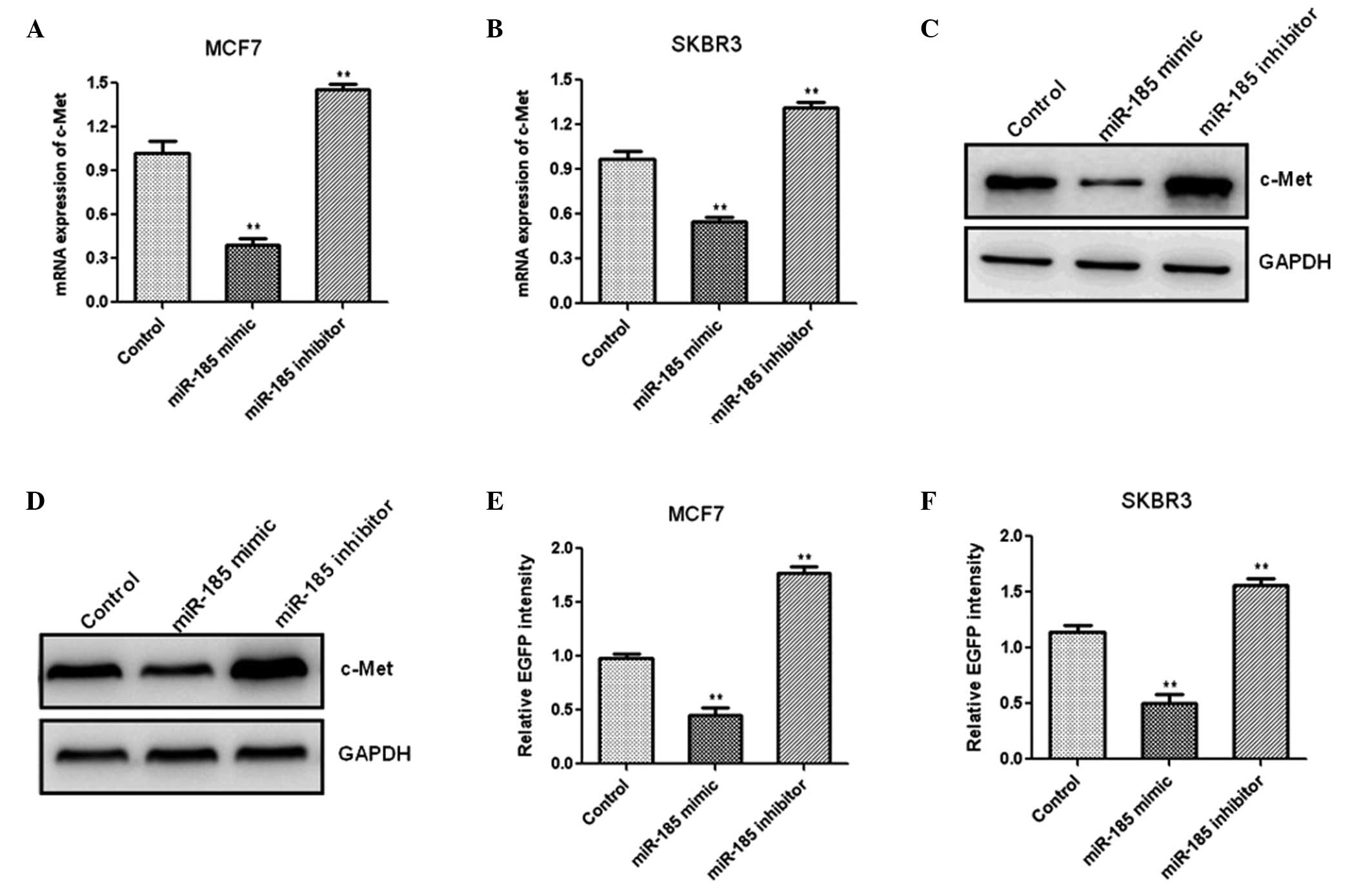
c-Met is a target of miR-185 in breast
cancer cells
To investigate the mechanism by which miR-185
inhibits cell proliferation in breast cancer tissues, putative
miR-185 targets were analyzed using the miRanda, TargetScan and
PicTar software. The 3′-UTR of c-Met, containing the putative
miR-185 binding sites, was identified. To determine whether miR-185
targeted c-Met in vitro, the MCF7 cells were transfected
with miR-185 mimics or an inhibitor, and the mRNA expression of
c-Met was detected using qPCR. Overexpression of miR-185
significantly decreased the mRNA expression of c-Met when compared
with the controls, whereas inhibition of miR-185 resulted in an
increase in c-Met mRNA expression in MCF7 (Fig. 4A) and SKBR3 (Fig. 4B) cells. Western blotting
demonstrated that overexpression of miR-185 resulted in an evident
decrease in c-Met protein expression, while a reduction in miR-185
markedly increased the protein expression of c-Met in the MCF7
(Fig. 4C) and SKBR3 cells
(Fig. 4D). These results indicated
that miR-185 regulated the mRNA and protein expression levels of
c-Met. Fluorescent reporter assays were performed to determine
whether c-Met was a direct target of miR-185. The 3′-UTR of c-Met
with the predicted binding site for miR-185 was cloned into a
fluorescent reporter vector. Upregulation of miR-185 expression
reduced the intensity of EGFP in the cells transfected with a
vector containing the c-Met 3′-UTR when compared with the control
groups, whereas in the miR-185 inhibitor group, the intensity of
EGFP in the MCF7 and SKBR3 cells increased significantly (Fig. 4E and F). These results indicated
that miR-185 binds to the 3′-UTR of c-Met directly.
