Introduction
Mitochondrial dysfunction plays a major role in the
process of aging and in aging-related neurodegenerative disorders.
This is due to the crucial role of mitochondria in producing
adenosine triphosphate (ATP), the main source of cellular energy
(1–4). Furthermore, mitochondria are the
target organelles for reactive oxygen species (ROS), which are a
major source of physiologically produced oxidative stress during
aging (3). A number of studies
have demonstrated that mitochondrial dysfunction is closely
associated with several aging-related diseases, including
Alzheimer’s disease, Huntington’s disease (HD) and Parkinson’s
disease (PD) (5–7). It has been confirmed that
mitochondrial protection and the consequent reduction of oxidative
stress are important targets for the prevention and treatment of
the early stages of these aging-related diseases (8,9).
D-galactose (D-gal) is a natural reducing sugar in the body that is
normally metabolized by D-galactokinase and galactose-1-phosphate
uridyltransferase in animals. An excess of D-gal results in
abnormal metabolism (10). The
progressive deterioration in learning and memory skills, as well as
the production of ROS in the brain tissue of rodents, has been
previously reported in the literature (11). It has been shown that the
administration of D-gal for 6–10 weeks induces mimetic aging
changes in the brain tissue of rats. This has been utilized to
establish animal models in studies investigating potential
therapies and prevention strategies for certain age-associated
diseases (12–15).
In recent years, the most frequently used
antioxidant food supplements have included certain lactic acid
bacteria (LAB) and medicinal plants. It has been revealed that
several dietary supplements, including spinach and citrus fruits
extracts, may be beneficial in protecting against age-related
neurological disorders (16,17).
A number of LAB strains have the ability to scavenge free radicals,
improve the activity of antioxidant enzymes and inhibit lipid
oxidation. Hathout et al (18) demonstrated that treatments with
Lactobacillus casei or Lactobacillus reuteri
protected rats fed an aflatoxin-contaminated diet from oxidative
stress. Bay et al (19)
reported that a skimmed-milk culture of LAB reduced lipid
peroxidation in rat livers and brains. The abnormal expression of
γ-aminobutyric acid (GABA), an important neurotransmitter in the
brain, is implicated in the pathogenesis of anxiety and depression
(20). A previous study showed
that chronic administration of D-gal markedly decreased the number
of GABA-immunoreactive neurons in the cortical layers of rats with
D-gal-induced aging, which further contributed to their behavioral
deficits (21). This is one of the
suggested mechanisms by which LAB regulates brain function
(22). In a previous study, the
LAB strain Lactobacillus plantarum NDC 75017 produced high
levels of GABA and exhibited anti-inflammatory,
cholesterol-lowering and antioxidant properties (23–25).
In the present study, the potential protective effect of L.
plantarum NDC 75017 was investigated through the establishment
of a D-gal-induced aging model in rats. The behavioral changes were
examined and the ATP levels, mitochondrial function and
mitochondrial ultrastructural changes in the cerebral cortical
neurons of the rats were examined to further investigate the
potential mechanism underlying the neuroprotective effect of L.
plantarum NDC 75017.
Materials and methods
Materials
D-gal, rhodamine 123 (Rh123), rotenone and
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Bicinchoninic
acid protein and ATP assay kits were purchased from Wuhan Boster
Bioengineering Co. Ltd. (Wuhan, China) and Beyotime Institute of
Biotechnology (Beijing, China), respectively. All other chemicals
used were of the highest quality that is commercially available.
The JSM25610LV transmission electron microscope used in the study
was produced by Japan Electron Optics Laboratory Co., Ltd. (Tokyo,
Japan).
Lactobacillus strain and growth
conditions
The L. plantarum NDC 75017 were isolated from
a traditional Chinese fermented yogurt (from the Tongliao range of
Inner Mongolia, China). The bacteria were anaerobically grown at
30°C overnight in de Man-Rogosa-Sharpe broth (Difco™,
Beckman-Coulter, Miami, FL, USA). The bacterial cells were
collected by centrifugation at 8,000 × g for 5 min, washed three
times with phosphate-buffered saline and adjusted to
1×108, 1×109 and 1×1010 CFU/ml for
oral administration to the rats.
Animals and experimental design
A total of 50 male Wistar rats (weighing 180–200 g)
were obtained from the Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China). The rats were housed in separate cages
and had free access to food and water for ≥1 week to acclimate
prior to the initiation of treatment. The animals were housed in a
limited-access animal facility where the room temperature and
relative humidity were set to 22±2°C and 55±10%, respectively.
Artificial lighting provided a 24-h cycle of 12-h light/12-h dark
(light from 7:00 a.m. to 7:00 p.m.). All of the animal experiments
were approved by the Animal Care and Use Committee of Heilongjiang
Province, China. After the one-week acclimation period, the rats
were randomly divided into five groups, with 10 rats in each group.
The rats were orally administered 1 ml/100 g body weight of
different concentrations (CFU/ml) of L. plantarum once per
day for 49 days (seven weeks). The rats in the control and aging
model groups were only administered a vehicle (0.9% saline) or
D-gal, respectively. The treatments were as follows: Group I, 0.9%
normal saline (control group); Group II, D-gal (100 mg/kg)
subcutaneously (D-gal group); Group III, low-dose L.
plantarum [1×108 CFU/100 mg, per oral (p.o.)] plus
D-gal (100 mg/kg) (L + D-gal group); Group IV, medium-dose L.
plantarum (1×109 CFU/100 mg, p.o.) plus D-gal (100
mg/kg) (M + D-gal group); Group V, high-dose L. plantarum
(1×1010 CFU/100 mg, p.o.) plus D-gal (100 mg/kg) (H +
D-gal group).
Water maze test
Spatial learning was investigated after the seven
weeks of D-gal injection using the Morris water escape task
according to a previous study (26). From the 44th day, the rats were
trained for four days until the 49th day, when the time taken to
climb onto the platform (escape latency) was recorded for each
rat.
