Introduction
Paraquat (1,1′-dimethyl-4,4′-bipyridinium
dichloride; PQ) is a widely-utilized non-selective herbicide for
the control of broadleaf weed. Available therapies for PQ poisoning
lack efficacy, and it is a major cause of herbicide-related
mortality, which occurs due to respiratory failure secondary to
lung injury (1). Although the
specific mechanisms of PQ toxicity have not been fully defined, it
is hypothesized that PQ toxicity involves the generation of
reactive oxygen species (ROS), leading to subsequent oxidative
stress, which results in cell death and lung tissue damage
(1,2). Antioxidants that directly inhibit ROS
generation have been proposed as possible therapeutic agents in
PQ-induced lung injury. However, traditional antioxidants, such as
glutathion and vitamin C, have failed to protect against the
characteristic pathological lung changes or mortality observed in
PQ poisoning (1). Therefore,
mechanisms other than oxidative stress may also be responsible for
the pulmonary toxicity of PQ. The identification of such mechanisms
may yield insight into the development of novel therapeutic agents
and is an important area of research.
Adiponectin (also termed Acrp30, AdipoQ and GBP28)
is a protein predominantly secreted from adipose tissue (3–5).
Primary sequence analysis has revealed that full-length adiponectin
has four main domains, with the globular segment (gAd) at the
carboxy terminus being much more potent than the full protein
(6,7). Adiponectin has been demonstrated to
exhibit anti-oxidative and anti-inflammatory effects (8). The mechanisms of its actions are
varied and depend upon the site of activity. In endothelial cells,
adiponectin enhances nitric oxide (NO) production, suppresses the
production of ROS and protects against inflammation mediated by
hyperglycemic states or tumor necrosis factor, via activation of
AMP-activated protein kinase and cyclic AMP-dependent protein
kinase signaling cascades (9). It
was hypothesized that globular domain adiponectin (gAd) may protect
against PQ-induced lung injury by attenuating oxidative/nitrative
stress.
The present study aimed to determine whether gAd
protects against PQ-induced pulmonary injury in BALB/c mice and to
determine the underlying mechanism or mechanisms of action.
Ultimately the aim would be to assess the potential of adiponectin
as a novel therapeutic agent in PQ-induced lung injury.
Materials and methods
Materials
PQ was obtained from Tokyo Kasei Kogyo Co., Ltd.
(Tokyo, Japan). Recombinant mouse gAd was obtained from
Adipobiotech Inc. (Beijing, China). Enzyme-linked immunosorbent
assay (ELISA) kits for 3-Nitrotyrosine (3-NT) were obtained from
Abcam (Cambridge, MA, USA). 8-hydroxy-2-dydeoxy guanosine (8-OHdG)
EIA and superoxide dismutase (SOD) assay kits were obtained from
Cayman Chemical Co. (Ann Arbor, MI, USA). Dihydroethidium (DHE)
probes were obtained from Merck Millipore (Darmstadt, Germany).
Malondialdehyde (MDA) EIA kits were obtained from BioVision, Inc,
(Milpitas CA, USA). The NO ELISA kit was obtained from 4A Biotech
Co., Ltd. (Beijing, China).
Animals and treatment
Male BALB/c mice were obtained from Dossy Biological
Technology Co., Ltd. (Chengdu, China). They were housed at 22±2°C
in a humidity-controlled room with free access to fresh water and
standard laboratory food. Following 1 week of conditioning in a 12
h light/dark cycle, eighty male mice were randomly divided into
four groups: (i) Control group (saline injection); (ii) PQ +
low-gAd group (PQ exposure combined with gAd pre-treatment at 500
μg/kg by tail vein injection at 12 and 36 h prior to PQ
administration); (iii) PQ + high-gAd group (PQ exposure combined
with gAd pre-treatment at 1000 μg/kg by tail vein injection at 12
and 36 h prior to PQ administration); and (iv) PQ (20 mg/kg) group,
intraperitoneal administration (IP). The dose for high/low gAd was
determined from preliminary experiments (data not shown). Mice were
anesthetized with 50 mg/kg pentobarbital (Hanlim Pharm. Co. Ltd.,
Seoul, Korea), IP. Serum and pulmonary samples were collected at 3,
6, 12, 24 and 72 h post-PQ injection. The study was conducted in
accordance with the ethical standards in the 1986 Directive
86/609/EEC, European Convention for the Protection of Vertebrate
Animals Used for Experimental and other Scientific Purposes, and
the Guiding Principles in the Use of Animals in Toxicology, adopted
by the Society of Toxicology in 1989. The study was approved by the
Committee on the Ethics of Animal Experiments of the Sichuan
University (permit no. 26).
Histopathological assessment of pulmonary
tissue
Pulmonary tissue, fixed in 10% neutral-buffered
formalin, was blocked in paraffin using an automated processor
(Leica, Nussloch□Germany), with graded alcohol, xylene and
paraffin. Sections (4 μm) were stained using hematoxylin and eosin.
Digital images of the stained glass slides were obtained using a
ScanScope Digital slide scanner (Aperio Technologies, Inc., Vista,
CA, USA) at a magnification of ×100.
