Introduction
Ovarian hyperstimulation syndrome (OHSS) is a
serious iatrogenic complication that may occur following ovarian
stimulation/superovulation. Symptoms include hemoconcentration,
pleural effusion, hypercoagulation and multiple organ dysfunction,
while severe cases of OHSS can be life-threatening (1,2). An
increasing incidence of OHSS has been observed due to the rapid
development of assisted reproductive technologies and the
widespread application of ovulation-induction drugs (3). Despite numerous years of clinical
experience, the pathophysiology of OHSS remains obscure. Delaying
embryo transfer with embryo cryopreservation reduces the occurrence
of pregnancy-associated late OHSS; however, there are still no
precise methods to completely eliminate the incidence of human
chorionic gonadotrophin (HCG)-induced severe early-onset OHSS.
Gonadotropin-releasing hormone antagonist (GnRH-ant)
has been widely used in the past two decades in in vitro
fertilization-embryo transfer (IVF-ET) to prevent luteinizing
hormone (LH) surge and the suppression of estradiol (E2)
levels. The use of GnRH-ant has been associated with a
significantly lower incidence of OHSS and E2
concentrations as compared with GnRH agonist (GnRH-a) (4). It has previously been reported that
luteal-phase GnRH-ant administration prevents patient
hospitalization for patients with established severe early-onset
OHSS and results in the quick regression of the syndrome on an
outpatient basis (5,6). However, the LH values fall rapidly in
the luteal phase of the stimulated cycles, and it remains to be
determined whether the exogenous suppression of LH levels in the
luteal phase is necessary. Furthermore, whether luteal-phase
GnRH-ant administration can block the pathogenesis of OHSS and
reduce the risk of severe OHSS has yet to be verified.
In the present study, Cetrotide, a GnRH-ant, was
administered to patients at high risk of OHSS, in whom embryo
transfer was canceled. The efficacy of Cetrotide in the prevention
and treatment of early-onset OHSS in patients undergoing embryo
cryopreservation was subsequently examined.
Materials and methods
Patients
A perspective, nonrandom, case-controlled study was
performed at the Reproductive Medical Center, Renmin Hospital of
Wuhan University (Wuhan, China) between January 2012 and June 2013.
A total of 135 patients receiving IVF-ET treatment were included in
the study. All participating patients met the following criteria:
(i) Number of retrieved oocytes was ≥25; (ii) mean number of
follicles with a diameter of >14 mm was ≥25; (iii) serum
E2 concentrations of ≥8,000 pg/ml; (iv) ovarian diameter
on the day of ovum retrieval of >10 cm; and (v) presentation of
evident symptoms of OHSS on the day of aspiration. Counseling was
provided to all the individuals recruited regarding the high risks
and symptoms of OHSS, and all the patients agreed to cancel the
fresh embryo transfer. The cases were permitted to enter the study
only once. The study protocol was approved by the Ethical Research
Committee of Renmin Hospital of Wuhan University, and patients were
included in the study following the provision of written
consent.
Stimulation protocol and IVF
procedure
In all the cases, a long mid-luteal GnRH-a protocol
was adopted for superovulation. Downregulation was performed with
daily subcutaneous administration of the GnRH-a, triptorelin (0.1
mg; Ferring Pharmaceuticals, Kiel, Germany), beginning on day 21 of
the previous menstrual cycle, as confirmed by a blood test. After
2–3 weeks of downregulation, confirmed by a blood test and
ultrasound, gonadotropin (Gn; Gonal-F; 75 IU; Merck Serono,
Darmstadt, Germany) was administered intramuscularly at 150–225
IU/day, beginning on days 5–8 of the menstrual cycle. The Gn dose
was adjusted according to the ovarian response. All the patients
were monitored using transvaginal ultrasound and the serum
E2 concentrations during superovulation. Final oocyte
maturation was achieved by the administration of 6,000–8,000 IU HCG
(1,000 IU; Lizhu Pharmaceuticals, Zhuhai, China), as soon as three
or more follicles of ≥17 mm were observed by ultrasound.
Transvaginal oocyte aspiration was performed 36 h later by an
ultrasound-guided follicle puncture.