Observation of mitochondrial
ultrastructure
After seven weeks of treatment, the mitochondrial
ultrastructure of the rat cerebral cortices was observed using a
transmission electron microscope as previously described (27). A total of 15 rats (n=3 from each
group) were sacrificed through an intraperitoneal injection of an
overdose of sodium pentobarbital (80 mg/kg). The cerebral cortices
were isolated, fixed and perfused with 2.5% glutaraldehyde. The
cortices were subsequently stored overnight at 4°C. Following
post-fixation in 2% osmium tetroxide for 2 h at 4°C, the tissues
were dehydrated in an ascending graded ethanol and acetone series
and immersed in an acetone/Epon 812 mixture at ratios of 1:1, 1:2
and 1:3 for 0.5, 2 and 10 h, respectively. Ultra thin sections (70
nm) were prepared, counterstained with uranyl acetate and lead
citrate and examined using the JSM25610LV transmission electron
microscope.
Isolation and purification of
mitochondria
Mitochondria were isolated from the cerebral
cortices of the rats through homogenization and differential
centrifugation according to the methods performed in a previous
study (28). The protein content
of the isolated mitochondria samples was determined using the
Bradford protein assay. Bovine serum albumin was used to construct
a standard curve.
Determination of ATP content
The levels of ATP in the mitochondria were measured
using the ATP Bioluminescence Assay kit (Beyotime Institute of
Biotechnology) according to the manufacturers’ instructions.
Briefly, the levels of ATP were determined by mixing 50 μl
mitochondrial solution with 50 μl luciferase solution, which
catalyzes ATP-mediated light production from luciferin. The amount
of emitted light, measured using a microplate luminometer (Promega,
Madison, WI, USA), was linearly associated with the ATP
concentration.
Measurement of the mitochondrial
permeability transition (MPT)
The MPT value was determined using an ultraviolet
spectrophotometer to measure the absorbance at 540 nm (A540
nm), as previously described (29). Briefly, the isolated mitochondria
were diluted to 0.5 mg/ml and incubated in the assay buffer (125 mm
sucrose, 65 mm KCl, 5 mm succinate, 5 mm rotenone and 10 mm
Tris-HCl; pH 7.4). MPT was initiated and monitored prior to and
following the addition of 50 μM calcium chloride for 5 min. The
results were expressed as the decrease in the absorbance at 540
nm.
Detection of mitochondrial membrane
potential (Δψm)
Δψm was detected according to the methods
of a previous study (29), with
modifications. Briefly, fluorescence (excitation at 503 nm and
emission at 527 nm) occurring in the reaction buffer (250 mm