Measurement of superoxide anion in
pulmonary tissue
Lung segments obtained from control and PQ-treated
mice were embedded in tissue freezing medium (Tris-buffered saline;
Thermo Fisher Scientific, Waltham, MA, USA). Following freezing,
30-μm segments were cut, and mounted and cover-slipped on glass
slides. Sections were treated with 2 μmol/l DHE in dimethyl
sulfoxide buffer. Slides were incubated in a light-protected
chamber at 37°C for 30 min. Ethidium-stained tissue was observed by
inverted fluorescent microscopy at a magnification of ×100 (Olympus
IX71, Olympus, Tokyo, Japan) following excitation (485 nm) and
emission (535 nm). Control and PQ-treated pulmonary samples were
processed and imaged in parallel.
Measurement of pulmonary tissue MDA, SOD,
NO and 8-OHdG levels, and blood 3-NT levels
Pulmonary tissue levels of MDA, SOD, NO and 8-OHdG,
and blood levels of 3-NT from control and PQ-treated mice were
determined using ELISA kits, according to the manufacturer’s
instructions.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Comparisons between groups at each time point were made
by one-way analysis of variance, followed by Student-Newman-Keul’s
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
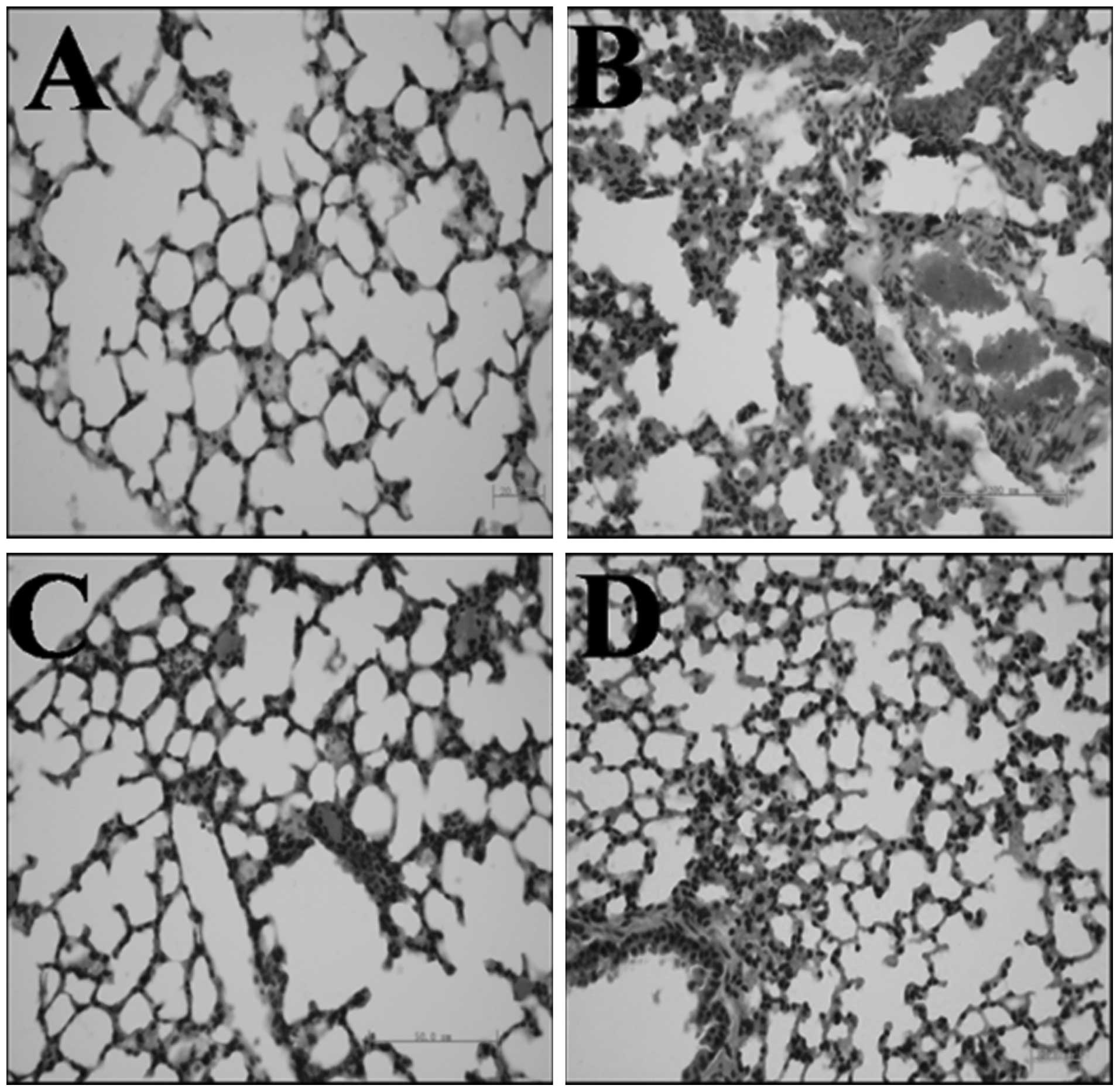
gAd attenuated PQ-mediated pulmonary
interstitial edema and inflammatory cell infiltration in a
dose-dependent manner
Compared with the control group (Fig. 1A), PQ administration caused acute
injury to pulmonary tissue, as demonstrated by the interstitial
edema and inflammatory cell infiltration (lymphocytes and
histiocytes) observed in the alveolar space and septum (Fig. 1B). Pre-treatment with gAd inhibited
these changes (Fig. 1C and D) in a
dose-dependent manner.
