Introduction
Acute myocardial infarction (AMI) is one of the
major diseases causing clinical mortality (1). In the past decade, doctors have been
able to make the ischemic heart regain blood perfusion and restore
oxygen supply in a short time using modern medical technologies,
including thrombolytic therapy and percutaneous coronary vessel
intervention; however, coronary reperfusion typically causes
myocardial ischemia-reperfusion injury (MIRI), leading to
conditions including arrhythmia, expansion of the infarct size and
persistent ventricular systolic dysfunction. MIRI is the main
reason for the poor prognosis of AMI (2,3).
Apoptosis is the process of programmed cell death
that occurs in multicellular organisms. Biochemical events lead to
characteristic cell changes and death. Previous studies have shown
that cardiomyocyte apoptosis plays an important role in the
processes of AMI and MIRI (4–6);
therefore, the development of drugs that can attenuate MIRI,
inhibit cardiomyocyte apoptosis and improve cardiac function has an
important clinical significance.
The medicinal herb Panax quinquefolium, also
known as American ginseng, has a long history of use in a number of
countries, including China (7,8).
Ginsenosides are the major active components of P.
quinquefolium (7,8), which has been described to possess
anti-stress, anti-diabetic and antitumor effects (9–12).
Ginsenoside-Rb3 (G-Rb3), an extract from the stems and leaf of
P. quinquefolium, has been demonstrated to exhibit a variety
of protective effects in the cardiovascular system by our
laboratory (13–15), including attenuating MIRI in rats
(15).
In our previous study (15), Sprague Dawley rats were orally
treated with G-Rb3 daily for three days, prior to being subjected
to left anterior descending coronary artery ligation for 30 min and
reperfusion for 24 h. The results showed that G-Rb3 treatment
resulted in a reduction in myocardial infarct size, as well as in
creatine kinase-MB (CK-MB) activity and lactate dehydrogenase (LDH)
activity in the serum. The cardioprotective effect of G-Rb3 was
further confirmed by histopathological examination. Whether G-Rb3
protects the myocardium from ischemia-reperfusion injury via
inhibition of apoptosis, however, is yet to be elucidated. Previous
studies (4–6) showed that MIRI and apoptosis appear
in the reperfused myocardium within ≤4 h; therefore, reperfusion
for 24 h is not the optimal time-period to observe the
anti-apoptotic effects of drugs. In the present study, the time of
reperfusion was reduced to 120 min to observe the functional and
histopathological changes in the early stage of MIRI. The aim of
this study was to verify the anti-apoptotic effects of G-Rb3 and
analyze the mechanism underlying its cardioprotective effects.
Ischemic postconditioning (IP), which has been proven to attenuate
MIRI (16), was selected to be the
positive control.
Materials and methods
Chemicals and reagents
A terminal deoxynucleotidyl-transferase-mediated
dUTP nick end labeling (TUNEL) apoptosis detection kit was
purchased from Roche Diagnostics (Basel, Switzerland). Antibodies
against B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein
(Bax) for immunohistochemistry (IHC) were purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Malondialdehyde
(MDA) and superoxide dismutase (SOD) assay kits were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Tumor
necrosis factor-α (TNF-α) and interleukin-6 (IL-6) radioimmunoassay
kits were purchased from Beijing Kangyuan Ruide Biotechnology Co.,
Ltd. (Beijing, China). The G-Rb3 was obtained from Dr Yanping Chen
(Department of Natural Medicinal Chemistry, School of Chemistry,
Jilin University, Changchun, China) and dissolved in
double-distilled (dd)H2O for use. All other chemicals
were analytical reagents.
Animals
Male Sprague Dawley rats (weight, 200–220 g) were
purchased from the Experimental Animal Center of Jilin University
(Changchun, China). All rats were allowed free access to food and
water. The experiments were performed in accordance with the Guide
for the Care and Use of Laboratory Animals of Jilin University, and
approved by the Ethics Committee of Jilin University.
The rats were randomly divided into four groups (10
rats in each group): i) Sham surgery (sham), ii) MIRI, iii) G-Rb3
and iv) IP. Rats in the G-Rb3 group were orally administered 20
mg/kg G-Rb3 (dissolved in ddH2O) and the other three
groups were treated with ddH2O. The drug or
ddH2O treatment was administered once a day for three
consecutive days. Thirty minutes after the final treatment, the
rats were anesthetized for surgery.
Surgical procedures
In the MIRI and G-Rb3 groups, myocardial ischemia
was induced by exposing the heart through a left thoracic incision
and placing a 6/0 silk suture with a slipknot around the left
anterior descending coronary artery. After 30 min ischemia, the
slipknot was released and the myocardium reperfused for 2 h. In the
IP group, the rats were treated three times with 30 sec reperfusion
and 30 sec ligation before the 2 h reperfusion, while the sham
group underwent left thoracotomy only.
