Introduction
Ankylosing spondylitis, a chronic inflammatory
rheumatic disease, is characterized by inflammation of the spine
and the sacroiliac joints, which may induce new bone formation in
the affected areas (1,2). The development of spinal
syndesmophytes, resulting in ankylosis between vertebral bodies, is
a common symptom of the disease. Upon ankylosis of the vertebral
bodies, the entire spine is distorted and may form a ‘bamboo spine’
(3). Ankylosing
spondylitis-induced structural damage triggers further damage
involving changes in the physical pathology and mobility of the
spine. Clinical therapy and diagnosis is mainly based on the
radiographic progression of ankylosing spondylitis (4). Radiographic imaging is the most
popular and convenient method used in the diagnosis and prognosis
estimation of the disease. However, only severe ankylosing
spondylitis-induced spinal damage can be identified through
radiographic detection (5). Thus,
understanding the molecular progression of ankylosing spondylitis
would aid early diagnosis and treatment during pathogenesis.
To date, the cause of the disease remains unclear.
The two main characteristics of ankylosing spondylitis are
inflammation and bone reformation (6,7).
Previous studies have demonstrated that inflammation is induced by
bone reformation (8). In addition,
several studies have indicated that genetic influence of the
HLA-B27 gene is responsible for ankylosing spondylitis (9,10).
HLA-B27 and bacterial infection have been considered to play a
crucial role in the pathogenesis of spondyloarthritis (11). This viewpoint was supported by the
findings of studies on Chlamydia trachomatis,
Shigella, Salmonella, Yersinia and
Campylobacter, which were shown to result in
spondyloarthritis (12).
Furthermore, T cell have been found to respond to aggrecan in
ankylosing spondylitis (13),
indicating that T cells plays a key role in the pathogenesis of
ankylosing spondylitis. Previous studies demonstrated that the T
cell subtypes, CD4+ and CD8+, may be
triggered by aggrecan in the blood and synovial fluid specimens
(13,14). In addition, tumour necrosis
factor-α (TNF-α), an indicator of inflammation, has been found to
be highly expressed in the sacroiliac joints, indicating that
ankylosing spondylitis progression may be associated with the
degree of inflammation (15).
While the pathogenic cause of ankylosing spondylitis remains
unclear, previous studies have revealed a T cell response to
aggrecan, which provides valuable information on the disease
pathogenesis.
T lymphocytes are considered to be crucial cells in
the regulation of the immune system (16). Based on the receptors on T
lymphocyte membranes, the cells are divided into a number of
subtypes, including CD4+ and CD8+ cells.
CD4+ cells are divided further into T helper (Th)1, Th2,
Th17 and regulatory T (Treg) subsets, while CD8+ cells
are divided into T cytotoxic (Tc)1, Tc2 and Tc17 subsets (17). Furthermore, Th1 and Tc1 cells
secrete type 1 cytokines, including interleukin (IL)-2, TNF-α and
interferon (IFN)-γ, while Th2 and Tc2 cells secrete type 2
cytokines, including IL-4, IL-5 and IL-13 (18). Other cytokines, including IL-10 and
transforming growth factor (TGF)-β, are secreted by Treg cells,
while IL-17 is secreted by Th17 and Tc17 cells (19,20).
The specific secretion of different T lymphocytes cells is balanced
in healthy individuals.
The aim of the present study was to: (i) Understand
the balance changes in the secretion of T lymphocyte subtypes in
ankylosing spondylitis patients; and (ii) illustrate the expression
level changes of inflammation mediators, including IFN-γ, IL-17A,
IL-4 and TGF-β, in ankylosing spondylitis patients. The study
results provide evidence for future diagnostic and therapeutic
strategies of ankylosing spondylitis.
Materials and methods
Clinical samples and preparation
All the clinical samples were obtained from the
Department of Orthopaedics, Xiangya Hospital of Central South
University (Changsha, China). In total, 55 patients, confirmed (via
physical, X-ray and blood examinations) to suffer from ankylosing
spondylitis, participated in the study from June 2011 to July 2013
and the disease severity was diagnosed according to the guidelines
of the German Spondyloarthritis Inception Cohort (Table I) (Rudwaleit). Spinal radiographs
(Multix Select DR; Siemens AG Healthcare, Erlangen, Germany), were
obtained from the 55 ankylosing spondylitis patients. In addition,
20 healthy individuals with no symptoms of ankylosing spondylitis
were enrolled into the study as the control group. Informed written
consent was obtained from all patients/patients’ families and
healthy individuals prior to participation in the present study.