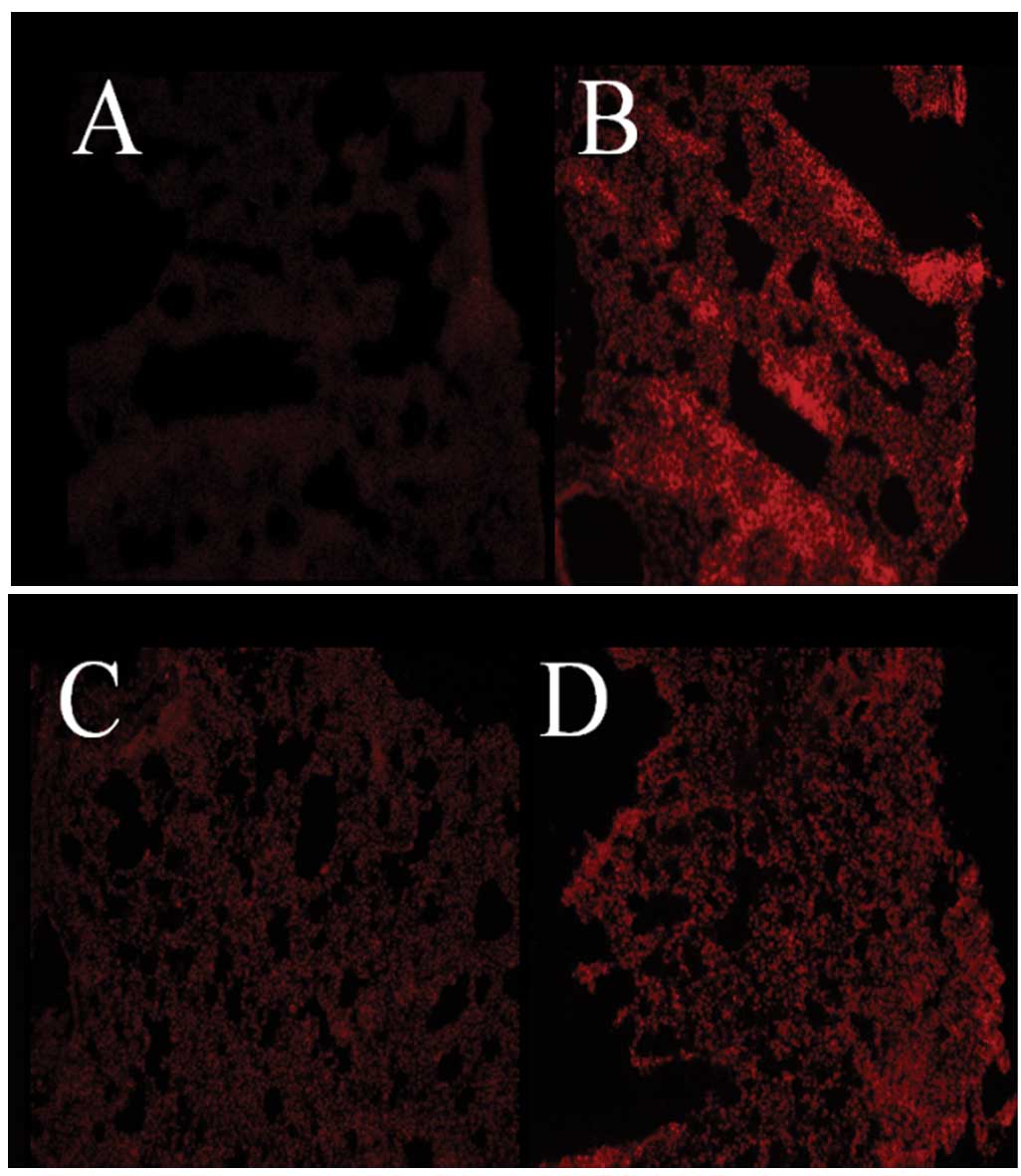
gAd reduced PQ-induced pulmonary
oxidative injury in a dose-dependent manner
Three series of experiments were performed to
determine the effects of PQ and gAd on oxidative stress in the
mouse lung. In the first series, in situ
O2•− generation was detected by DHE staining.
Weak DHE staining was observed in the pulmonary tissue of control
animals, indicating the basal pulmonic O2•−
production (Fig. 2A). The staining
was significantly intensified in mice subjected to PQ
administration (Fig. 2B). By
contrast, gAd (at high and low doses, Fig. 2C and D) decreased this augmented
staining.