After 2 h reperfusion, heart rate (HR) and blood
pressure were measured, and then the blood and myocardium tissue
samples were obtained. Six out of the 10 rat hearts in every group
had the apex cordis removed for assay of the MDA levels and the SOD
activity, while the remaining cardiac tissue samples were fixed in
4% buffered paraformaldehyde solution for histopathological
examination. The other four rat hearts were prepared for infarct
size measurement.
Measurement of infarct size
To distinguish between the normal and infarcted
myocardium, the hearts were cut into four horizontal slices and
incubated with p-nitro-blue tetrazolium (NBT; 0.5 mg/ml, 10
min at 37°C). In the presence of intact dehydrogenase enzyme
systems (normal myocardium), NBT forms a dark blue formazan; by
contrast, areas of necrosis lack dehydrogenase activity and
therefore do not stain (17). The
normal and infarcted myocardium were weighed together and then
separated by following the line of demarcation between the dark
blue stained and unstained tissue. The infarcted myocardium was
then weighed. The infarct size was calculated as infarcted
myocardium mass divided by total myocardium mass, and expressed as
a percentage.
Histopathological examination: Histology,
TUNEL and IHC
The myocardial tissue samples were fixed in 4%
buffered paraformaldehyde solution, and then embedded in paraffin.
Four-micrometer thick paraffin sections were stained with
hematoxylin and eosin (HE). TUNEL staining with the paraffin
sections was performed according to the kit manufacturer’s
instructions (Roche Diagnostics). The sections were examined using
light microscopy (Nikon E100; Nikon Corporation, Tokyo, Japan), and
photomicrograph images were captured.
For the IHC, endogenous peroxidase activity was
blocked with 3% methanol-H2O2. Nonspecific
sites were treated with a blocking buffer (5% albumin and 10%
specific serum in phosphate-buffered saline). Primary antibody
(against Bcl-2 or Bax) were added to the sections in the blocking
buffer and incubated overnight at 4°C. Subsequent to washing,
polyclonal goat anti-rat antibody (Beijing Dingguo Changsheng
Biotech Co., Ltd., Beijing, China) was added and washed, prior to
development with avidin-biotin-streptavidin complex and
diaminobenzidine chromogen. Photomicrograph images were then
captured.
The Motic Images Advanced 3.2 image analysis system
(Motic, Causeway Bay, Hong Kong) was used to analyze the
photomicrographs of the TUNEL and IHC staining, and the results
were expressed by grayscale value. Higher grayscale values
correlated with lighter TUNEL or IHC staining, which indicated
fewer apoptotic cells or lower protein (Bcl-2 or Bax)
expression.
Assay of MDA levels and SOD activity
The preparation of the myocardium tissue was as
follows: 1 g apex cordis was homogenized in nine volumes of
ice-cold saline and centrifuged (1,500 × g) at 4°C for 15 min. The
supernatant was obtained (stored at −80°C) to analyze the MDA
levels and the SOD activity using assay kits and a
spectrophotometer (7202B; Unico Instrument Co., Ltd., Shanghai,
China), in accordance with the kit manufacturer’s instructions
(Nanjing Jiancheng Bioengineering Institute).
Assay of the TNF-α and IL-6 levels and
the activities of aspartate aminotransferase (AST), CK-MB and
LDH
Blood samples were collected and left at room
temperature for 2 h to allow complete clotting and then centrifuged
(1,500 × g) at 4°C for 15 min. The serum was removed and stored at
−80°C for radioimmunoassay and biochemical assay. The TNF-α and
IL-6 levels were analyzed using radioimmunoassay kits purchased
from Beijng Kangyuan Ruide Biotechnology Co., Ltd. The activities
of AST, LDH and CK-MB were analyzed by the Clinical Laboratory in
the First Hospital of Jilin Academy of Traditional Chinese Medicine
(Changchun, China), using commercial kits by employing an automatic
biochemical analyzer (Cobas-Fara; Roche Diagnostics, Basel,
Switzerland).
Statistical analysis
All results are presented as the mean ± standard
deviation. The Student’s t-test was applied when two or more values
were compared.