Discussion
miRNAs are a group of small non-coding RNAs that
modulate the expression of genes by targeting mRNAs for
translational repression (13).
Thus, the key to understanding the function of miRNA is the
elucidation of functional targets, which usually involves analyzing
changes in the target proteins following a gain or loss of function
in the specific miRNA. In the present study, for the first time,
miR-185 was demonstrated to inhibit the proliferation of breast
cancer cells by regulating the expression of c-Met.
Aberrant expression of miRNAs plays a critical role
in cell proliferation, apoptosis and cell cycle arrest in various
cancer types (14,15). A recent study demonstrated that
miR-155 promoted the proliferation of human breast cancer MCF-7
cells through targeting tumor protein 53-induced nuclear protein 1;
thus, provided a new therapeutic strategy for breast cancer
(16). An additional study
revealed that miR-24 regulated cell proliferation and DNA repair
directly. The study hypothesized that enhancing miR-24 function in
cancer cells by introducing miR-24 mimics may be an attractive
therapeutic method, as miR-24 may potentially block dysregulated
cell proliferation and sensitize cancer cells to DNA damage from
chemotherapy and radiotherapy (17). In the present study, the MTT assay
revealed that overexpression of miR-185 significantly inhibited the
proliferation of MCF7 and SKBR3 cells. In addition, human breast
cancer cells transfected with an miR-185 inhibitor exhibited
increased proliferation, indicating that miR-185 inhibited the
proliferation of breast cancer cells in vitro.
Apoptosis is the process of programed cell death,
which is associated with cell growth and maintaining cellular
homeostasis (18). Increasing
evidence has shown that miRNAs play a critical role in cell
proliferation, differentiation and apoptosis (14). A recent study revealed that
downregulation of miR-155 induced cell apoptosis by targeting a
number of antiapoptotic factors and causing cell cycle arrest
(19). Furthermore, a previous
study demonstrated that miR-185 targeted the expression of RhoA and
Cdc42, and inhibited the proliferation potential of human
colorectal cells (20). The
results of the present study demonstrated that overexpression of
miR-185 promoted the apoptosis of MCF7 and SKBR3 cells.
miRNAs control cellular biological functions by
targeting the expression of genes; therefore, the elucidation of
functional targeted genes is crucial. Studies on signal
transduction pathways have generated various promising molecular
targets for therapeutic inhibition in cancer therapy (13). Receptor tyrosine kinases represent
an important class of such therapeutic targets. c-Met is a receptor
tyrosine kinase that has been shown to be overexpressed in a
variety of malignancies (21,22).
Increased c-Met signaling promotes cell migration and invasion
through several pathways, including the extracellular
signal-regulated kinase, phosphatidyl inositol 3-kinase and focal
adhesion kinase pathways (23,24).
Consistent with observations of previous studies, the present study
demonstrated that the expression level of c-Met increased
significantly in breast cancer samples. In addition, transfection
with miR-185 mimics resulted in decreased luciferase activity and
c-Met expression in breast cancer cells, indicating that c-Met is
the target gene of miR-185.