The clinical sample collection was approved by the Ethics Committee
of the Xiangya Hospital of Central South University.
 | Table ISummary of study group characteristics
and observed expressions. |
Table I
Summary of study group characteristics
and observed expressions.
| Variable | Control group | Mild ankylosing
spondylitis group | Severe ankylosing
spondylitis group |
|---|
| Number of samples
(n) | 20 | 23 | 22 |
| Age (years) | 55.05±6.42 | 52.31±8.24 | 51.22±6.78 |
| Gender, male/female
(n) | 10/10 | 12/11 | 11/11 |
| Duration of disease
(years) | - | 2.7±2.56 | 3.4±2.45 |
| α-globulin
(g/cl) | 0.22±0.05 | 0.52±0.12a | 0.68±0.08a |
| γ-globulin
(g/cl) | 0.37±0.12 | 0.98±0.29a | 0.78±0.11a |
| IgG (mg/cl) | 827.23±121.69 | 1328±48.90a | 1438±104.87a |
| IgA (mg/cl) | 12.55±1.95 | 21.69±2.62a | 29.62±3.58a |
| IgM (mg/cl) | 10.64±2.69 | 25.62±1.29a | 31.57±2.02a |
| Serum complement C3
(U/ml) | 88.65±10.62 |
152.70±26.62a |
180.59±20.55a |
| Serum complement C4
(U/ml) | 70.52±20.64 |
198.85±38.25a |
201.92±44.52a |
Punctured cells were collected from the patient
lesions and stored at −80°C prior to the assay. A total of 5 ml
serum was obtained from each patient which was subsequently
centrifuged at 724 × g for 10min and 10–25°C (Eppendorf 5415C;
Eppendorf, Hamburg, Germany). The lymphocytes were prepared using a
lymphocyte kit (Lymphoprep™ Axis-Shield PoC AS, Oslo,
Norway). The peripheral blood mononuclear cells were prepared in
RPMI 1640 containing 10% fetal bovine serum (Gibco Life
Technologies, Grand Island, NY, USA).
Quantitative polymerase chain reaction
(PCR)
Gene expression levels were detected using
quantitative PCR. The samples were homogenized and total RNAs were
isolated using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Gene expression level detection was performed
using the 7500 Real-Time PCR System (Applied Biosystems Life
Technologies, Foster City, CA, USA). The PCR conditions were as
follows: DNA degeneration at 95°C for 5 sec, followed by primer
annealing at 60°C for 40 sec and primer extension at 70°C for 90
sec. These steps were repeated for 40 cycles. The gene expression
levels were calculated using the SDS software 1.3 (Applied
Biosystems, Grand Island, NY, USA) of the PCR device. Melting-curve
analysis was performed to confirm the specificity of the
amplification products.
Enzyme-linked immunosorbent assay
(ELISA)
The serum levels of IFN-γ, IL-4, TGF-α (all
Quantikine; R&D Systems, Inc., Minneapolis, MN, USA) and IL-17A
(Heterodimer DuoSet, 5 Plate; R&D Systems, Inc.) were detected
using ELISA kits according to the manufacturer’s instructions. The
expression levels of the immune factors were assayed in triplicate
to improve accuracy.
Flow cytometric analysis
T lymphocyte subsets were analyzed by flow cytometry
(MoFlo® Astrios™; Beckman Coulter, Fullerton,
CA, USA). The cells were stained using PerCP-Cy5.5-labeled
anti-human CD3 (Anti-Human CD3 PerCP-Cyanine5.5; eBioscience, San
Diego, CA, USA) and fluorescein isothiocyanate (FITC)-labeled
anti-human CD8 (Anti-Human CD8a; eBioscience). Treg cells were
identified using FITC-labeled anti-human CD4 (eBioscience). The
various T lymphocyte subsets were calculated using the antibody
signals of the specific proteins.