Results
Effects of G-Rb3 on heart rate and blood
pressure
Compared with the sham surgery control, MIRI induced
heart function impairment; this was demonstrated by the
significantly reduced HR and systolic blood pressure (SBP) in the
MIRI group (P<0.05). Compared with the MIRI group, the HRs of
the G-Rb3 and IP groups were significantly reduced (P<0.05), but
no significant differences were observed in the SBP and diastolic
blood pressure (Table I).
 | Table IEffects of G-Rb3 on heart rate and
blood pressure. |
Table I
Effects of G-Rb3 on heart rate and
blood pressure.
| Group | Heart rate
(beats/min) | Systolic blood
pressure (mmHg) | Diastolic blood
pressure (mmHg) |
|---|
| Sham | 477.1±36.1 | 154.4±22.4 | 104.4±34.4 |
| MIRI | 436.9±22.1a | 120.0±9.3a | 82.5±11.6 |
| G-Rb3 | 406.0±34.9b | 116.3±16.9 | 82.5±19.8 |
| IP | 402.4±35.5b | 125.0±16.0 | 80.6±20.4 |
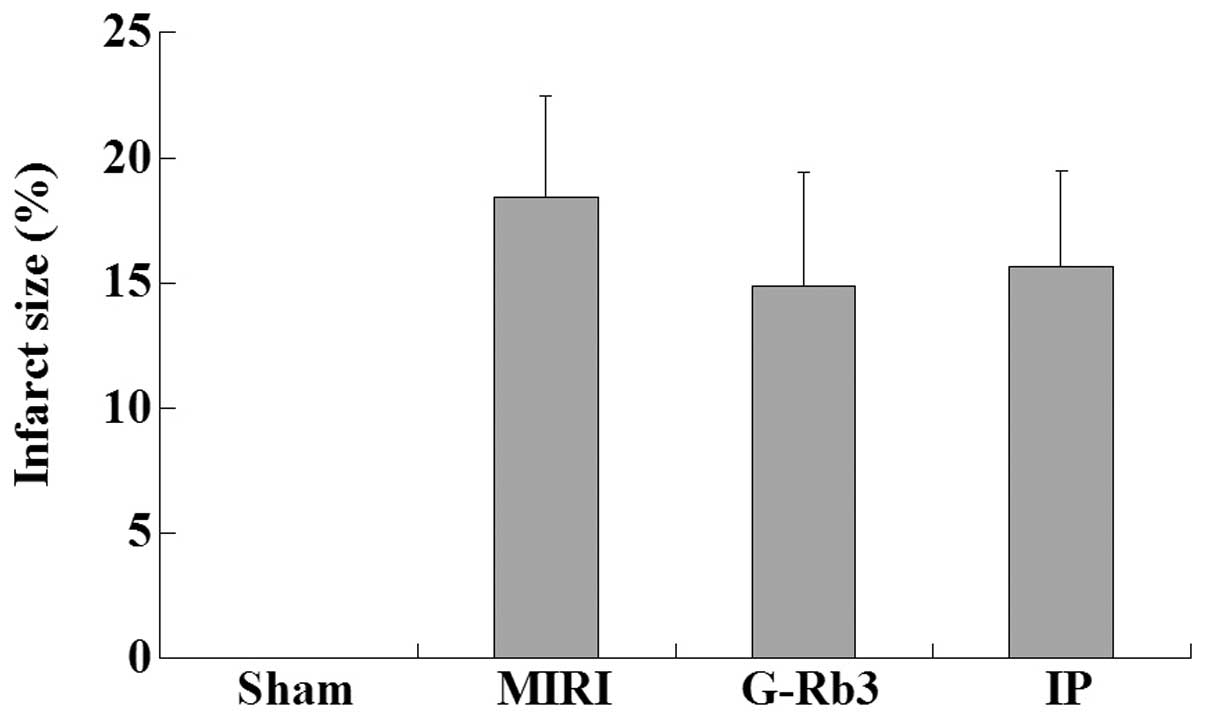
Effects of G-Rb3 on infarct size
The infarct size of the myocardium in the sham group
was zero, while the size in the MIRI group was 18.42±4.05%.
Compared with the MIRI group, the infarct size was reduced in the
G-Rb3 (14.85±4.58%) and IP (15.66±3.80%) groups, although the
difference was not significant (Fig.
1).