sucrose, 2 mm HEPES, 0.5 mm KH2PO4, 4.2 mm
sodium succinate at pH 7.4 and 0.3 mm Rh123) was measured using an
F-4500FL spectrophotometer (Hitachi High-Technologies Co., Tokyo,
Japan). The diluted mitochondria (0.5 mg/ml) were added to the
buffer and incubated for 3 min. The fluorescence was measured again
using the F-4500FL spectrophotometer. Finally, the change in
Δψm was expressed by the decrease in fluorescence.
Activities of the mitochondrial
respiratory chain
MTT reduction was used to assess the activities of
the mitochondrial respiratory chain. The methods and procedures
utilized in the present study were identical to those of a previous
study (28). Briefly, 0.02 ml MTT
(0.1 mg/ml) was added to the mitochondrial solution containing 60
μg protein. The reaction mixture was co-incubated at 37°C for 30
min and centrifuged at 1,000 g for 5 min at room temperature. The
obtained pellet was dissolved in 1 ml of acidic isopropanol and
re-centrifuged at 1,000 g for 5 min at room temperature to obtain
the supernatant. The absorbance was measured at 595 nm and the
results were presented as A595 nm/mg protein.
Statistical analysis
Data are presented as the mean ± standard deviation.
The data were analyzed using one-way analysis of variance followed
by least significant difference post hoc tests to compare the
different treatment groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
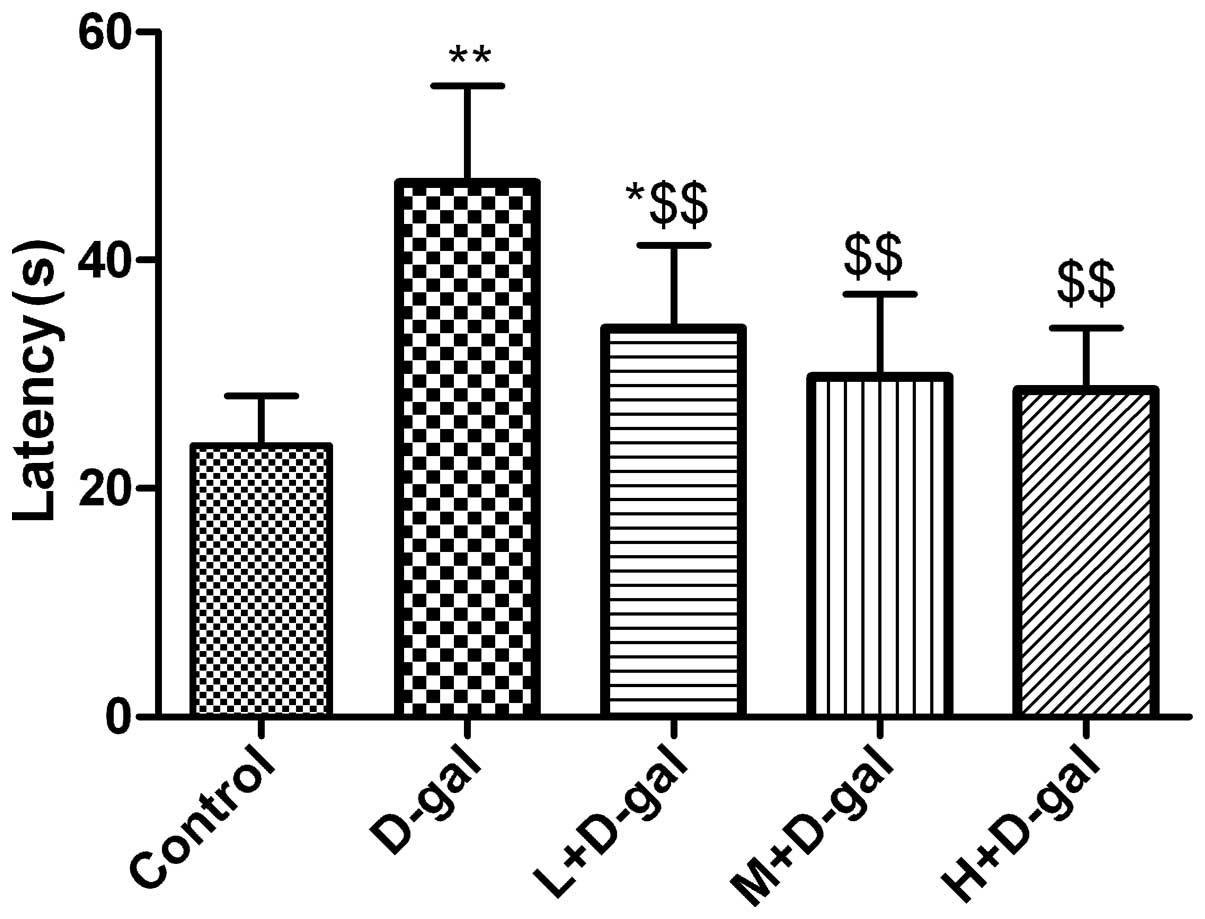
Effect of L. plantarum NDC 75017 on the
spatial learning of aging rats induced by D-gal
From the 44th day, the rats were trained for four
days, following which the water maze test was carried out. The
escape times of the rats in D-gal group were significantly higher
(P<0.01) compared with those of the control group rats. However,
the escapes times of rats in the L + D-gal, M + D-gal and H + D-gal
groups were significantly lower (P<0.01) compared with those of
rats in the D-gal group (Fig.
1).
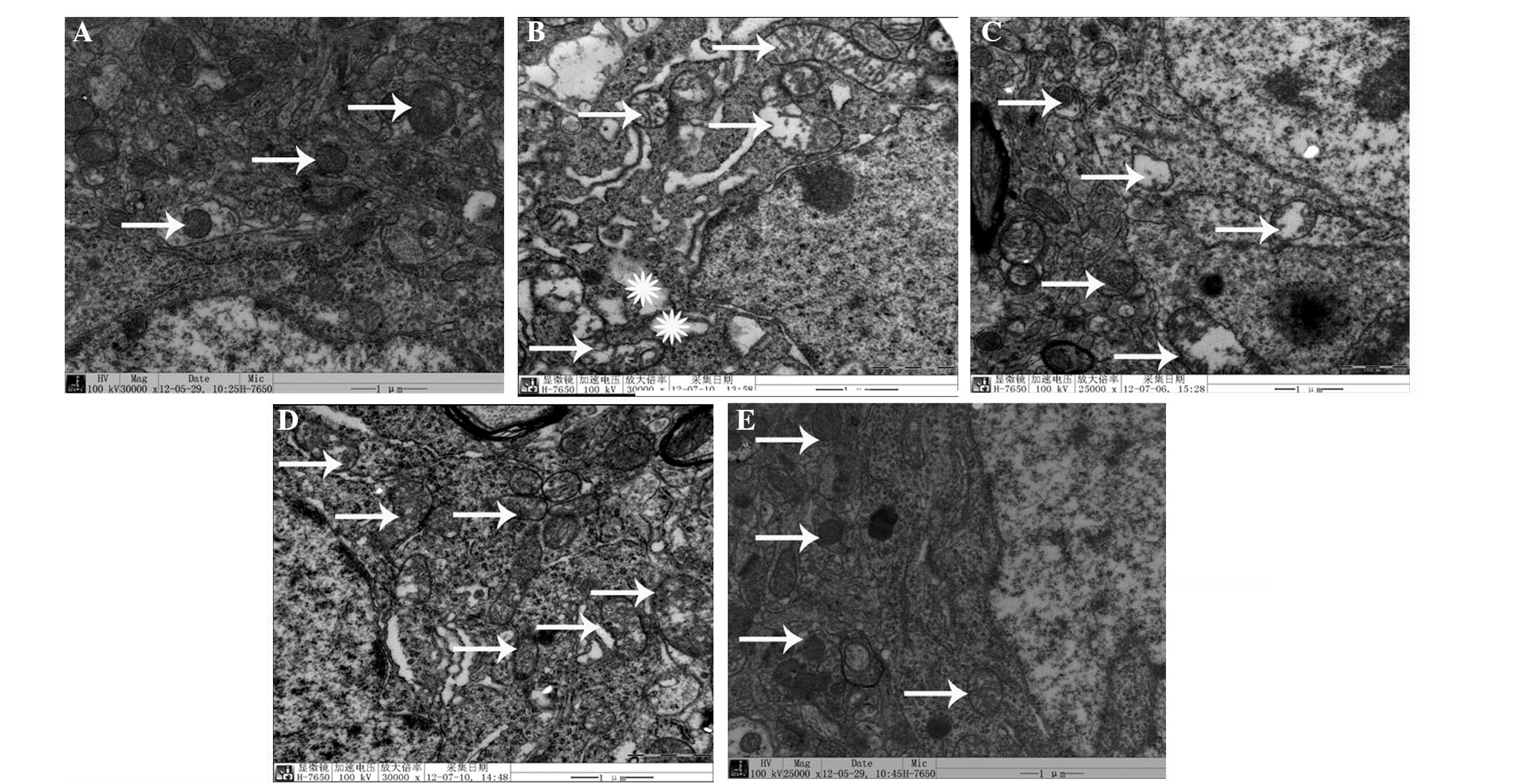
D-gal-induced ultrastructural changes in
neuronal mitochondria and the effect of L. plantarum NDC 75017
Ultrastructural changes in the mitochondria of the
cerebral cortical neurons were observed and the results are shown
in Fig. 2. After seven weeks of
D-gal treatment, marked pathological changes were observed in the
mitochondria of the cerebral cortical neurons of the D-gal-induced
aging group compared with the control group, including rupture and
scarcity of the cristae and vacuolization (Fig. 2B). Coadministration of L.
plantarum NDC 75017 and D-gal decreased the neuronal
mitochondria injury in rats with dose-dependent effects (Fig. 2C–E).