In conclusion, miR-185 was found to be significantly
downregulated in breast cancer tissues. Moreover, miR-185 was
demonstrated to inhibit the proliferation of breast cancer cells by
regulating the expression of c-Met, which indicates the therapeutic
potential of miR-185 in breast cancer treatment.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
2
|
Serrano MJ, Rovira PS, Martinez-Zubiaurre
I, et al: Dynamics of circulating tumor cells in early breast
cancer under neoadjuvant therapy. Exp Ther Med. 4:43–48.
2012.PubMed/NCBI
|
|
3
|
Yamaguchi M, Kwong YL, Kim WS, et al:
Phase II study of SMILE chemotherapy for newly diagnosed stage IV,
relapsed, or refractory extranodal natural killer (NK)/T-cell
lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin
Oncol. 29:4410–4416. 2011. View Article : Google Scholar
|
|
4
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTG). Peto R, Davies C, Godwin J, et al:
Comparisons between different polychemotherapy regimens for early
breast cancer: meta-analyses of long-term outcome among 100,000
women in 123 randomised trials. Lancet. 379:432–444. 2012.
View Article : Google Scholar
|
|
5
|
Fang YX and Gao WQ: Roles of microRNAs
during prostatic tumorigenesis and tumor progression. Oncogene.
33:135–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nazarov PV, Reinsbach SE, Muller A, et al:
Interplay of microRNAs, transcription factors and target genes:
linking dynamic expression changes to function. Nucleic Acids Res.
41:2817–2831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Kouwenhove M, Kedde M and Agami R:
MicroRNA regulation by RNA-binding proteins and its implications
for cancer. Nat Rev Cancer. 11:644–656. 2011.PubMed/NCBI
|
|
8
|
Marcucci G, Maharry KS, Metzeler KH, et
al: Clinical role of microRNAs in cytogenetically normal acute
myeloid leukemia: miR-155 upregulation independently identifies
high-risk patients. J Clin Oncol. 31:2086–2093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Voorhoeve PM: MicroRNAs: Oncogenes, tumor
suppressors or master regulators of cancer heterogeneity? Biochim
Biophys Acta. 1805:72–86. 2010.PubMed/NCBI
|
|
11
|
Xiang Y, Ma N, Wang D, et al: MiR-152 and
miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity
by targeting DNMT1 directly: a novel epigenetic therapy independent
of decitabine. Oncogene. 33:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Östling P, Leivonen SK, Aakula A, et al:
Systematic analysis of microRNAs targeting the androgen receptor in
prostate cancer cells. Cancer Res. 71:1956–1967. 2011.PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cimmino A, Calin GA, Fabbri M, et al:
miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou M, Liu Z, Zhao Y, et al:
MicroRNA-125b confers the resistance of breast cancer cells to
paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist
killer 1 (Bak1) expression. J Biol Chem. 285:21496–21507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang CM, Zhao J and Deng HY: MiR-155
promotes proliferation of human breast cancer MCF-7 cells through
targeting tumor protein 53-induced nuclear protein 1. J Biomed Sci.
20:792013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lal A, Navarro F, Maher CA, et al: miR-24
Inhibits cell proliferation by targeting E2F2, MYC, and other
cell-cycle genes via binding to ‘seedless’ 3′UTR microRNA
recognition elements. Mol Cell. 35:610–625. 2009.PubMed/NCBI
|
|
18
|
Powell-Coffman JA and Coffman CR:
Apoptosis: Lack of oxygen aids cell survival. Nature. 465:554–555.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Higgs G and Slack F: The multiple roles of
microRNA-155 in oncogenesis. J Clin Bioinforma. 3:172013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M, Lang N, Chen X, et al: miR-185
targets RhoA and Cdc42 expression and inhibits the proliferation
potential of human colorectal cells. Cancer Lett. 301:151–160.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maroun CR and Rowlands T: The Met receptor
tyrosine kinase: a key player in oncogenesis and drug resistance.
Pharmacol Ther. 142:316–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Wu JJ, Hynes M, et al: c-Met is a
marker of pancreatic cancer stem cells and therapeutic target.
Gastroenterology. 141:2218–2227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Mendoza MC, Pei X, et al:
Down-regulation of CMTM8 induces epithelial-to-mesenchymal
transition-like changes via c-MET/extracellular signal-regulated
kinase (ERK) signaling. J Biol Chem. 287:11850–11858. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang MK, Zhou HY, Yam JW and Wong AS:
c-Met overexpression contributes to the acquired apoptotic
resistance of nonadherent ovarian cancer cells through a cross talk
mediated by phosphatidylinositol 3-kinase and extracellular
signal-regulated kinase 1/2. Neoplasia. 12:128–138. 2010.
|