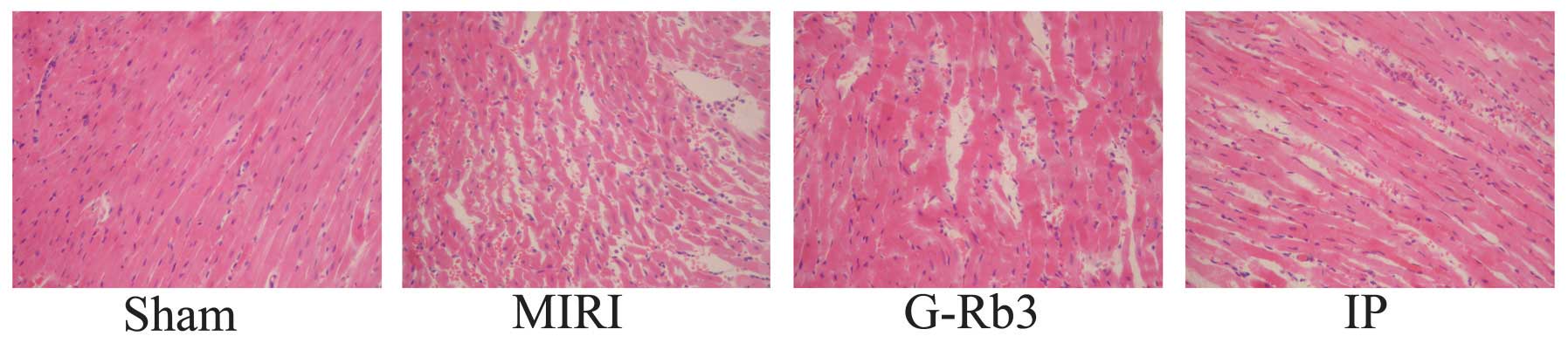
Effects of G-Rb3 on histology, TUNEL and
IHC
According to the HE staining, the myocardial tissue
samples of the sham group exhibited no focal separation of
myocardial fibers, but had a small amount of erythrocyte
infiltration. The samples of the other three groups exhibited focal
destruction of myocardial fibers with erythrocyte and neutrophil
infiltration; however, myocyte necrosis was rarely observed. The
condition of myocardial injury of these three groups was similar.
(Fig. 2).
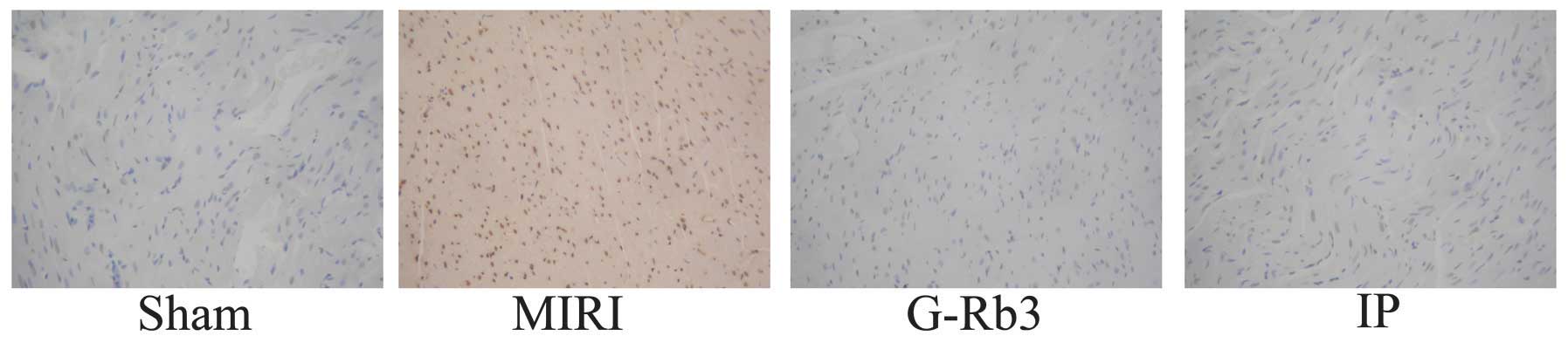
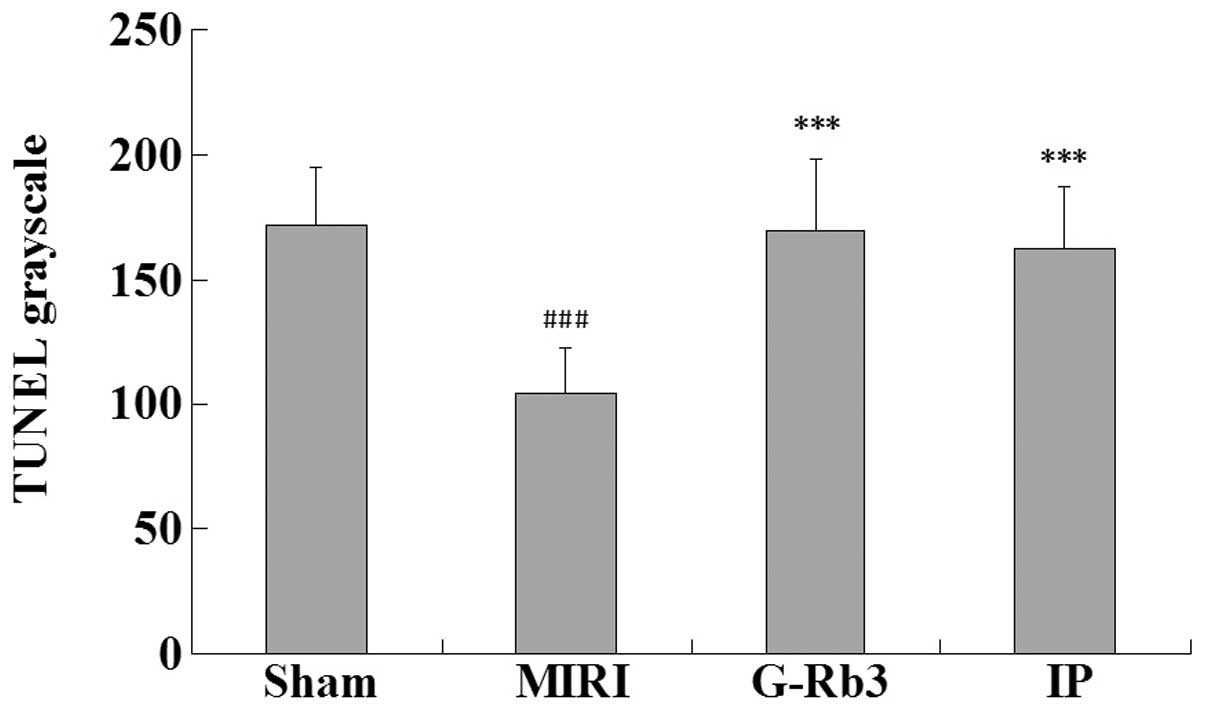
Compared with the sham group, the numbers of