In the second series of experiments, pulmonary
tissue MDA content was found to be significantly increased in mice
subjected to PQ administration at as early as 3 h following initial
PQ exposure (Fig. 3). This
increase was eliminated from 6 to 72 h following the initial PQ
exposure, by pre-treatment with gAd.
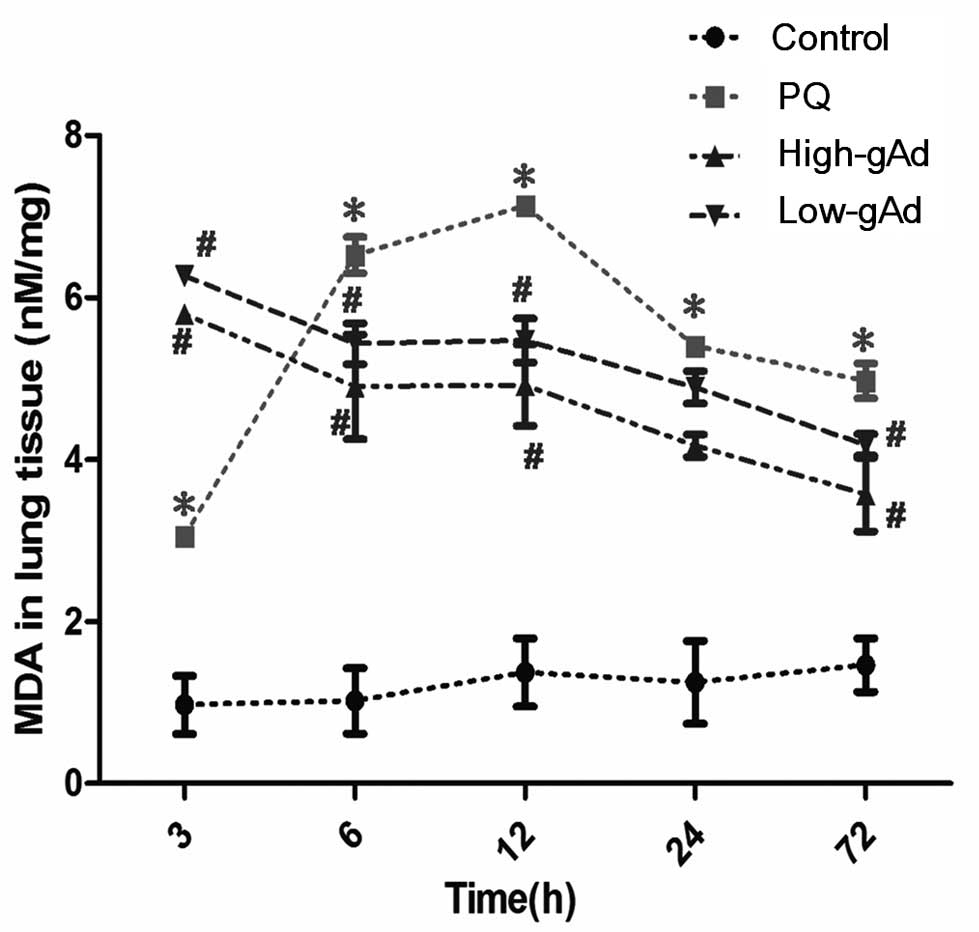 | Figure 3Effect of gAd pretreatment on
pulmonary MDA level in control animals or PQ-exposed animals,
treated with vehicle or gAd (high-dose or low-dose) at 0, 3, 6, 12,
24 and 72 h after PQ injection. MDA levels were determined by an
enzyme-linked immunosorbent assay. Values represent the mean ±
standard error of the mean of four parallel measurements.
*P<0.05, compared with controls and
#P<0.05, compared with the PQ group. gAd, globular
domain adiponectin; MDA, malondialdehyde; PQ, paraquat. |
In the third series of experiments, pulmonary SOD
levels were found to be significantly increased at all time points
measured in animals subjected to PQ administration compared with
the control group (Fig. 4).
Notably, the augmented SOD activity was further amplified by
pre-administration of gAd at 3 h, although it was decreased from 6
to 72 h, in accordance with the trend in MDA levels.