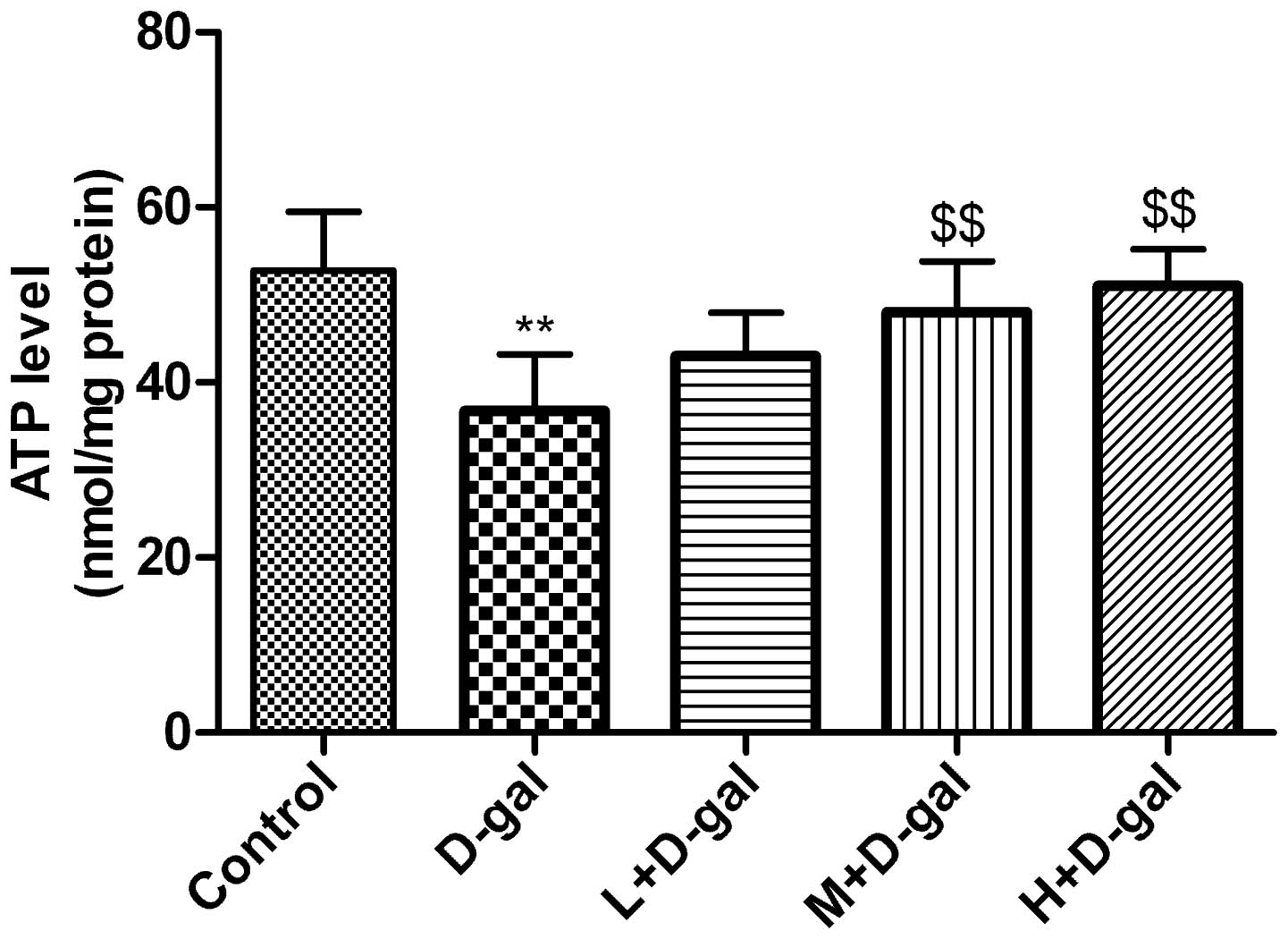
Effect of L. plantarum NDC 75017 on the
D-gal-induced changes in ATP content
The levels of ATP in the mitochondria in the
cerebral cortical neurons of the rats were determined. The results
are shown in Fig. 3. Compared with
the control group, the levels of ATP in the D-gal group were
significantly lower (P<0.01). However, significant increases in
the levels of ATP were observed in the M and H + D-gal groups
(P<0.01) compared with the D-gal group.