apoptotic cells were increased significantly in the MIRI group and
reduced significantly in the G-Rb3 and IP groups (P<0.001).
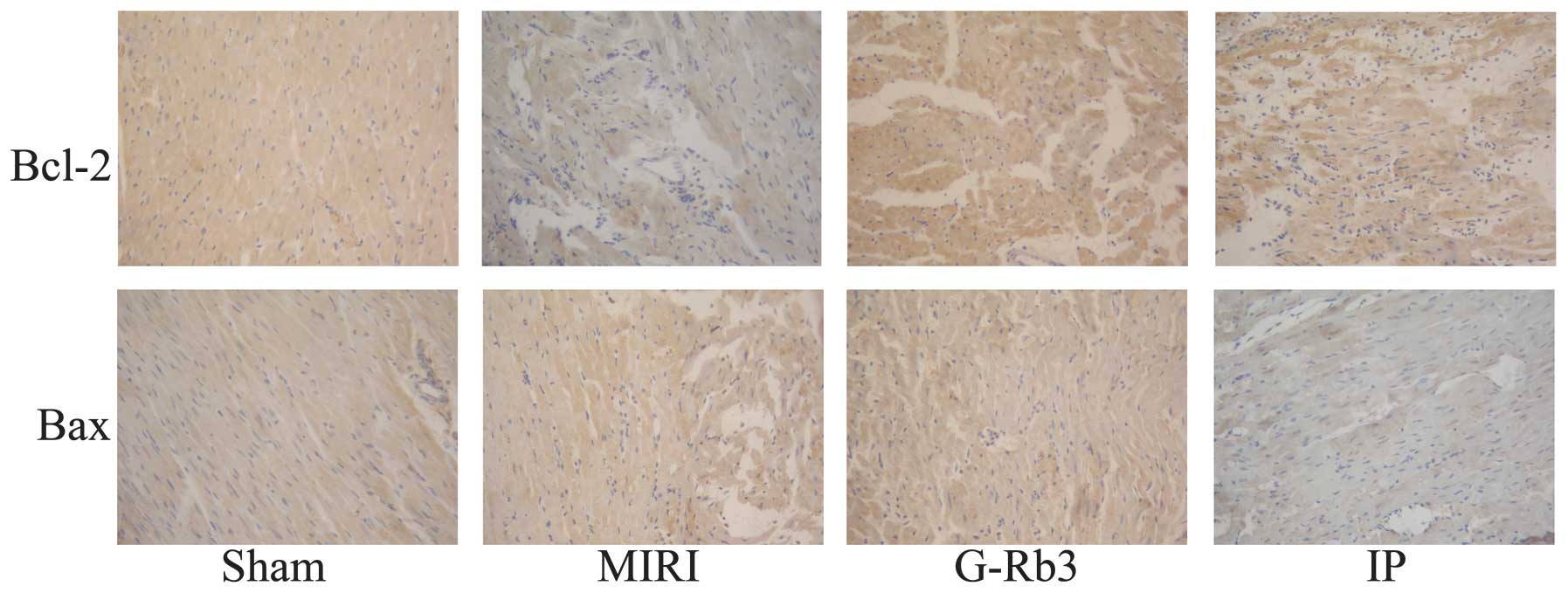
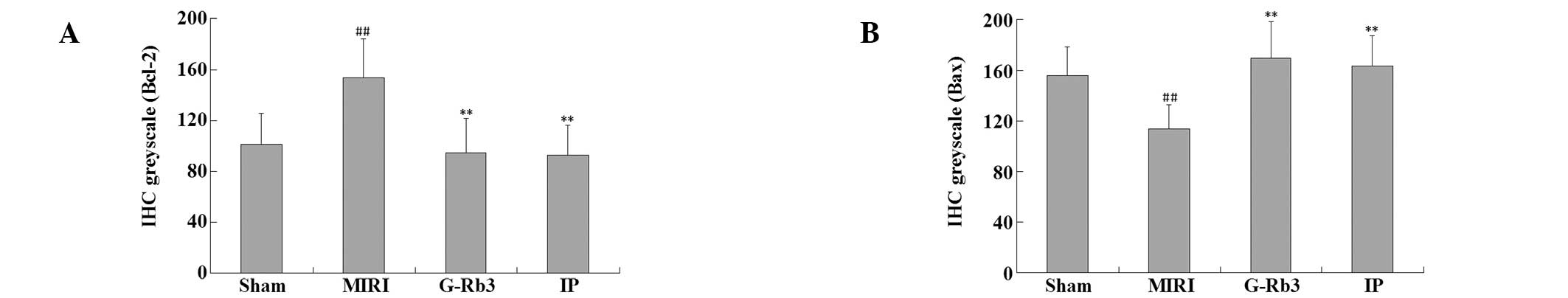
(Figs. 3 and 4) The expression of Bcl-2 in the
myocardial tissue samples was lower in the MIRI group than that in
the other three groups, and this difference was significant
(P<0.01). By contrast, the expression of Bax was higher in the
MIRI group than that in the other three groups; these differences
also showed significance (P<0.01) (Figs. 5 and 6).
Effects of G-Rb3 on MDA levels and SOD
activity
The MDA levels in the myocardial tissue of the MIRI