Statistical analysis
The data were statistically analyzed using the SPSS
version 17.0 software (SPSS, Inc., Chicago, IL, USA) and are
displayed as the mean ± standard error of mean. Analysis of the
significant differences among the study groups was performed by
one-way analysis of variance. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Case summary
In total, 23 patients were found to suffer from mild
ankylosing spondylitis (including normal or narrow joint clearance,
no ‘bamboo spine’ and a bone dense region present), whereas 22
patients were found to suffer from severe ankylosing spondylitis
(including no joint clearance, a ‘bamboo spine’ and no bone dense
region present). As shown in Table
I, no statistically significant differences were observed in
the age, gender and duration of the disease between the two
ankylosing spondylitis groups. The expression levels of α-globulin,
γ-globulin, immunoglobulin (Ig)G, IgA, IgM, serum complement C3 and
C4 were found to be significantly increased in ankylosing
spondylitis patients. In addition, the expression levels of these
indexes were found to be higher in severe ankylosing spondylitis
patients compared with mild ankylosing spondylitis patients
(Table I).
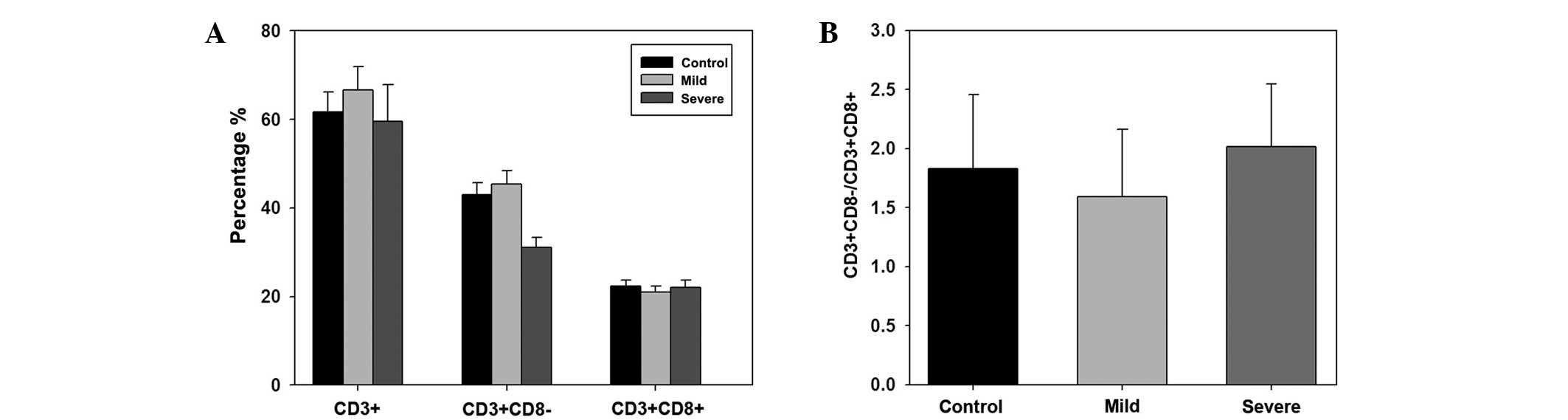
The T lymphocyte subset content was analyzed in the
control, mild ankylosing spondylitis and severe ankylosing
spondylitis groups. In total, in the control group, the percentage
of CD3+, CD3+CD8− and
CD3+CD8+ T lymphocyte subset cells were
61.60±4.61, 43.01±2.63 and 22.31±1.36%, respectively. In the mild
ankylosing spondylitis group, the percentage of CD3+,
CD3+CD8− and CD3+CD8+ T
lymphocyte subset cells were 66.62±5.32, 45.32±3.01 and
21.06±1.30%, respectively. In the severe ankylosing spondylitis
group, the percentage of CD3+,
CD3+CD8− and CD3+CD8+ T
lymphocyte subset cells were 59.61±8.20, 31.03±2.30 and
22.03±1.69%, respectively. However, no statistically significant
differences were observed among the groups (Fig. 1A). In addition, the
CD3+CD8−/CD3+CD8+ ratio
in each group was calculated. The results indicated no
statistically significant differences among the ratios of the three
groups (Fig. 1B).