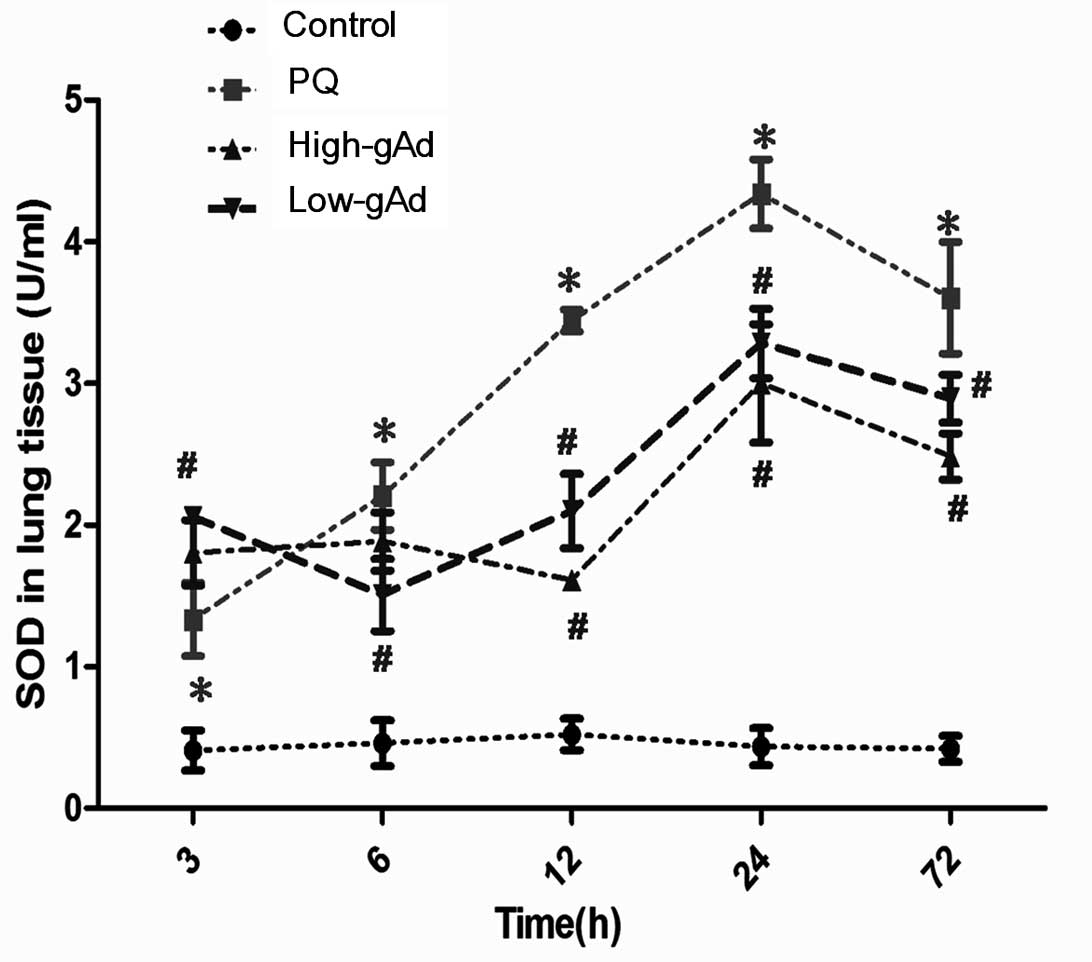 | Figure 4Effect of gAd pre-treatment on
pulmonary SOD level in control animals or PQ-exposed animals,
treated with vehicle or gAd (high-dose or low-dose) at 0, 3, 6, 12,
24 and 72 h following PQ injection. SOD levels were determined by
an enzyme-linked immunosorbent assay. Values represent the mean ±
standard error of the mean of four parallel measurements.
*P<0.05, compared with controls and
#P<0.05, compared with the PQ group. gAd, globular
domain adiponectin; SOD, superoxide dismutase; PQ, paraquat. |
gAd reduced PQ-induced pulmonary
nitrative injury in a dose-dependent manner
NO production was significantly increased in
PQ-exposed mice compared with the control group. This augmentation
was significantly reduced in gAd-pre-treated mice compared with the
PQ group (Fig. 5). As a
consequence of the induced NO production, blood 3-NT levels were