group were significantly higher than those of the sham group
(P<0.05), while the SOD activity was significantly lower in the
MIRI group than that in the sham group (P<0.05). G-Rb3 treatment
significantly reduced the MDA levels (P<0.01) and improved the
SOD activity (P<0.05); this was a similar trend to the IP group
(P<0.01, MDA and SOD) (Fig.
7).
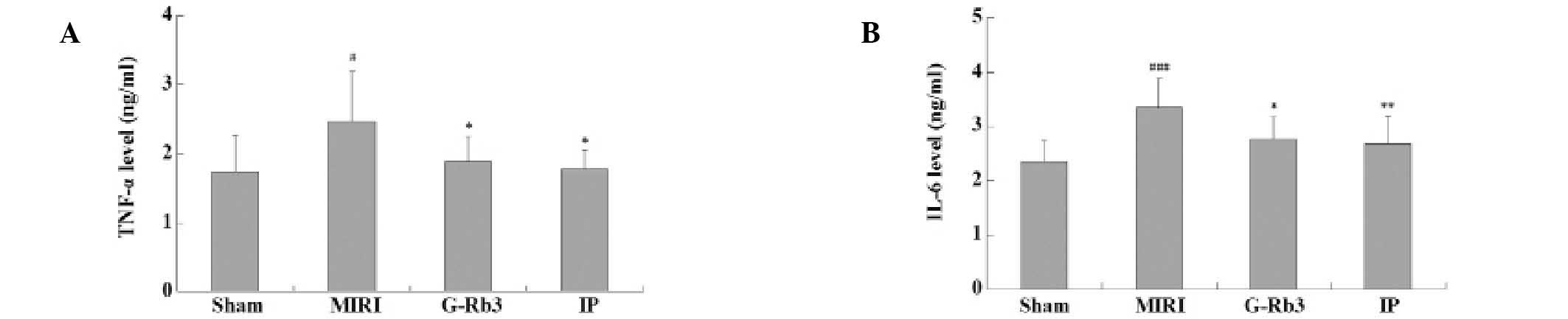
Effects of G-Rb3 on TNF-α and IL-6
levels
The serum TNF-α levels of the MIRI group were
significantly higher than those of the sham group (P<0.05), and
the TNF-α levels of the G-Rb3 (P<0.05) and IP (P<0.05) groups
were significantly reduced compared with those of the MIRI group.