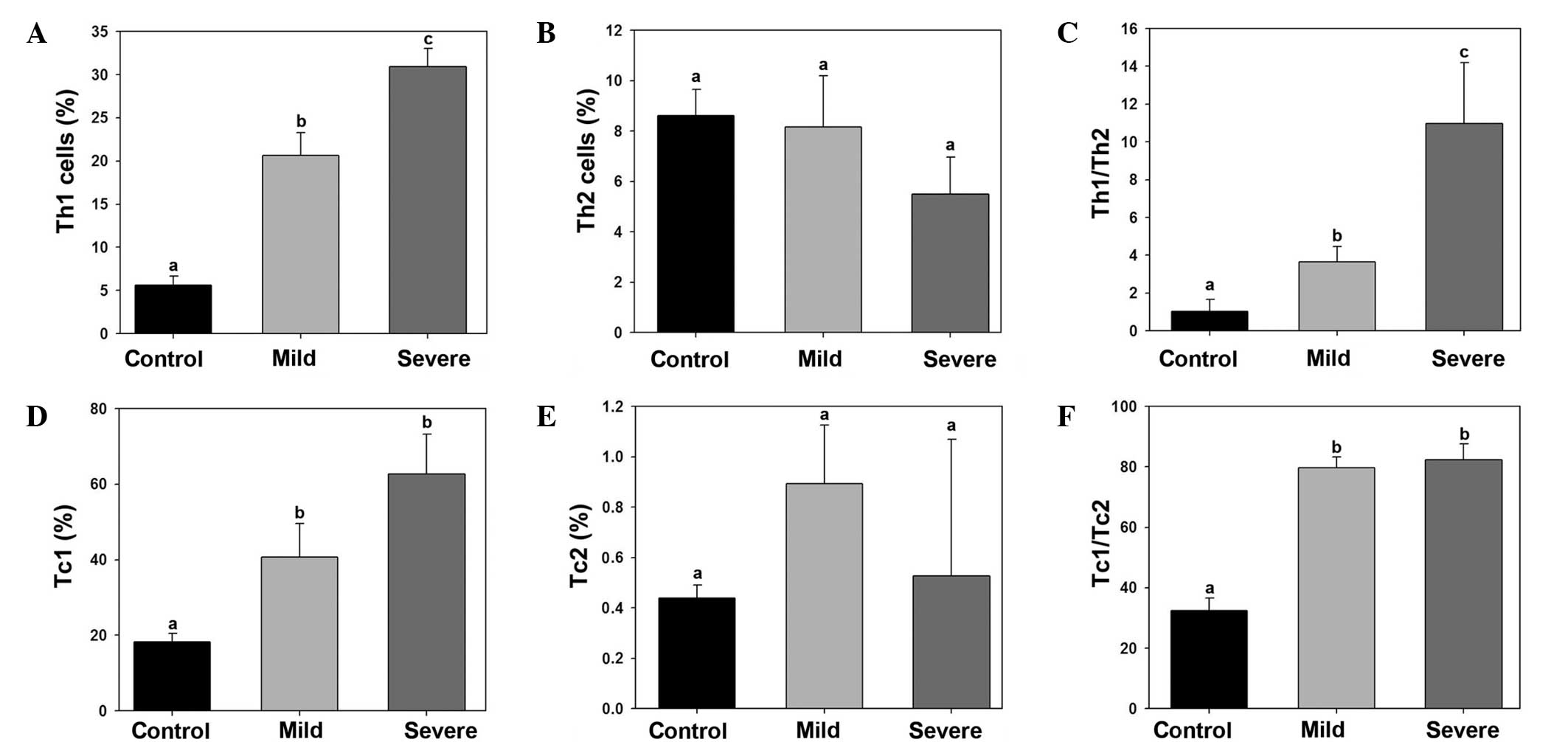
Imbalance of Th1/Th2 and Tc1/Tc2 in
ankylosing spondylitis patients
The percentage of Th1, Th2, Tc1 and Tc2 cells in the
control, mild ankylosing spondylitis and severe ankylosing
spondylitis samples were calculated. The Th1 cell percentage was
significantly increased in the mild and severe ankylosing
spondylitis group (Fig. 2A). By
contrast, no statistically significant differences were observed in
the percentage of Th2 cells among the groups. Thus, the Th1/Th2
ratio was significantly higher in the mild and severe ankylosing
spondylitis groups (Fig. 2C). The
number of Tc1 cells was found to be significantly higher in the
mild and severe ankylosing spondylitis groups compared with the
control group (Fig. 2D). However,
no statistically significant differences were observed in the
percentage of Tc2 cells among the groups. Therefore, the Tc1/Tc2
ratio was significantly higher in the mild and severe ankylosing
spondylitis groups compared with the control group (Fig. 2F).