Grouping and intervention
All the patients received routine preventive
intravenous volume expansion therapy on the day of oocyte
retrieval, and all embryos were cryopreserved due to the high risk
of OHSS and/or presence of severe early OHSS. The patients were
divided into two groups after being informed of the two treatment
options. The treatment group (n=39) received subcutaneous
injections of Cetrotide (0.25 mg/day; Merck Serono) for five
consecutive days, beginning on the day following oocyte retrieval,
while the control group (n=96) received no additional
medication.
Blood samples were collected from all the patients
on days 2, 5 and 8 following oocyte retrieval, and the serum
E2, LH and progesterone (P4) levels were measured. The
general patient information, medication prescribed for any untoward
signs associated with IVF, embryonic condition, hematocrit (HCT),
albumin (Alb) levels, pleural effusion, urine output and other
patient parameters were monitored and recorded. In addition, the
length of hospital stay, the performance of paracentesis and the
amount of Alb transfused were recorded. The patients were
followed-up until menstruation, for a maximum of 16 days.
The diagnostic criteria of OHSS were determined
according to the classification of Golan et al (7). Patients with mild OHSS presented with
symptoms of mild abdominal distension and discomfort, possibly
accompanied by nausea, vomiting and diarrhea, and an ovarian
diameter of ≤5 cm. Moderate OHSS was defined as an aggravation of
the aforementioned symptoms, associated with a weight gain of
>4.5 kg, ascites identified by ultrasound examination and an
ovarian diameter of 5–10 cm. Severe OHSS was defined as marked
ascites and/or hydrothorax, HCT >45%, white blood cell count
>15,000/mm3, dyspnea, oliguria or abnormal liver
function tests and large ovaries (>10 cm maximum diameter).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA), according to
the intention-to-treat principle. All the analyses were two-sided
and tested at the 5% significance level; P<0.05 was considered
to indicate a statistically significant difference. Continuous
variables were analyzed using the F-test in the case of normal
distribution. The results of the two groups were compared using the
t-test or Mann-Whitney U-test for parametric and nonparametric
data, respectively. Qualitative variables were compared using the
χ2 test with Yates correction or Fisher’s exact test. In
the present study, serum P4 levels of >60 ng/ml were considered
to be 60 ng/ml exactly, due to a lack of further testing to
establish the exact values.
Results
Comparison of general information between
the two groups
Age, body mass index (BMI), number of cases of
polycystic ovary syndrome (PCOS), duration of infertility, baseline
follicle-stimulating hormone levels, baseline E2 levels,
duration of Gn stimulation and Gn dose were compared between the
treatment and control groups. However, no statistically significant
differences were observed for any of the parameters (P>0.05;
Table I). In addition, no
statistically significant differences were identified between the
two groups with regard to the mean E2 concentration on
the day of HCG administration, the number of follicles with a
diameter of ≥14 mm, the number of oocytes retrieved, and the
fertilization, cleavage and good embryo rates (P>0.05; Table II).
 | Table IComparison of general information
between the control and treatment groups. |
Table I
Comparison of general information
between the control and treatment groups.
| Parameter | Treatment group
(n=39) | Control group
(n=96) | P-value |
|---|
| Age, years | 29.9±4.2 | 30.1±4.0 | >0.05 |
| BMI,
kg/m2 | 21.7±3.0 | 21.4±2.9 | >0.05 |
| PCOS cases, n | 5 | 12 | |
| Duration of
infertility, years | 4.6±3.2 | 4.3±3.5 | >0.05 |
| Baseline FSH,
IU/l | 5.9±1.8 | 6.1±1.6 | >0.05 |
| Baseline
E2, pg/ml | 50.9±17.8 | 48.2±18.2 | >0.05 |
| Duration of Gn
administration, days | 10.8±1.5 | 11.0±1.6 | >0.05 |
| Gn dose, IU | 25.3±6.5 | 25.7±6.4 | >0.05 |
 | Table IIEffect of treatment on in vitro
fertilization parameters. |
Table II
Effect of treatment on in vitro
fertilization parameters.