The findings were similar for IL-6 levels, which were significantly
increased in the MIRI group (P<0.001) and significantly reduced
in the G-Rb3 (P<0.05) and IP (P<0.01) groups (Fig. 8).
Effects of G-Rb3 on the activities of
AST, LDH and CK-MB
The activities of AST, LDH and CK-MB in the serum
were significantly increased in the MIRI group (P<0.01) compared
with those in the sham group. The G-Rb3 and IP treatments reduced
the activities of AST, LDH and CK-MB, and the difference was
significant (P<0.05) (Table
II).
 | Table IIEffects of G-Rb3 on AST, LDH and
CK-MB. |
Table II
Effects of G-Rb3 on AST, LDH and
CK-MB.
| Group | AST (U/ml) | LDH (U/ml) | CK-MB (U/ml) |
|---|
| Sham | 402.1±208.8 | 1246.7±744.0 | 2308.3±945.0 |
| MIRI | 820.1±198.2a | 2256.1±564.2a | 3799.3±487.2a |
| G-Rb3 | 598.3±209.9b | 1510.3±724.3b | 3072.0±850.1b |
| IP | 600.4±192.1b | 1727.0±315.3b | 3220.8±558.2b |
Discussion
Apoptosis has been studied for decades. In mammals,
there are two major pathways of apoptosis in vivo: The death
receptor pathway and the mitochondrial death pathway (18). MIRI results in the accumulation of
reactive oxygen species (ROS), which causes oxidative stress and
mitochondrial injury (19,20). With the development of MIRI, the
mitochondrial death pathway becomes activated, causing
cardiomyocyte apoptosis and irreversible myocardial injury. In
addition to ROS accumulation and oxidative stress, MIRI may also
cause the release of inflammatory factors (21,22),
particularly TNF-α, which is associated with the death receptor
pathway.
As described in the Introduction, G-Rb3 treatment
can attenuate MIRI in the rat heart with 30 min ischemia and 24 h
reperfusion. The infarct size was reduced significantly and there
was a significant difference in the histological result (15). In the present study, however, when
the reperfusion time was reduced to 2 h, the differences in the
infarct size and histological results between the MIRI and G-Rb3
groups were no longer significant. A possible reason is that in the
early stage of MIRI, myocardial injury is reversible and the
organic change is slight. During this stage, drug or IP/ischemic
preconditioning treatment can inhibit cardiomyocyte apoptosis and
attenuate MIRI by protecting the mitochondria from oxidative stress
and reducing inflammation. When the reperfusion is continued, MIRI
without treatment can therefore cause serious conditions, including
arising arrhythmia, expansion of the infarct size and persistent
ventricular systolic dysfunction. These serious conditions can be
prevented with treatment, as shown in our previous study (15).
According to the histopathological examination,
although the MIRI and G-Rb3 groups exhibited similar conditions in
the photomicrographs of HE staining, the TUNEL results show that
cardiomyocyte apoptosis was inhibited in the G-Rb3 group compared
with the MIRI group. This was further verified by the IHC results,
which showed that the expression of Bcl-2, one of the most
important anti-apoptotic proteins (23), was increased with G-Rb3 treatment,
and the expression of Bax, which has the opposite effect to Bcl-2,
was reduced with G-Rb3 treatment. The results of the assays for MDA
levels and SOD activity showed that G-Rb3 treatment attenuated ROS
accumulation and oxidative stress, which may have been a major
mechanism underlying its anti-apoptotic effect. The serum TNF-α and
IL-6 levels were also reduced with G-Rb3 treatment, which may also
have been associated with its anti-apoptotic effect. Further study
is required to determine these mechanisms, particularly at the
molecular level, and apoptosis-related genes and proteins, such as
the caspase family, require analysis (24). The cardioprotective effect of G-Rb3
was confirmed by the AST, LDH and CK-MB results. Although G-Rb3
treatment did not improve the SBP reduced by MIRI, it did reduce
the HR further, thus reducing the myocardial oxygen consumption and
protecting the heart. According to the majority of the results, the
cardioprotective effects of G-Rb3 are similar to those of IP.
In conclusion, G-Rb3 treatment can inhibit
cardiomyocyte apoptosis in the early stage of MIRI in rats,
consequently preventing serious conditions occurring when the
reperfusion is continued. This is one of the major mechanisms of
its cardioprotective effects, which is similar to IP. Attenuating
ROS accumulation and oxidative stress may be a possible mechanism
underlying the anti-apoptotic effect of G-Rb3, which is also
associated with the reduction of inflammatory factor levels.