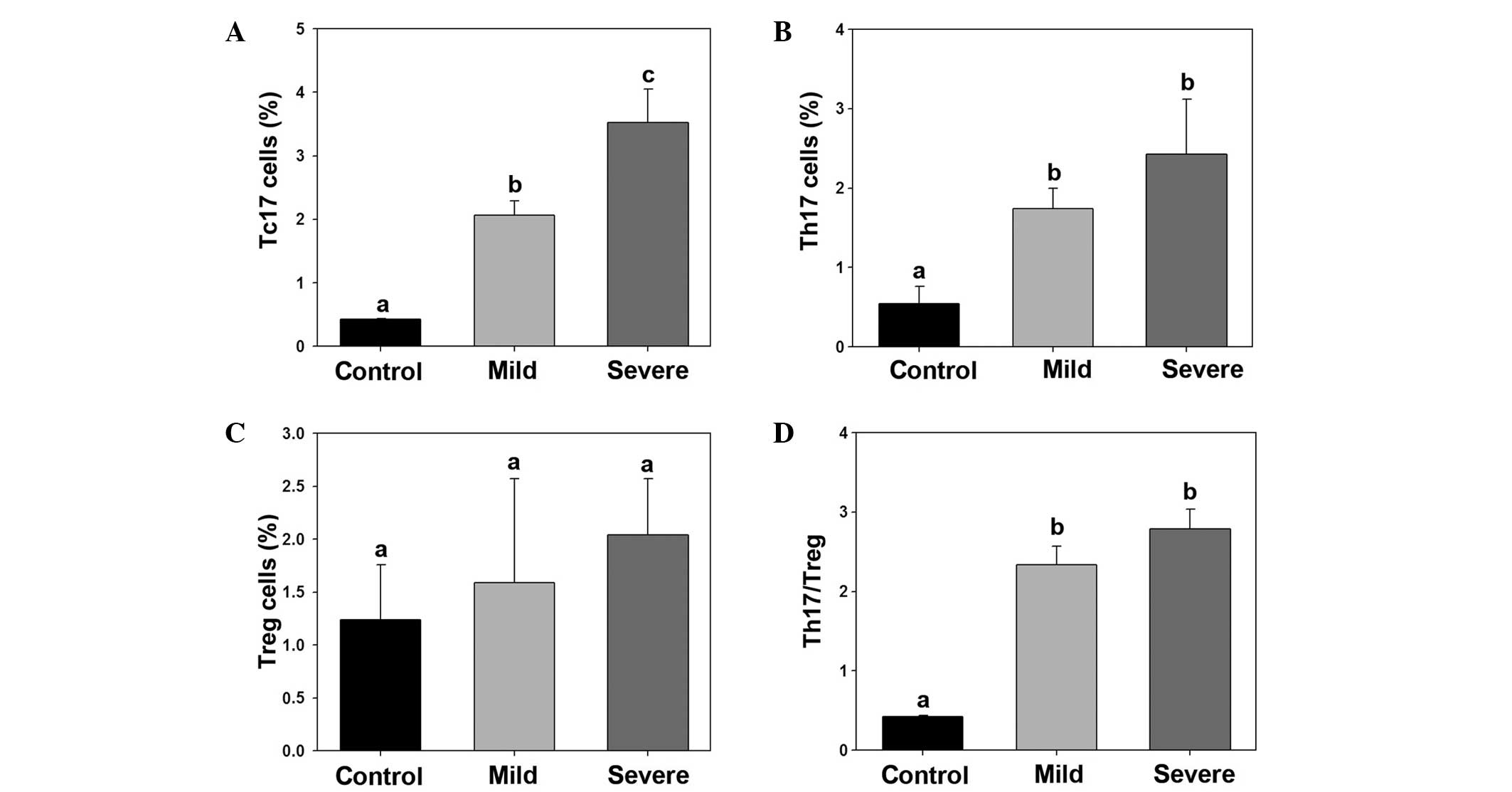
Imbalance of Th17/Treg in ankylosing
spondylitis patients
The percentage of Tc17, Th17 and Treg cells was
calculated in the control, mild ankylosing spondylitis and severe
ankylosing spondylitis groups. The percentage of Tc17 and Th17
cells were significantly increased in the mild and severe
ankylosing spondylitis groups, whereas the percentage of Treg cells
was not found to be significantly different among the groups. Thus,
the Th17/Treg ratio was found to be significantly higher in the
mild and severe ankylosing spondylitis groups (Fig. 3).