| Fertilization
parameter | Treatment group
(n=39) | Control group
(n=96) | P-value |
|---|
| Estradiol,
pg/mla | 9349.3±2391.8 | 8837.1±2885.9 | >0.05 |
| Follicles with a
diameter of >14 mm, n | 30.6±7.3 | 31.1±6.9 | >0.05 |
| Ova retrieved, n | 27.1±6.2 | 26.4±6.1 | >0.05 |
| Fertilization rate,
% | 81.1 | 83.2 | >0.05 |
| Cleavage rate, % | 98.3 | 97.9 | >0.05 |
| Good embryo rate,
% | 63.1 | 61.9 | >0.05 |
Comparison of serum steroid hormone
levels on different days after oocyte retrieval between the two
groups
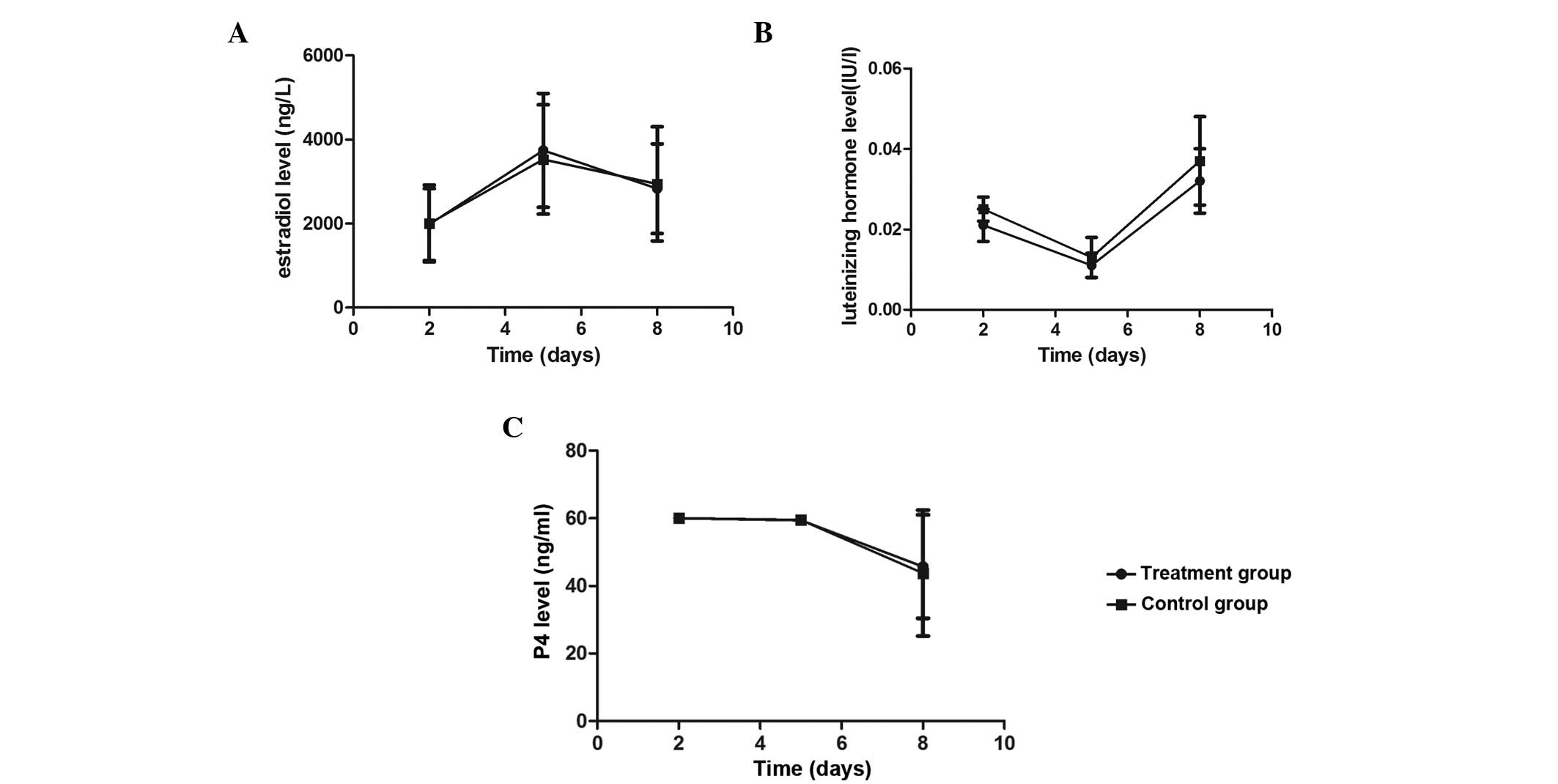
The serum E2, LH and P4 levels were
measured on days 2, 5 and 8 following oocyte retrieval. The results
revealed that the serum E2 levels of the two patient