also increased in the PQ-exposed mice compared with the control
group. gAd administration decreased the blood 3-NT level,
particularly from 24 to 72 h following PQ-exposure (Fig. 6).
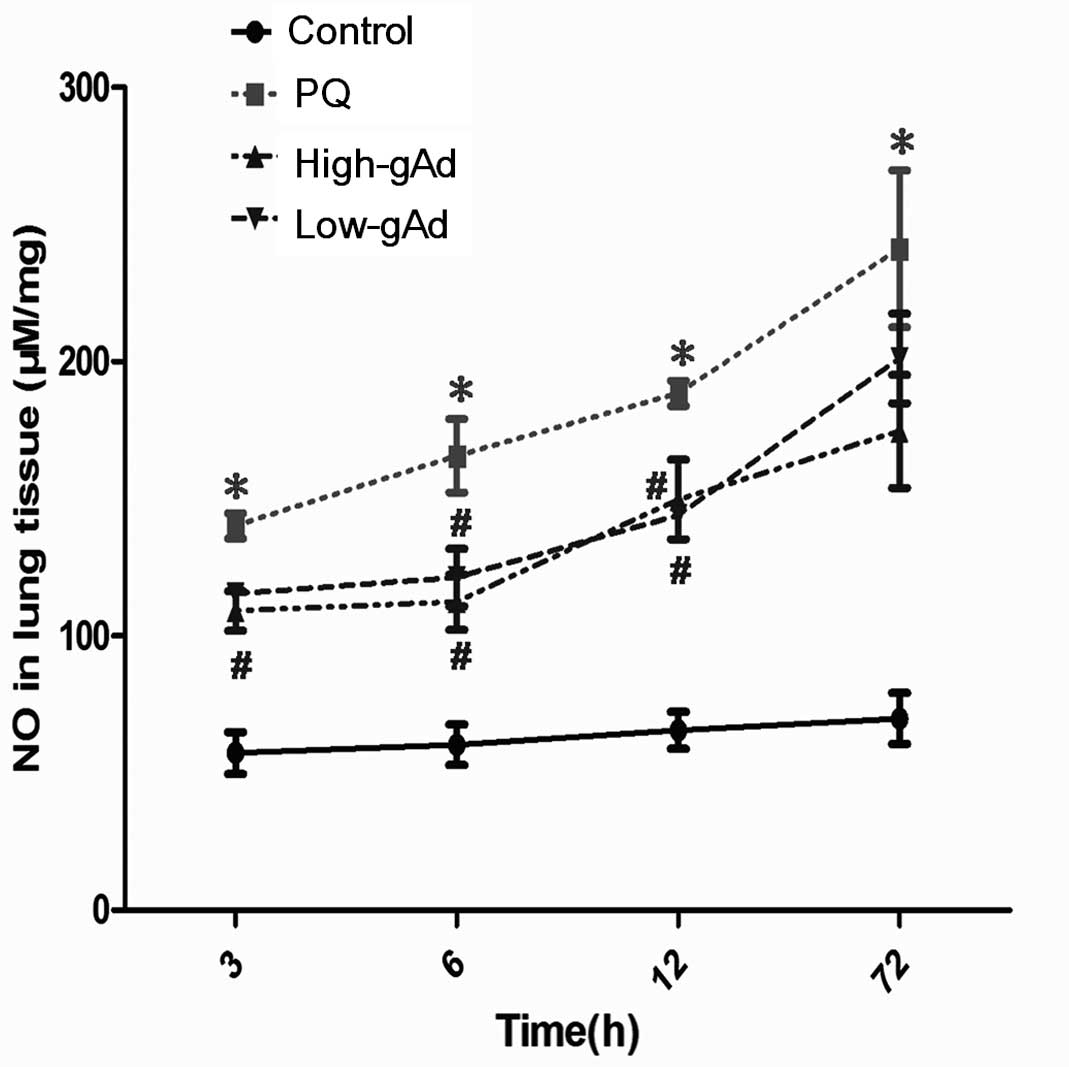 | Figure 5Effect of gAd pretreatment on
pulmonary NO level in control animals or PQ-exposed animals,
treated with vehicle or gAd (high-dose or low-dose) at 0, 3, 6, 12,
24 and 72 h following PQ injection. NO levels were determined by an
enzyme-linked immunosorbent assay. Values represent the mean ±
standard error of the mean of four parallel measurements.
*P<0.05, compared with controls and
#P<0.05, compared with the PQ group. gAd, globular
domain adiponectin; NO, nitric oxide; PQ, paraquat. |
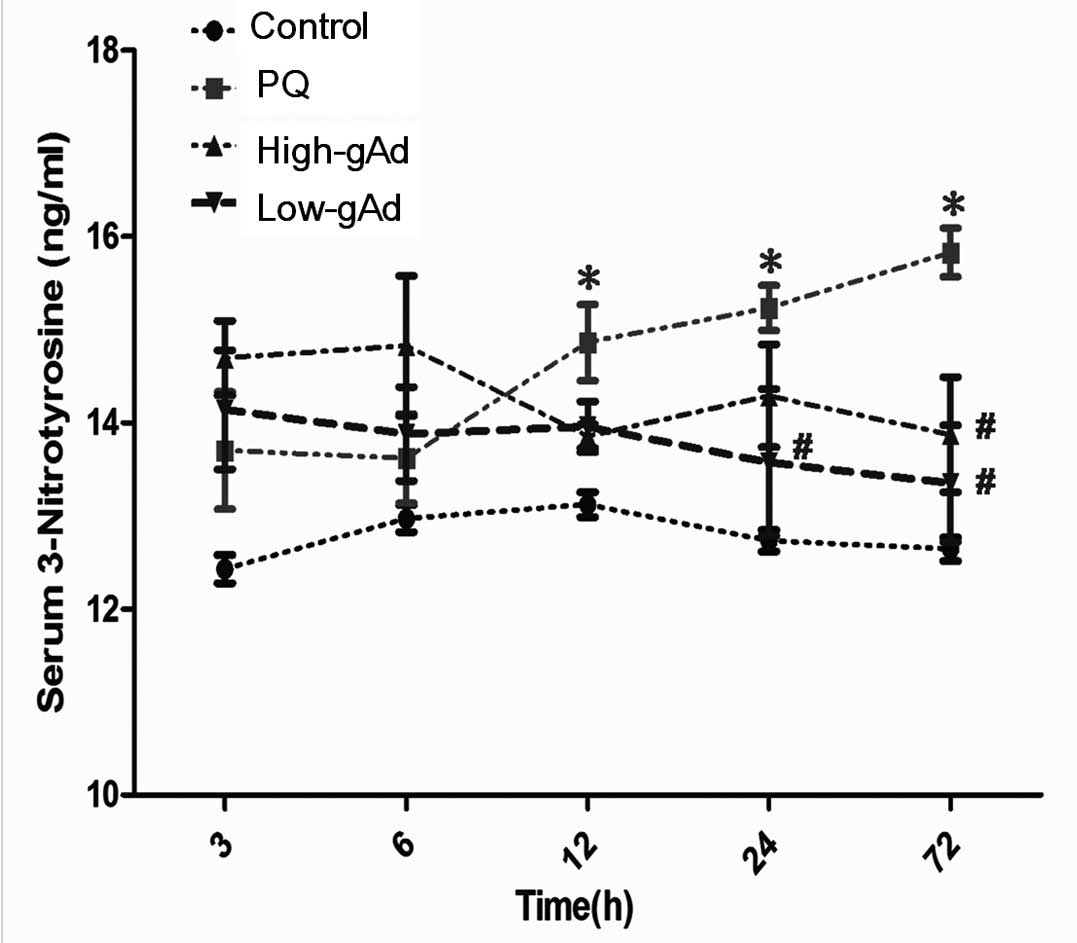 | Figure 6Effects of gAd pretreatment on blood
3-NT level in control animals or PQ-exposed animals, determined by
an enzyme-linked immunosorbent assay at 0, 3, 6, 12, 24 and 72 h.