Cytokine expression level changes in
ankylosing spondylitis patients
The expression levels of IFN-γ, IL-17A, IL-4 and
TGF-β were detected in the lesion punctured cells and the serum
using quantitative PCR and ELISA, respectively. The mRNA expression
levels in punctured cells and the serum protein expression levels
of IFN-γ and IL-17A were found to be significantly higher in the
mild and severe ankylosing spondylitis groups compared with the
control group. In addition, the mRNA expression levels of IL-4 and
TGF-β were found to be higher in punctured cells of the mild and
severe ankylosing spondylitis groups. By contrast, no statistically
significant differences were observed in the serum protein
expression levels of these cytokines among the three groups.
Discussion
A number of studies have indicated that certain
pathogenic inflammation is associated with the development of
ankylosing spondylitis (21,22).
Although understanding the inflammatory mechanism of the disease
progression is essential, the adaptive immunity of ankylosing
spondylitis in patients has been rarely studied. The results of the
present study demonstrate the variations in T lymphocyte subsets in
mild and severe ankylosing spondylitis patients. From the results
of this study, there does not appear to be an association between
the number of T lymphocyte subset cells (CD3+, CD3+CD8− and
CD3+CD8+) and the occurrence or severity of ankylosing spondylitis.
Th1, Th17, Tc1 and Tc17 cell percentages were found to be
significant higher in ankylosing spondylitis patients compared with
the control group. In addition, the specific expression of immune
mediators, such as IFN-γ and IL-17A, was found to be significantly
increased in the plasma of ankylosing spondylitis patients compared
with the control group. The results indicate that the progression
of ankylosing spondylitis is associated with the imbalance of T
lymphocyte subset. In 2009, Lin et al investigated the
imbalance of blood B-cell subsets in ankylosing spondylitis
patients (23). The imbalance of
serum B-cell subsets was found to promote the development of
ankylosing spondylitis and result in joint proliferation.
Therefore, the increasing number of CD19+ cells was
considered to be responsible for the progression of ankylosing
spondylitis. Wu et al identified Th/Treg cell imbalance in
ankylosing spondylitis patients and hypothesized that
immunomodulation may contribute to the pathogenesis of ankylosing
spondylitis through Th/Treg cell misbalance (24). Therefore, the T lymphocyte subset
imbalance is a key factor in the incidence of ankylosing
spondylitis pathogenesis.
In 2003, Kidd hypothesized that Th1/Th2 is
associated with human health and disease progression development
(25). Th1 and Th2 cells
participate in various pathways of the immune system. Th1 cells
drive the cellular immunity, namely the type-1 pathway, while Th2
cells drive the humoral immunity, namely the type-2 pathway. Th1
cells play a crucial role in disease pathogenesis and can cause
cell death in tumors, while Th2 cells have been shown to upregulate
antibody production. Changes in the Th1/Th2 ratio may alter the
immunological balance, which is a suitable model for a number of
diseases, including diabetes, cancer and pathogen infection
(26–29). However, the Th1/Th2 ratio is not
suitable for certain diseases, such as rheumatoid arthritis or
asthma (30). In the present
study, the Th1/Th2 ratio was significantly increased in patients
with ankylosing spondylitis. In addition, the ratio was higher in
severe ankylosing spondylitis patients compared with mild
ankylosing spondylitis patients. Therefore, the Th1/Th2 balance and
Th17/Treg balance were disturbed during the disease. A number of
studies have reported that the Treg and Th17 subsets are associated
with multidirectional immunology, which is in accordance with the
results of the current study (31–33).
Thus, Th1/Th2 and Th17/Treg imbalances may be due to the
development of ankylosing spondylitis, indicating that these
subsets may be crucial in the progression of the disease,
particularly in severe ankylosing spondylitis. In the present
study, the percentage of Th17 cells was found to increase in
patients with ankylosing spondylitis. Subsequently, the specific
expression of IL-17A (expressed by Th17 cells) was significantly
stimulated. Considering that IL-17A plays an important role in the
inflammatory response induced by neutrophil activation, the
upregulation of Th17 cells in patients with ankylosing spondylitis
is a crucial inflammatory pathway (34). The observations in the present
study indicate that changes in the Th1/Th2 and Th17/Treg ratios are
key evidence for the suffering of ankylosing spondylitis.