groups increased on days 2 and 5, while an evident decrease was
observed on day 8. By contrast, the serum LH levels remained at a
relatively low level on days 2,5 and 8 (<0.1 IU/l). The serum P4
levels were found to be >60 ng/ml on days 2 and 5, but a
decrease was observed on day 8. No statistically significant
differences were observed in the serum E2, LH and P4
levels between the treatment and control groups (P>0.05;
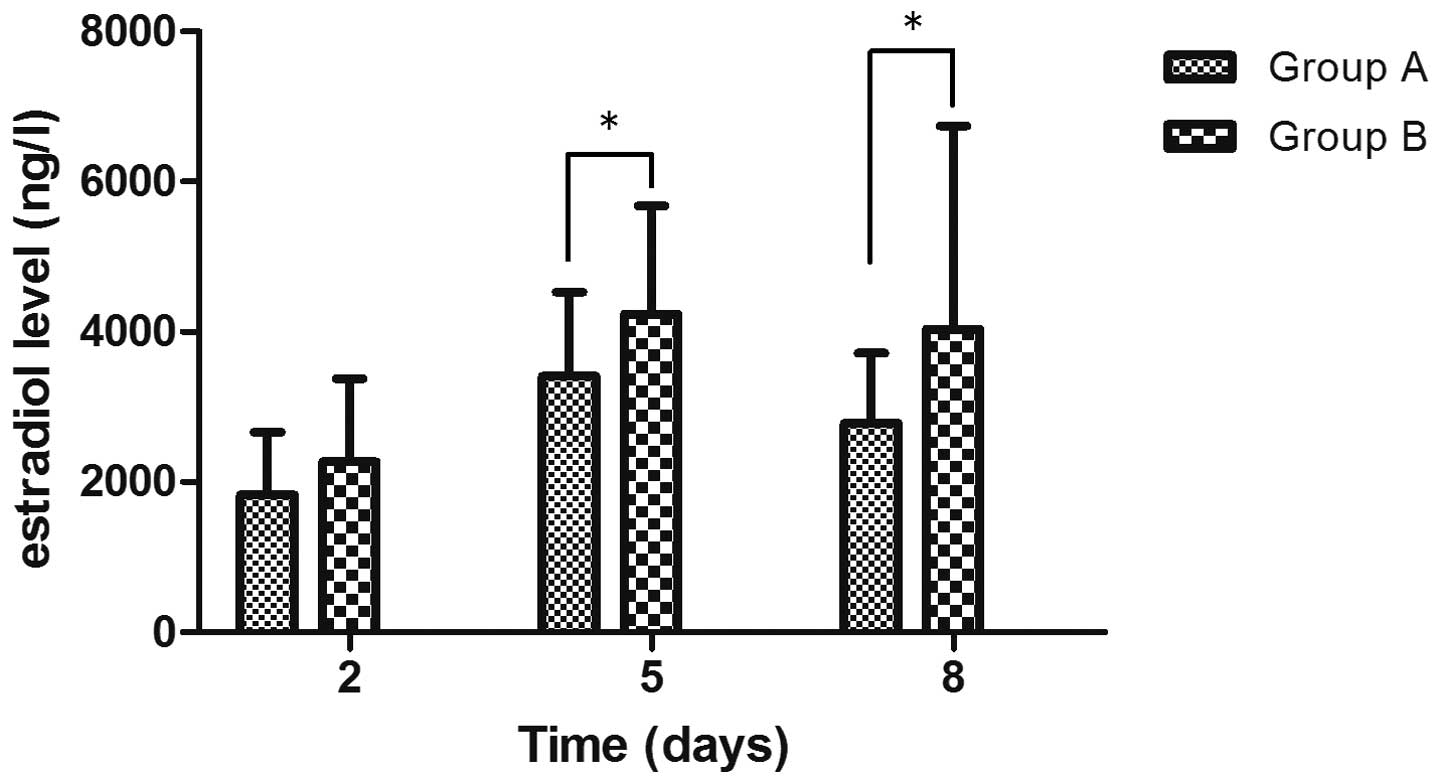
Fig. 1) When comparing the serum
E2 levels of the control patients with mild OHSS and
moderate/severe OHSS on days 2, 5 and 8, the moderate or severe
OHSS patients were shown to have significantly higher serum
E2 levels (P<0.05) and a slower decline in the serum
E2 level (Fig. 2).