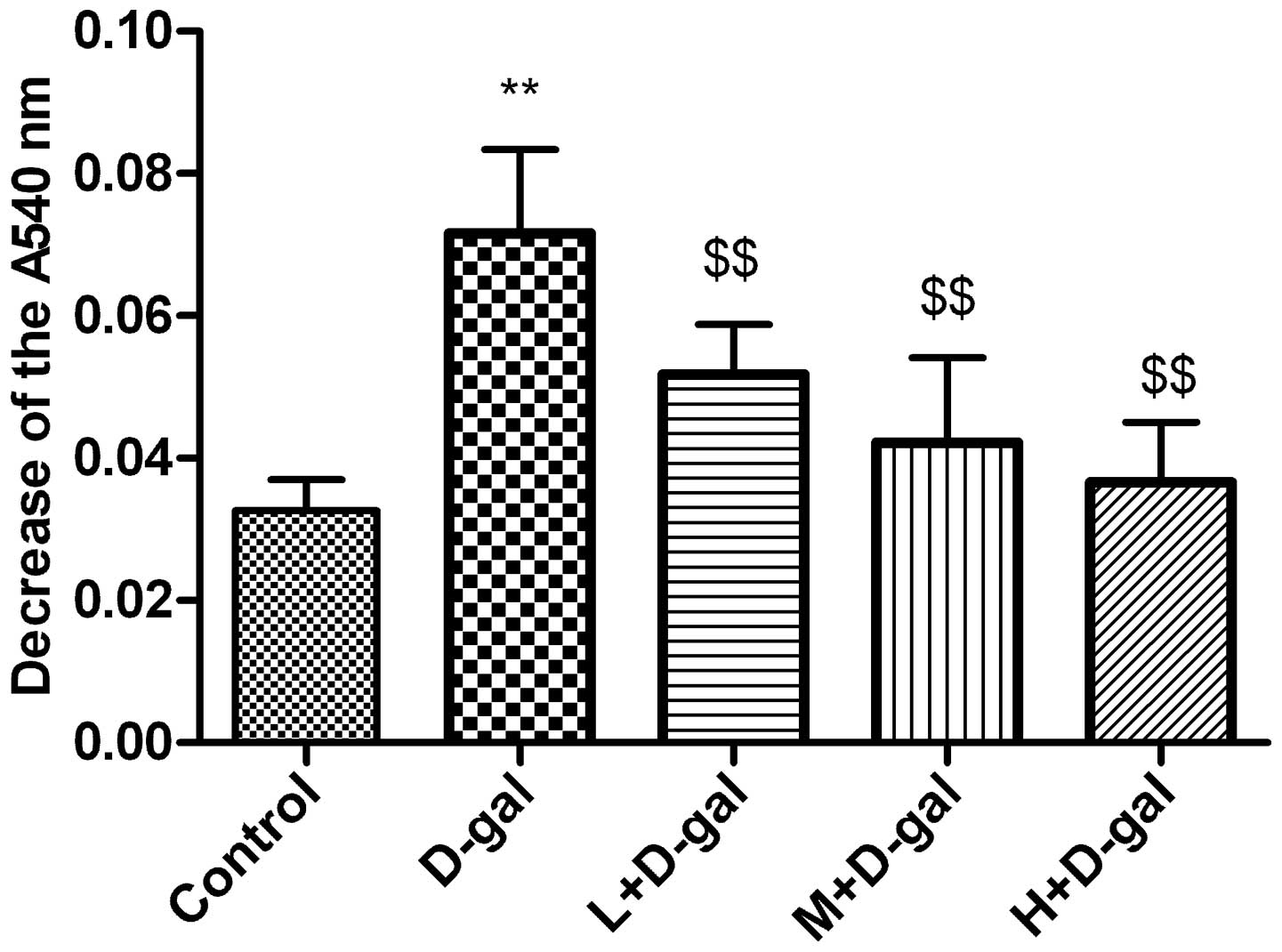
Effect of L. plantarum NDC 75017 on
Ca2+-induced changes in MPT
Ca2+-induced changes in MPT were assessed
and the results are shown in Fig.
4. The MPT in the mitochondria of rat cerebral cortical neurons
was significantly higher (P<0.01) in the D-gal group compared
with that in the control group. However, the MPT in the L, M and H
+ D-Gal groups was significantly lower than that in the D-gal
group, with decreases of 27.7, 41.1, and 48.9%, respectively,
relative to the D-gal model group (all P<0.01).
Effect of L. plantarum NDC 75017 on
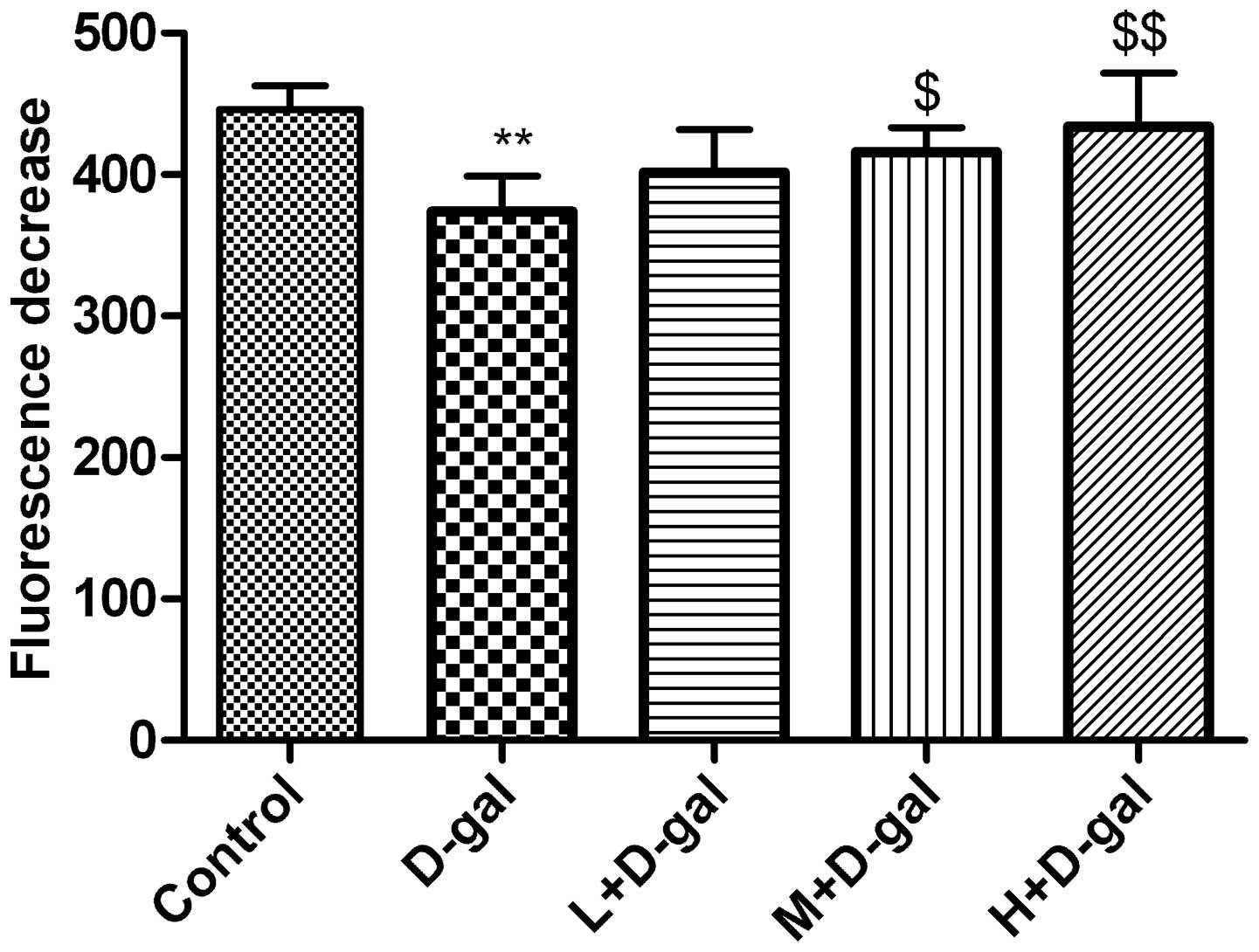
D-gal-induced changes in Δψm
Δψm was determined by monitoring the
dynamic fluorescence quenching of Rh123. The initial fluorescence
(714±36) was markedly reduced following the addition of
mitochondria. As shown in Fig. 5,
mitochondria of the rats in the control group quenched the