Furthermore, the percentage of Th17 cells and expression level of
IL-17A were found to be significantly higher in ankylosing
spondylitis patients, while the protein levels of IL-4 and TGF-β
were unchanged. The percentage of Treg cells was also unchanged in
ankylosing spondylitis patients compared with the control group,
indicating that Treg cells may not participate in the disease
progression, as opposed to Th17 cells.
In the present study, the protein levels of IL-4 and
TGF-β were found to be unchanged in ankylosing spondylitis patients
(mild and severe) compared with the control group, which is in
accordance with a previous study (35). Therefore, IL-4 and TGF-β may not
participate in the progression of ankylosing spondylitis. Further
studies are required to fully investigate the effect and underlying
mechanism of T lymphocyte subsets in patients with ankylosing
spondylitis.
In conclusion, imbalances in the T lymphocyte subset
ratios, Th1/Th2 and Th17/Treg, were demonstrated in patients with
ankylosing spondylitis. These imbalances resulted in increased mRNA
and protein expression levels of immune mediators, particularly
IFN-γ and IL-17A. The present study provided further evidence on
the function and underlying mechanism of T lymphocyte subsets,
which may be useful in the diagnosis and treatment of ankylosing
spondylitis.
References
|
1
|
Calin A, Porta J, Fries JF and Schurman
DJ: Clinical history as a screening test for ankylosing
spondylitis. JAMA. 237:2613–2614. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gran JT and Skomsvoll JF: The outcome of
ankylosing spondylitis: a study of 100 patients. Br J Rheumatology.
36:766–771. 1997. View Article : Google Scholar
|
|
4
|
Van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Graham B and Van Peteghem PK: Fractures of
the spine in ankylosing spondylitis. Diagnosis, treatment, and
complications. Spine (Phila Pa 1976). 14:803–807. 1989. View Article : Google Scholar
|
|
6
|
Braun J, Brandt J, Listing J, et al:
Treatment of active ankylosing spondylitis with infliximab: a
randomised controlled multicentre trial. Lancet. 359:1187–1193.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akhtar S, O’Flynn PE, Kelly A and
Valentine PM: The management of dysphasia in skeletal hyperostosis.
J Laryngol Otol. 114:154–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imhof H and El-Khoury GY: Traumas of the
Axial Skeleton. Musculoskeletal Diseases. Springer; Milan: pp.
112–120. 2005, View Article : Google Scholar
|
|
9
|
Van der Linden SM, Valkenburg HA, de Jongh
BM and Cats A: The risk of developing ankylosing spondylitis in
HLA-B27 positive individuals. A comparison of relatives of
spondylitis patients with the general population. Arthritis Rheum.
27:241–249. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brown MA, Kennedy LG, MacGregor AJ, et al:
Susceptibility to ankylosing spondylitis in twins: the role of
genes, HLA, and the environment. Arthritis Rheum. 40:1823–1828.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobs JC, Berdon WE and Johnston AD:
HLA-B27-associated spondyloarthritis and enthesopathy in childhood:
clinical, pathologic, and radiographic observations in 58 patients.
J Pediatr. 100:521–528. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leirisalo-Repo M: Reactive arthritis.
Scand J Rheumatol. 34:251–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atagunduz P, Appel H, Kuon W, et al:
HLA-B27-restricted CD8+ T cell response to
cartilage-derived self peptides in ankylosing spondylitis.
Arthritis Rheum. 52:892–901. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Braun J, Khan MA and Sieper J: Enthesitis
and ankylosis in spondyloarthropathy: what is the target of the
immune response? Ann Rheum Dis. 59:985–994. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brandt J, Haibel H, Cornely D, et al:
Successful treatment of active ankylosing spondylitis with the
anti-tumor necrosis factor alpha monoclonal antibody infliximab.
Arthritis Rheum. 43:1346–1352. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reinherz EL and Schlossman SF: The
differentiation and function of human T lymphocytes. Cell.
19:821–827. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sallusto F, Lenig D, Förster R, Lipp M and
Lanzavecchia A: Two subsets of memory T lymphocytes with distinct
homing potentials and effector functions. Nature. 401:708–712.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lucey DR, Clerici M and Shearer GM: Type 1
and type 2 cytokine dysregulation in human infectious, neoplastic,
and inflammatory diseases. Clin Microbiol Rev. 9:532–562.