Comparison of OHSS outcome between the
two groups
All the patients were followed-up until their next
menses. Among the 39 patients in the treatment group, 19 cases of
mild OHSS, 13 cases of moderate OHSS and seven cases of severe OHSS
were identified. In the seven patients with sever OHSS,
paracentesis was performed for the drainage of abdominal fluid. In
the treatment group, the mean length of hospital stay was 7.0±2.8
days and the mean interval between oocyte retrieval and the onset
of the next menses was 10.7±2.4 days. Among the 96 patients of the
control group, 50 cases of mild OHSS, 25 cases of moderate OHSS and
21 cases of severe OHSS were identified, with 19 patients
undergoing paracentesis. In the control group, the mean length of
hospital stay was 7.3±3.5 days and the mean interval between oocyte
retrieval and the next menses was 11.3±3.0 days. No statistically
significant difference was observed in the incidence of severe OHSS
between the treatment (18%) and control groups (21.8%; P>0.05).
The mean length of hospital stay for the treatment group was
slightly shorter compared with the control group; however, the
difference was not statistically significant. Furthermore, no
statistically significant difference was observed between the two
groups in the number of days between oocyte retrieval and the onset
of the next menses (P>0.05; Table
III). Notably, no serious adverse complications were observed
in either group.
 | Table IIIComparison of OHSS outcome between the
control and treatment groups. |
Table III
Comparison of OHSS outcome between the
control and treatment groups.
| Parameter | Treatment group
(n=39) | Control group
(n=96) | P-value |
|---|
| Paracentesis, n
(%) | 7 (17.9) | 19 (19.8) | >0.05 |
| Length of hospital
stay, days | 7.0±2.8 | 7.3±3.5 | >0.05 |
| Severity of OHSS, n
(%) |
| Mild | 19 (48.7) | 50 (52.1) | >0.05 |
| Moderate | 13 (33.3) | 25 (26.1) | >0.05 |
| Severe | 7 (18.0) | 21 (21.8) | >0.05 |
| Complications, n
(%) | 0 (0) | 0 (0) | |
| Luteal phase,
daysa | 10.7±2.4 | 11.3±3.0 | >0.05 |
Discussion
Superovulation is a significant component of the
IVF-ET process, aiming to provide numerous healthy ova and embryos.
However, superovulation also increases the risk of OHSS. The main
pathological features of OHSS include an increased ovarian volume
and increased systemic capillary proliferation and permeability,
resulting in fluid exudation, hemoconcentration and electrolyte
imbalance (8,9). Although the pathogenesis of OHSS is
not entirely clear, OHSS is known to be self-limiting, transient
and occasionally lethal. Effectively preventing and reducing the
occurrence of OHSS has become an important topic in assisted
reproductive technology. The primary preventive measures include
the identification of populations at high risk of OHSS and the
performance of ovarian stimulation with caution. A secondary
preventive measure is immediate action for early control or
mitigation of OHSS development in the event of an over-reaction or
tendency towards OHSS (10,11).
For patients at high risk of OHSS, freezing all the embryos can
effectively avoid the occurrence of late-onset OHSS. However, no
effective countermeasure exists for moderate and severe early-onset
OHSS, with the exception of conventional volume expansion and other
symptomatic treatments. In previous studies, supplementation with
cabergoline, letrozole, calcium or other drugs has been reported
following ovum retrieval for the prevention and treatment of
early-onset OHSS (12–16).