fluorescence to 446±17, and the extent of quenching exhibited by
mitochondria in the D-gal group was significantly lower (374±25)
(P<0.01). In the M and H + D-gal groups, fluorescence quenching
was significantly increased to 416±17 and 434±38, respectively
(P<0.05 and P<0.01, respectively) compared with the D-gal
group (Fig. 5).
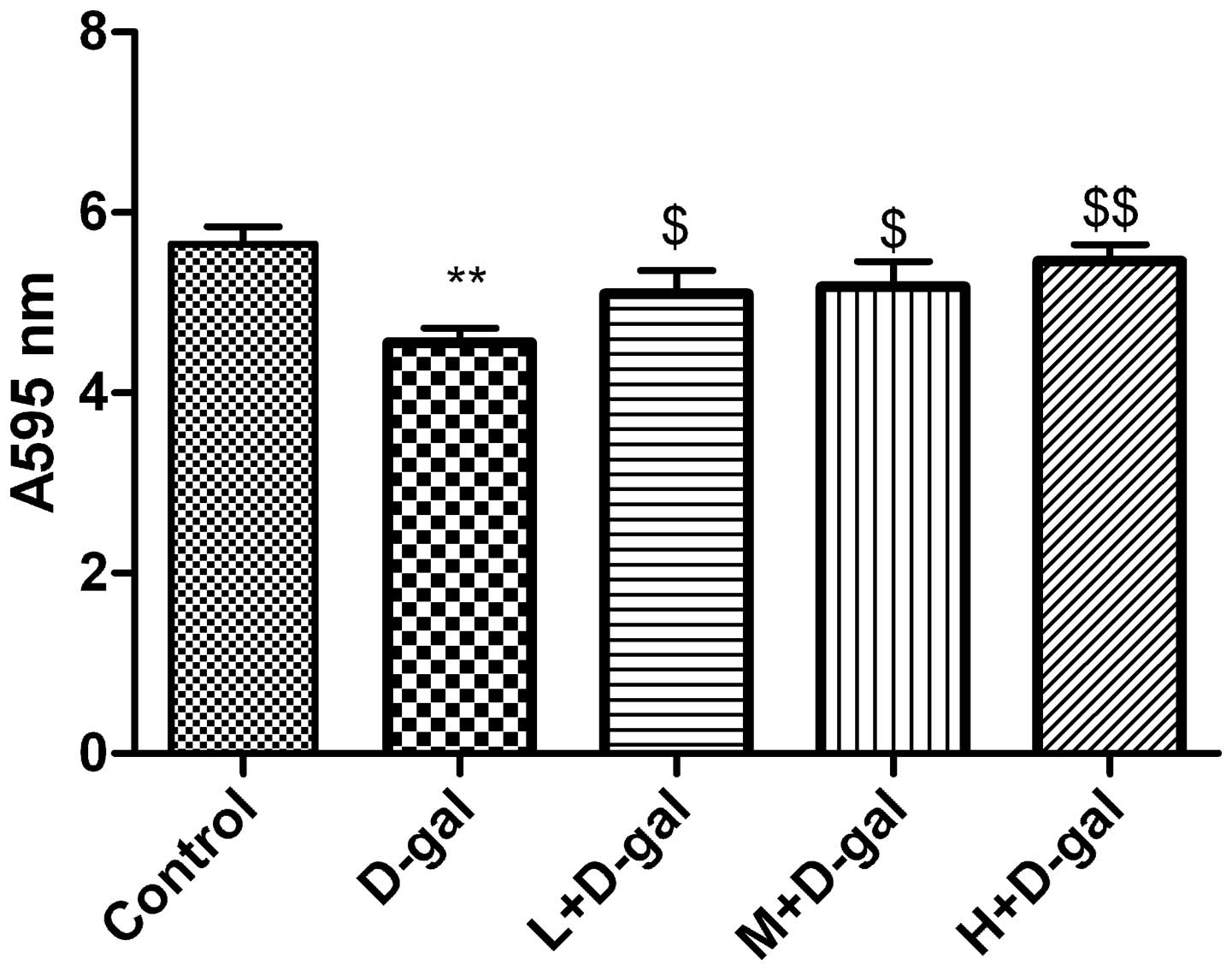
Effect of L. plantarum NDC 75017 on the
D-gal-induced changes in the activity of the mitochondrial
respiratory chain
The reduction of the water-soluble tetrazolium salt
MTT to formazan is regarded as an indicator of mitochondrial
respiration, particularly the activity of mitochondrial succinate
dehydrogenase. Compared with the control group, mitochondrial
enzymatic activity was significantly lower (P<0.01) in the D-gal
group, and progressive improvements were observed in the L, M and H
+ D-gal groups (Fig. 6).