Values represent the mean ± standard error of the mean of four
parallel measurements. *P<0.05, compared with
controls and #P<0.05, compared with the PQ group.
gAd, globular domain adiponectin; 3-NT, 3-nitrotyrosine; PQ,
paraquat. |
Similarly, a marked increase in pulmonary 8-OHdG
levels was observed in PQ-exposed mice compared with the control
group. The increase was decreased by gAd pre-treatment to a degree
at all time points measured, and this reduction was significant
compared with the PQ group at 72 h (Fig. 7).
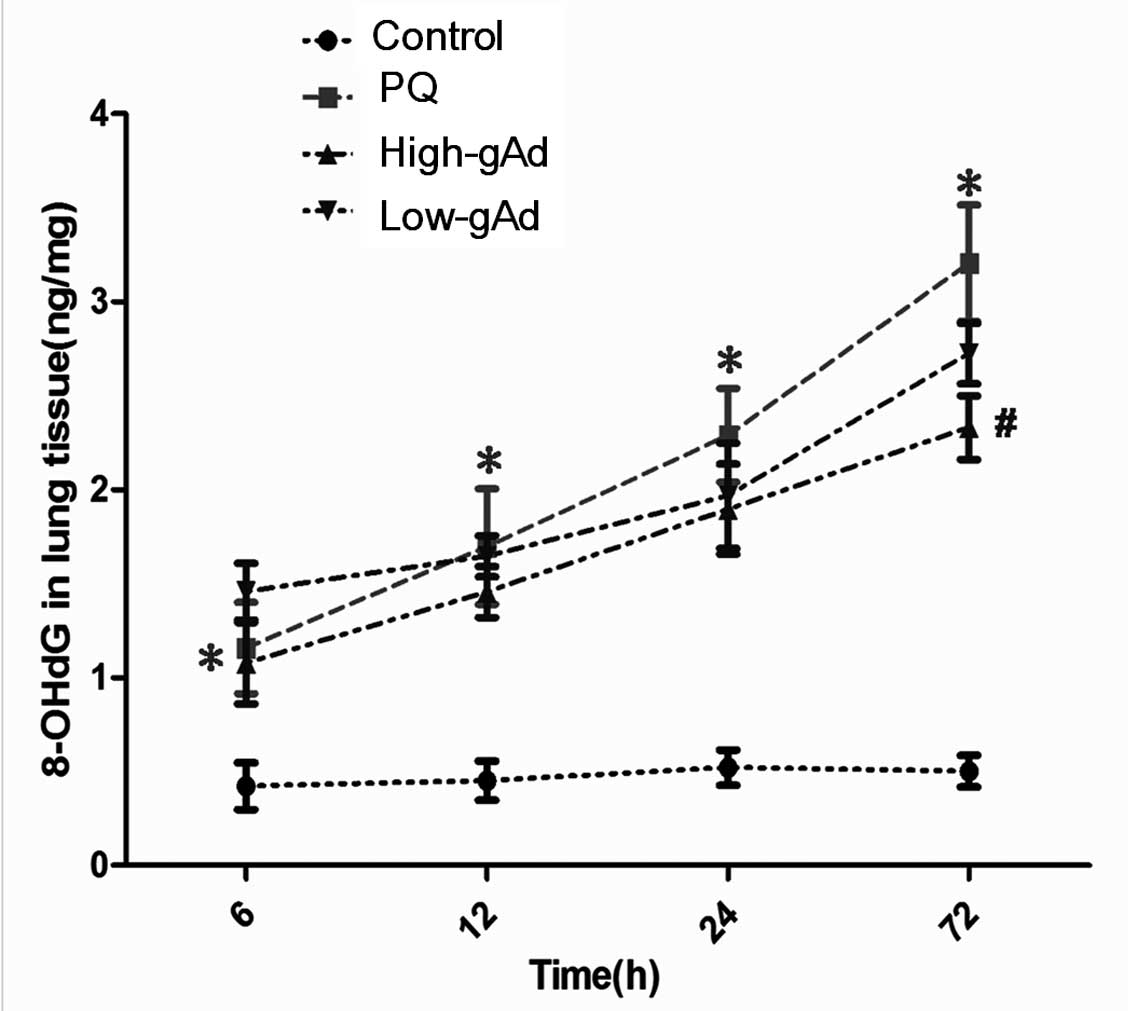 | Figure 7Effects of gAd pretreatment on
pulmonary 8-OdGH in control animals or PQ-exposed animals,
determined by an enzyme-linked immunosorbent assay at 0, 3, 6, 12,
24 and 72 h. Values represent the mean ± standard error of the mean
of four parallel measurements. *P<0.05, compared with
controls and #P<0.05, compared with the PQ group.