Cetrotide is a type of GnRH-ant that has been widely
used in recent years for ovulation induction in IVF. Cetrotide
rapidly binds to GnRH receptors in the anterior pituitary,
inhibiting Gn release and quickly suppressing the endogenous LH
surge during controlled ovarian hyperstimulation. Previous studies
have demonstrated that, compared with GnRH-a protocols, GnRH-ant
protocols significantly lower the serum E2 levels and
the incidence of OHSS on the day of HCG administration (17). Hill et al (18) demonstrated that, even with a
long-term GnRH-a protocol, the daily injection of 0.25 mg GnRH-ant
prior to HCG stimulation could effectively suppress the increase in
serum E2 levels when the serum E2
concentration was >4,000 pg/ml. In 2007, Lainas et al
(5) first reported the use of an
antagonist protocol for the treatment of three patients with PCOS
accompanied by early-onset OHSS, with cancellation of fresh embryo
transfer. Ganirelix was administered on day 3 following ovum
retrieval at 0.25 mg/day for seven consecutive days. Outpatient
follow-ups revealed that following the treatment, the HCT, white
blood cell count, ovarian volume and ascites of the patients were
significantly reduced. In addition, small sample studies performed
by Hosseini et al (19) and
Bonilla-Musoles et al (6)
revealed that the symptoms of OHSS and the degree of severity
significantly decreased following the administration of a GnRH-ant
to severe early-onset OHSS patients during the luteal phase. Thus,
GnRH-ant administration during the luteal phase was hypothesized to
be potentially applicable for the prevention and treatment of
early-onset OHSS (6,19).
Based on the aforementioned studies, the present
study examined the efficacy of GnRH-ant administration during the
early luteal phase in patients at high risk of early-onset OHSS who
had undergone embryo cryopreservation. All the patients were at
high risk of OHSS due to the relatively large number of ova
retrieved during each IVF cycle, and exhibited a relatively high
E2 level. No statistically significant differences were
observed between the control and treatment groups with regard to
the age, BMI, total amount of Gn administered, E2 level
on high-HCG days, number of ova retrieved, fertilization rate or
number of usable embryos. Similarly, no statistically significant
difference was identified in the incidence of severe OHSS between
the two groups. Since the occurrence of OHSS is associated with
high levels of serum steroids, the serum E2 levels of
patients with mild OHSS on days 2, 5 and 8 following ovum retrieval
were compared with patients with moderate and severe OHSS. The
serum E2 levels of patients with moderate and severe
OHSS were significantly higher and the decrease in the
E2 levels was slower when compared with the patients
with mild OHSS. Thus, the results indicate that the serum steroid
level is, to a certain degree, associated with the severity of
OHSS, and that embryo transfer during the IVF treatment of patients
with a relatively high estrogen level on day 2 following ovum
retrieval should be performed with caution. Furthermore, no
statistically significant differences were observed in the
E2, LH and P4 levels of the patients in the treatment
and control groups on days 2, 5 and 8. Therefore, GnRH-ant
administration during the luteal phase does not appear to affect
the secretion of steroid hormones, which is consistent with the
results of Asimakopoulos et al (20). However, coculture of granulosa
lutein cells with a GnRH-a or GnRH-ant for 48 h revealed that the
concentration of vascular endothelial growth factor (VEGF) in the
GnRH-ant group was significantly lower compared with the GnRH-a
group, and that the VEGF level was closely associated with the
manifestation of OHSS (21–23).