1996.PubMed/NCBI
|
|
19
|
Wilke CM, Bishop K, Fox D and Zou W:
Deciphering the role of Th17 cells in human disease. Trends
Immunol. 32:603–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yen HR, Harris TJ, Wada S, et al: Tc17 CD8
T cells: functional plasticity and subset diversity. J Immunol.
183:7161–7168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szalay B, Mészáros G, Cseh Á, et al:
Adaptive immunity in ankylosing spondylitis: phenotype and
functional alterations of T-cells before and during infliximab
therapy. Clin Dev Immunol. 2012:8087242012. View Article : Google Scholar
|
|
22
|
Raffeiner B, Dejaco C, Duftner C, et al:
Between adaptive and innate immunity: TLR4-mediated perforin
production by CD28null T-helper cells in ankylosing spondylitis.
Arthritis Res Ther. 7:R1412–R1420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Q, Gu JR, Li TW, et al: Value of the
peripheral blood B-cells subsets in patients with ankylosing
spondylitis. Chin Med J (Eng). 122:1784–1789. 2009.
|
|
24
|
Wu Y, Ren M, Yang R, et al: Reduced
immunomodulation potential of bone marrow-derived mesenchymal stem
cells induced CCR4+CCR6+ Th/Treg cell subset
imbalance in ankylosing spondylitis. Arthritis Res Ther.
13:R292011. View
Article : Google Scholar
|
|
25
|
Kidd P: Th1/Th2 balance: the hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
26
|
Yao S, Hong CC, McCann SE, et al: Combined
effects of circulating levels of 25-hydroxyvitamin d and Th1 and
th2 cytokines on breast cancer estrogen receptor status. Cancers
(Basel). 6:211–225. 2014. View Article : Google Scholar
|
|
27
|
Hou N, Zhang X, Zhao L, et al: A novel
chronic stress-induced shift in the Th1 to Th2 response promotes
colon cancer growth. Biochem Biophys Res Commun. 439:471–476. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gut W, Pancer K, Abramczuk E, et al: RSV
respiratory infection in children under 5 y.o - dynamics of the
immune response Th1/Th2 and IgE. Przegl Epidemiol. 67:17–22.
2013.
|
|
29
|
Vitry MA, De Trez C, Goriely S, et al:
Crucial role of gamma interferon-producing CD4+ Th1 cells but
dispensable function of CD8+ T cell, B cell, Th2, and Th17
responses in the control of Brucella melitensis infection in mice.
Infect Immun. 80:4271–4280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steinman L: A brief history of T(H)17, the
first major revision in the T(H)1/T(H)2 hypothesis of T
cell-mediated tissue damage. Nat Med. 13:139–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yasukawa S, Dainichi T, Kokuba H, et al:
Bullous pemphigoid followed by pustular psoriasis showing Th1, Th2,
Treg and Th17 immunological changes. Eur J Dermatol. 19:69–71.
2009.
|
|
32
|
Bryant C, Suen H, Brown R, et al:
Long-term survival in multiple myeloma is associated with a
distinct immunological profile, which includes proliferative
cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood
Cancer J. 3:e1482013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaur P, Qadir GA, Upadhyay S, Singh AK,
Shukla NK and Das SN: Skewed immunological balance between Th17
(CD4(+)IL17A (+)) and Treg (CD4 (+)CD25 (+)FOXP3 (+)) cells in
human oral squamous cell carcinoma. Cell Oncol (Dordr). 35:335–343.
2012. View Article : Google Scholar
|
|
34
|
Iwakura Y, Nakae S, Saijo S and Ishigame
H: The roles of IL-17A in inflammatory immune responses and host
defense against pathogens. Immunol Rev. 226:57–79. 2008. View Article : Google Scholar
|
|
35
|
Cañete JD, Martínez SE, Farrés J, et al:
Differential Th1/Th2 cytokine patterns in chronic arthritis:
interferon gamma is highly expressed in synovium of rheumatoid
arthritis compared with seronegative spondyloarthropathies. Ann
Rheum Dis. 59:263–268. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rudwaleit M, Haibel H, Baraliakos X, et
al: The early disease stage in axial spondylarthritis: results from
the German Spondyloarthritis Inception Cohort. Arthritis Rheum.
60:717–27. 2009. View Article : Google Scholar : PubMed/NCBI
|