Further studies at the molecular level to determine these
mechanisms are a research direction of the future.
Acknowledgements
The authors would like to thank Dr Yanping Chen for
providing G-Rb3, and Dr Min Li (Jilin University, Changchun, China)
and Dr Xin Zhou (Fudan University, Shanghai, China) for their
useful comments during the preparation of this manuscript.
References
|
1
|
Chambless L, Keil U, Dobson A, et al:
Population versus clinical view of case fatality from acute
coronary heart disease: results from the WHO MONICA Project
1985–1990. Multinational MONItoring of Trends and Determinants in
CArdiovascular Disease. Circulation. 96:3849–3859. 1997.PubMed/NCBI
|
|
2
|
Buja LM: Myocardial ischemia and
reperfusion injury. Cardiovasc Pathol. 14:170–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saraste A, Pulkki K, Kallajoki M, et al:
Apoptosis in human acute myocardial infarction. Circulation.
95:320–323. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haunstetter A and Izumo S: Apoptosis:
basic mechanisms and implications for cardiovascular disease. Circ
Res. 82:1111–1129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu H, Yu X, Qu S, Chen Y, Wang Z and Sui
D: Protective effect of Panax quinquefolium
20(S)-protopanaxadiol saponins, isolated from Panax
quinquefolium, on permanent focal cerebral ischemic injury in
rats. Exp Ther Med. 7:165–170. 2014.
|
|
8
|
Lin G, Yu X, Wang J, Qu S and Sui D:
Beneficial effects of 20(S)-protopanaxadiol on antitumor activity
and toxicity of cyclophosphamide in tumor-bearing mice. Exp Ther
Med. 5:443–447. 2013.PubMed/NCBI
|
|
9
|
Wang LC and Lee TF: Effect of ginseng
saponins on cold tolerance in young and elderly rats. Planta Med.
66:144–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin JY, Song JY, Yun YS, et al:
Immunostimulating effects of acidic polysaccharides extract of
Panax ginseng on macrophage function. Immunopharmacol
Immunotoxicol. 24:469–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishijo H, Uwano T, Zhong YM and Ono T:
Proof of the mysterious efficacy of ginseng: basic and clinical
trials: effects of red ginseng on learning and memory deficits in
an animal model of amnesia. J Pharmacol Sci. 95:145–152. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G and Wang Z, Sun Y, Liu K and Wang Z:
Ginsenoside 20(S)-protopanaxadiol inhibits the proliferation and
invasion of human fibrosarcoma HT1080 cells. Basic Clin Pharmacol
Toxicol. 98:588–592. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang T, Yu X, Qu S, Xu H, Han B and Sui D:
Effect of ginsenoside Rb3 on myocardial injury and heart function
impairment induced by isoproterenol in rats. Eur J Pharmacol.
636:121–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang T, Yu XF, Qu SC, Xu HL and Sui DY:
Ginsenoside Rb3 inhibits angiotensin II-induced vascular smooth
muscle cells proliferation. Basic Clin Pharmacol Toxicol.
107:685–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi Y, Han B, Yu X, Qu S and Sui D:
Ginsenoside Rb3 ameliorates myocardial ischemia-reperfusion injury
in rats. Pharm Biol. 49:900–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao ZQ, Corvera JS, Halkos ME, et al:
Inhibition of myocardial injury by ischemic postconditioning during
reperfusion: comparison with ischemic preconditioning. Am J Physiol
Heart Circ Physiol. 285:H579–H588. 2003.PubMed/NCBI
|
|
17
|
Nachlas MM and Shnitka TK: Macroscopic
identification of early myocardial infarcts by alterations in
dehydrogenase activity. Am J Pathol. 42:379–405. 1963.PubMed/NCBI
|
|
18
|
Gupta S: Molecular steps of death receptor
and mitochondrial pathways of apoptosis. Life Sci. 69:2957–2964.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Werns SW and Lucchesi BR: Myocardial
ischemia and reperfusion: the role of oxygen radicals in tissue
injury. Cardiovasc Drugs Ther. 2:761–769. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bolli R: Oxygen-derived free radicals and
postischemic myocardial dysfunction (‘stunned myocardium’). J Am
Coll Cardiol. 12:239–249. 1988.
|
|
21
|
Ren G, Dewald O and Frangogiannis NG:
Inflammatory mechanisms in myocardial infarction. Curr Drug Targets
Inflamm Allergy. 2:242–256. 2003. View Article : Google Scholar
|
|
22
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antonsson B: Bax and other pro-apoptotic
Bcl-2 family ‘killer-proteins’ and their victim the mitochondrion.
Cell Tissue Res. 306:347–361. 2001.
|
|
24
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|