In theory, early-onset OHSS occurs at the same time
as the zenith of corpus luteum (CL) formation, and GnRH-ants are
known to exert certain luteolytic effects (24). A study by Duffy et al, using
a monkey model, revealed a decrease in P4 levels following antide
administration during the luteal phase, and the next menstrual
cycle began earlier than usual (25). The CL was removed 10 days following
the antide treatment and was found to weigh less than the
respective control. In addition, a reduction in the number of
luteal cells was observed. By contrast, Ortmann et al
demonstrated that a low-dose of GnRH-ant was unable to exert an
antagonistic effect locally in the ovary, despite GnRH receptor
expression being demonstrated in the ovary (26). In the present study, the serum LH
and P4 levels of the two groups were not found to differ
significantly. In addition, follow-up examination did not reveal a
statistically significant difference in the interval between ovum
retrieval and the onset of the next menses, which is the duration
of the luteal phase. When compared with the control group, the mean
length of hospital stay and the incidence of paracentesis for the
moderate and severe OHSS patients in the treatment group were not
found to be significantly reduced. Therefore, OHSS is hypothesized
to induce a cascade of effects following activation by HCG, and the
application of a single GnRH-ant at a late stage does not
effectively treat severe OHSS. Preventing OHSS is preferable, and
the best preventive measure appears to be mild stimulation, which
reduces the incidence of adverse reactions.
In conclusion, the current study presents the
preliminary results of a prospective, nonrandomized clinical study.
Cetrotide administration of 0.25 mg/day for five consecutive days
during the early luteal phase was not found to significantly
improve the clinical conditions of patients at high risk of severe
early-onset OHSS. However, the number of patients in the present
study was limited, and no experiments were performed to compare the
effects at different dosing, timing and duration of treatment, or
different types of GnRH-ant. Therefore, elucidation of the efficacy
of GnRH-ant administration during the luteal phase for the
prevention and treatment of early-onset moderate and severe OHSS
merits further large-scale, multicenter random-controlled
trials.
Acknowledgements
The authors thank Ai-Bin Li, Dan Chen, Zhuo-Ni Xiao
and Jie Li for their assistance during the trial, and Jin Luo for
reading and revising the manuscript.
References
|
1
|
Aboulghar M: Prediction of ovarian
hyperstimulation syndrome (OHSS). Estradiol level has an important
role in the prediction of OHSS. Hum Reprod. 18:1140–1141. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamath MS, Joshi A, Kamath AM and Aleyamma
T: Management of severe ovarian hyperstimulation syndrome with
thawed plasma. J Hum Reprod Sci. 6:82–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Mouzon J, Goossens V, Bhattacharya S,
Castilla JA, Ferraretti AP, Korsak V, Kupka M, Nygren KG and Nyboe
Andersen A: European IVF-monitoring (EIM) Consortium, for the
European Society of Human Reproduction and Embryology (ESHRE):
Assisted reproductive technology in Europe, 2006: results generated
from European registers by ESHRE. Hum Reprod. 25:1851–1862.
2010.
|
|
4
|
Gilliam ML: Gonadotrophin-releasing
hormone antagonists for assisted reproductive technology. Obstet
Gynecol. 118:706–707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lainas TG, Sfontouris IA, Zorzovilis IZ,
Petsas GK, Lainas GT and Kolibianakis EM: Management of severe
early ovarian hyperstimulation syndrome by re-initiation of GnRH
antagonist. Reprod Biomed Online. 15:408–412. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonilla-Musoles FM, Raga F, Castillo JC,
Sanz M, Dolz M and Osborne N: High doses of GnRH antagonists are
efficient in the management of severe ovarian hyperstimulation
syndrome. Clin Exp Obstet Gynecol. 36:78–81. 2009.PubMed/NCBI
|
|
7
|
Golan A, Ron-el R, Herman A, Soffer Y,
Weinraub Z and Caspi E: Ovarian hyperstimulation syndrome: an
update review. Obstet Gynecol Surv. 44:430–440. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aboulghar MA and Mansour RT: Ovarian
hyperstimulation syndrome: classifications and critical analysis of
preventive measures. Hum Reprod Update. 9:275–289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gómez R, Soares SR, Busso C,
Garcia-Velasco JA, Simón C and Pellicer A: Physiology and pathology
of ovarian hyperstimulation syndrome. Semin Reprod Med. 28:448–457.