Discussion
Several theories have been proposed to explain
age-related mitochondrial dysfunction. The oxidative stress theory
is the most important theory of aging proposed in past few decades
(30). The targeted accumulation
of ROS damages the mitochondria, which are more sensitive and
vulnerable to oxidative stress than other organelles in cells due
to their structural and functional characteristics (6). The decrease in mitochondrial function
and the increase in mitochondrial DNA damage suggests that the
progressive accumulation of oxidative DNA damage is a contributing
factor to cell apoptosis or necrosis, with the generation of more
ROS during aging (10,20,28,29).
Chronic administration of D-gal has been widely used to mimic the
process of brain aging. The D-gal-induced model of brain aging is
important in the development of suitable anti-aging drug strategies
(31,32). A number of studies have revealed
that low doses of D-gal (such as 50 or 100 mg/kg) decrease the
learning and memory abilities of mice and rats, as demonstrated
through the T-maze, Y-maze and Morris water maze tests (26,33).
In the present study, rats administered 100 mg/kg D-gal for seven
weeks exhibited a significant decrease in learning and memory
ability, as confirmed through their performance in the Morris water
maze test.
At present, mitochondrial Ca2+
homeostasis is the center of widespread interest in scientific
studies due to its modulatory role in numerous physiological
processes and its involvement in cell death (6,28,29).
Ca2+ uptake and release from the mitochondrial membrane
via a variety of mechanisms control the local regulation of
intracellular Ca2+ concentration. The mitochondrial
Ca2+ dysfunction can cause MPT pore opening, leading to
a change in the Δψm and resulting in mitochondrial
swelling and dysfunction, and even cell death (28). It has been demonstrated that
injecting rodents with D-gal for 6–10 weeks induces aging,
affecting mitochondrial bioenergetics. This leads to the activity
of the electron transport chain complex becoming compromised and a
decrease in the rate of ATP synthesis (6,28).
The results of the present study indicated that chronic
administration of D-gal impaired the activity of the mitochondrial
enzyme complex, Δψm, mitochondrial membrane permeability
and ATP production ability. These changes were alleviated with the
administration of L. plantarum NDC 75017, which may be
associated with its antioxidative properties.
GABA supplementation may activate the upstream
signal survival pathways that regulate mitochondrial function, such
as the phosphoinositide 3-kinase-Akt signal survival pathway
(34). GABA also plays an
important role in the aging process and diseases including PD and
HD (22,34,35).
GABA increases the circulation of serum lipids and decreases
mitochondrial injury mediated by oxidative stress (21,36).
Certain LABs have been demonstrated to directly regulate GABA in
the microbiome-gut-brain axis in mice (22). L. plantarum NDC 75017 has
the ability to produce high levels of GABA in vitro, which
may contribute to its anti-aging and D-gal-induced mitochondrial
dysfunction-alleviating abilities. The precise mechanisms for this
should be investigated in further studies.
In conclusion, the results of the present study
revealed that L. plantarum NDC 75017 was able to alleviate
learning and memory-associated injuries in aging rats by reducing
mitochondrial dysfunction induced by D-gal. This may be associated
with its antioxidant and GABA-producing activities.
Acknowledgements
This study was supported by grants from the National
Science and Technology Project (no. 2011AA100902), the National
Natural Science Foundation of China (no. 31171718), the China
Postdoctoral Science Foundation (no. 2012M510911), the Science and
Technology Project for the Universities of Shandong Province (no.
J13LE55) and the Characteristic Course of Adult Education in
Shandong Province (no. 20131206).
References
|
1
|
Miyoshi N, Oubrahim H, Chock PB and
Stadtman ER: Age-dependent cell death and the role of ATP in
hydrogen peroxide-induced apoptosis and necrosis. Proc Natl Acad
Sci USA. 103:1727–1731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moreira PI, Santos MS and Oliveira CR:
Alzheimer’s disease: a lesson from mitochondrial dysfunction.
Antioxid Redox Signal. 9:1621–1630. 2007.
|
|
3
|
Morais VA and De Strooper B: Mitochondria
dysfunction and neurodegenerative disorders: cause or consequence.
J Alzheimers Dis. 20:S255–S263. 2010.PubMed/NCBI
|
|
4
|
Reddy PH: Mitochondrial dysfunction in
aging and Alzheimer’s disease: strategies to protect neurons.
Antioxid Redox Signal. 9:1647–1658. 2007.
|
|
5
|
Martin LJ: Biology of mitochondria in
neurodegenerative diseases. Prog Mol Biol Transl Sci. 107:355–415.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coskun PE and Busciglio J: Oxidative
stress and mitochondrial dysfunction in Down’s syndrome: relevance
to aging and dementia. Curr Gerontol Geriatr Res.
2012:3831702012.
|
|
7
|
Bishop NA, Lu T and Yankner BA: Neural
mechanisms of ageing and cognitive decline. Nature. 464:529–535.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552:335–344. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Floyd RA and Hensley K: Oxidative stress
in brain aging. Implications for therapeutics of neurodegenerative
diseases. Neurobiol Aging. 23:795–807. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang XL, An LJ, Bao YM, Wang JY and Jiang
B: D-galactose administration induces memory loss and energy
metabolism disturbance in mice: protective effects of catalpol.
Food Chem Toxicol. 46:2888–2894. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen B, Zhong Y, Peng W, Sun Y and Kong
WJ: Age-related changes in the central auditory system: comparison
of D-galactose-induced aging rats and naturally aging rats. Brain
Res. 1344:43–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prisila Dulcy C, Singh HK, Preethi J and
Rajan KE: Standardized extract of Bacopa monniera (BESEB
CDRI-08) attenuates contextual associative learning deficits in the
aging rat’s brain induced by D-galactose. J Neurosci Res.
90:2053–2064. 2012.
|
|
13
|
Li WJ, Nie SP, Peng XP, et al:
Ganoderma atrum polysaccharide improves age-related
oxidative stress and immune impairment in mice. J Agric Food Chem.
60:1413–1418. 2012. View Article : Google Scholar
|
|
14
|
Zhang WW, Sun QX, Liu YH, et al: Chronic
administration of Liu Wei Dihuang protects rat’s brain against
D-galactose-induced impairment of cholinergic system. Sheng Li Xue
Bao. 63:245–255. 2011.PubMed/NCBI
|
|
15
|
Lei M, Hua X, Xiao M, et al: Impairments
of astrocytes are involved in the D-galactose-induced brain aging.
Biochem Biophys Res Commun. 369:1082–1087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Chang CF, Chou J, et al: Dietary
supplementation with blueberries, spinach, or spirulina reduces
ischemic brain damage. Exp Neurol. 193:75–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gemma C, Mesches MH, Sepesi B, et al:
Diets enriched in foods with high antioxidant activity reverse
age-induced decreases in cerebellar beta-adrenergic function and
increases in proinflammatory cytokines. J Neurosci. 22:6114–6120.
2002.PubMed/NCBI
|
|
18
|
Hathout AS, Mohamed SR, El-Nekeety AA, et
al: Ability of Lactobacillus casei and Lactobacillus
reuteri to protect against oxidative stress in rats fed
aflatoxins-contaminated diet. Toxicon. 58:179–186. 2011.
|
|
19
|
Bay BH, Lee YK, Tan BK and Ling EA: Lipid
peroxidative stress and antioxidative enzymes in brains of
milk-supplemented rats. Neurosci Lett. 277:127–130. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheng B, Gong K, Niu Y, et al: Inhibition
of gamma-secretase activity reduces Abeta production, reduces
oxidative stress, increases mitochondrial activity and leads to
reduced vulnerability to apoptosis: implications for the treatment
of Alzheimer’s disease. Free Radic Biol Med. 46:1362–1375.
2009.PubMed/NCBI
|
|
21
|
Gu X, Zhou Y, Hu X, et al: Reduced numbers
of cortical GABA-immunoreactive neurons in the chronic D-galactose
treatment model of brain aging. Neurosci Lett. 549:82–86. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bravo JA, Forsythe P, Chew MV, et al:
Ingestion of Lactobacillus strain regulates emotional
behavior and central GABA receptor expression in a mouse via the
vagus nerve. Proc Natl Acad Sci USA. 108:16050–16055.
2011.PubMed/NCBI
|
|
23
|
Liu Y, Man C, Lv X, et al:
Lactobacillus plantarum NDC 75017 affects il-6 gene
expression in Caco-2 cells. Wei Sheng Wu Xue Bao. 52:1237–1243.
2012.(In Chinese).
|
|
24
|
Wang JY MC and Yang XY: Study on
cholesterol-lowering ability of probiotic Lactobacillus
plantarum NDC 75017. Food Science. 34:243–247. 2013.
|
|
25
|
Guo Y, Shan Y, Man C, Yang S, et al:
Identification of a high γ-aminobutyric acid-producing
Lactobacillus plantarum from traditional dairy products in
Inner Mongolia of China. J Anim Sci. 90:262012.
|
|
26
|
Chen CF, Lang SY, Zuo PP, et al: Effects
of D-galactose on the expression of hippocampal peripheral-type
benzodiazepine receptor and spatial memory performances in rats.
Psychoneuroendocrinology. 31:805–811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hernández-Fonseca JP, Rincón J, Pedreañez
A, et al: Structural and ultrastructural analysis of cerebral
cortex, cerebellum, and hypothalamus from diabetic rats. Exp
Diabetes Res. 2009:3296322009.PubMed/NCBI
|
|
28
|
Xin X, Zeng T, Dou DD, et al: Changes of
mitochondrial ultrastructures and function in central nervous
tissue of hens treated with tri-ortho-cresyl phosphate (TOCP). Hum
Exp Toxicol. 30:1062–1072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai C and Li J and Li J: New insight in
colistin induced neurotoxicity with the mitochondrial dysfunction
in mice central nervous tissues. Exp Toxicol Pathol. 65:941–948.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marchi S, Giorgi C, Suski JM, et al:
Mitochondria-ros crosstalk in the control of cell death and aging.
J Signal Transduct. 2012:3296352012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsieh HM, Wu WM and Hu ML: Soy isoflavones
attenuate oxidative stress and improve parameters related to aging
and Alzheimer’s disease in C57BL/6J mice treated with D-galactose.
Food Chem Toxicol. 47:625–632. 2009.PubMed/NCBI
|
|
32
|
Ho SC, Liu JH and Wu RY: Establishment of
the mimetic aging effect in mice caused by D-galactose.
Biogerontology. 4:15–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen YX, Xu SY, Wei W, et al: Melatonin
reduces memory changes and neural oxidative damage in mice treated
with D-galactose. J Pineal Res. 32:173–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schapira AH: Mitochondrial involvement in
Parkinson’s disease, Huntington’s disease, hereditary spastic
paraplegia and Friedreich’s ataxia. Biochim Biophys Acta.
1410:159–170. 1999.
|
|
35
|
Leventhal AG, Wang Y, Pu M, Zhou Y and Ma
Y: GABA and its agonists improved visual cortical function in
senescent monkeys. Science. 300:812–815. 2003. View Article : Google Scholar
|
|
36
|
Poe BH, Linville C and Brunso-Bechtold J:
Age-related decline of presumptive inhibitory synapses in the
sensorimotor cortex as revealed by the physical disector. J Comp
Neurol. 439:65–72. 2001. View Article : Google Scholar
|