2010.
|
|
10
|
Egbase PE: Severe OHSS: how many cases are
preventable? Hum Reprod. 15:8–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Humaidan P, Quartarolo J and Papanikolaou
EG: Preventing ovarian hyperstimulation syndrome: guidance for the
clinician. Fertil Steril. 94:389–400. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fatemi HM, Popovic-Todorovic B, Donoso P,
Papanikolaou E, Smitz J and Devroey P: Luteal phase oestradiol
suppression by letrozole: a pilot study in oocyte donors. Reprod
Biomed Online. 17:307–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcia-Velasco JA: How to avoid ovarian
hyperstimulation syndrome: a new indication for dopamine agonists.
Reprod Biomed Online. 18(Suppl 2): 71–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gurgan T, Demirol A, Guven S, Benkhalifa
M, Girgin B and Li TC: Intravenous calcium infusion as a novel
preventive therapy of ovarian hyperstimulation syndrome for
patients with polycystic ovarian syndrome. Fertil Steril. 96:53–57.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papanikolaou EG, Polyzos NP, Humaidan P,
Pados G, Bosch E, Tournaye H and Tarlatzis B: Aromatase inhibitors
in stimulated IVF cycles. Reprod Biol Endocrinol. 9:852011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rollene NL, Amols MH, Hudson SB and
Coddington CC: Treatment of ovarian hyperstimulation syndrome using
a dopamine agonist and gonadotropin releasing hormone antagonist: a
case series. Fertil Steril. 92:1169.e15–1169.e17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Inany HG, Youssef MA, Aboulghar M,
Broekmans F, Sterrenburg M, Smit J and Abou-Setta AM:
Gonadotrophin-releasing hormone antagonists for assisted
reproductive technology. Cochrane Database Syst Rev.
CD0017502011.
|
|
18
|
Hill MJ, Chason RJ, Payson MD, Segars JH
and Csokmay JM: GnRH antagonist rescue in high responders at risk
for OHSS results in excellent assisted reproduction outcomes.
Reprod Biomed Online. 25:284–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hosseini MA, Mahdavi A, Aleyasin A,
Safdarian L and Bahmaee F: Treatment of ovarian hyperstimulation
syndrome using gonadotropin releasing hormone antagonist: a pilot
study. Gynecol Endocrinol. 28:853–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asimakopoulos B, Nikolettos N, Nehls B,
Diedrich K, Al-Hasani S and Metzen E: Gonadotropin-releasing
hormone antagonists do not influence the secretion of steroid
hormones but affect the secretion of vascular endothelial growth
factor from human granulosa luteinized cell cultures. Fertil
Steril. 86:636–641. 2006. View Article : Google Scholar
|
|
21
|
McDonough PG: Vascular endothelial growth
factor - mediator of OHSS? Fertil Steril. 79:1466–1467. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alvarez C, Alonso-Muriel I, García G,
Crespo J, Bellver J, Simón C and Pellicer A: Implantation is
apparently unaffected by the dopamine agonist Cabergoline when
administered to prevent ovarian hyperstimulation syndrome in women
undergoing assisted reproduction treatment: a pilot study. Hum
Reprod. 22:3210–3214. 2007. View Article : Google Scholar
|
|
23
|
Kosaka K, Fujiwara H, Yoshioka S and Fujii
S: Vascular endothelial growth factor production by circulating
immune cells is elevated in ovarian hyperstimulation syndrome. Hum
Reprod. 22:1647–1651. 2007. View Article : Google Scholar
|
|
24
|
Fridén BE and Nilsson L:
Gonadotrophin-releasing hormone antagonist luteolysis during the
preceding mid-luteal phase is a feasible protocol in ovarian
hyperstimulation before in vitro fertilization. Acta Obstet Gynecol
Scand. 84:812–816. 2005.
|
|
25
|
Duffy DM, Stewart DR and Stouffer RL:
Titrating luteinizing hormone replacement to sustain the structure
and function of the corpus luteum after gonadotropin-releasing
hormone antagonist treatment in rhesus monkeys. J Clin Endocrinol
Metab. 84:342–349. 1999.
|
|
26
|
Ortmann O, Weiss JM and Diedrich K: Embryo
implantation and GnRH antagonists: ovarian actions of GnRH
antagonists. Hum Reprod. 16:608–611. 2001. View Article : Google Scholar : PubMed/NCBI
|