gAd, globular domain adiponectin; 8-OdGH, 8-hydroxy-2-dydeoxy
guanosine; PQ, paraquat. |
Discussion
Previous experimental and clinical studies have
demonstrated that PQ directly contributes to lung injury by
inducing inflammation, edema and fibrosis (10–13).
In the present study, gAd pre-treatment mitigated the pathological
changes induced by PQ in a dose-dependent manner. This provides
evidence that the adipocytokine, adiponectin, which is known to
have antidiabetic (14),
anti-atherogenic (15), antitumor
(16) and anti-inflammatory
properties (17,18), also exerts a pulmonary protective
effect against PQ-induced injury.
The current study indicated that the possible
mechanisms by which gAd relieves PQ-induced lung injury may be
attributed to two effects. gAd protects mice against PQ-induced
lung injury by attenuating oxidative stress. In addition, gAd
protects against PQ-induced lung injury by mitigating nitrative
stress.
PQ is a toxic herbicide that particularly affects
the lungs, since the pulmonary polyamine uptake system
preferentially recruits PQ, resulting in a 6–10 fold increase in
lung levels compared with the plasma (19,20).
PQ is known to induce alveolar collapse and fibrosis (21). Such effects are due to the
imbalance between the formation and scavenging of ROS (1,2,22).
ROS overproduction damages cell membranes, resulting in lipid
peroxidation, for which MDA levels serve as a proxy measurement
(23). SOD is a mammalian
enzymatic defense system, which protects against oxidative injury
by scavenging excess O2−. It also indirectly
reflects the level of lipid peroxidation (24). The present study demonstrated that
PQ significantly increases MDA production and activates SOD, which
was consistent with a previous study (25) This suggests that PQ simultaneously
stimulates oxidative stress injury and anti-oxidative defenses to
ameliorate lipid peroxidation.
Consistent with previous reports, adiponectin
potently scavenges free radicals, leading to the inhibition of
lipid peroxidation (6). In the
current study, it was demonstrated that gAd administration 12 and
36 h prior to PQ exposure significantly attenuated
O2− generation and MDA production. To the
best of our knowledge, this study provides the first direct
evidence supporting the hypothesis that adiponectin modulates
antioxidant defense mechanisms in the lung, and could thus offer
protection against PQ-induced lung damage via attenuation of
oxidative and nitrative stresses.
Contrary to findings from previous studies (26,27),
gAd pre-treatment at high or low doses only augmented SOD
activities at 3 h following initial PQ exposure. By contrast, SOD
activity was lower in the gAd groups compared with the PQ group
following 6 to 72 h. There are a number of possible explanations
for this effect. The most plausible of these is that gAd may
stimulate anti-oxidative SOD activity in a burst-like manner, which
would diminish ROS and MDA levels such that the higher levels of
SOD producing the anti-oxidant effect were no longer required. An
alternative explanation is that the short half-life (~13–17.5 h) of
gAd in circulation (28) may limit
its anti-oxidative properties over a prolonged period.
Other than ROS, reactive nitrogen species (RNS),
including NO and ONOO−, mediate nitrative stress via the
nitration of various biomolecules, including proteins, lipids and
nucleic acids (29). Accumulating
evidence has demonstrated that PQ augments NO and ONOO−
production, suggesting that RNS are also involved in PQ-mediated
pulmonary injury (30–33). The current study showed that PQ
significantly increased NO production, which emphasized the
pathophysiological importance of NO in PQ-mediated lung injury. In
contrast to its effects in an endothelial model (34), pre-treatment with gAd significantly
reduced NO production in PQ-exposed mice lung. A possible
explanation may be that gAd causes different effects in different
cells or tissues under different stimulation.
Excess NO reacts with O2− to
produce ONOO− (35,36).
ONOO− is a highly toxic reactive species, which
nitratively modifies various proteins, for example, changing
tyrosine to 3-NT (37).
ONOO− also injures DNA via a number of mechanisms,
including the formation of 8-OHdG (38). The present study demonstrated an
increase in 8-OHdG levels in lung tissue from 6 to 72 h, and
increased 3-NT from 12 to 72 h, in PQ-exposed mice. These results
provide additional evidence in support of the hypothesis that
nitrative stress is involved in PQ-mediated injury (39). gAd pre-treatment markedly
attenuated 3-NT production at 24 and 72 h, and decreased 8-OHdG
after 72 h. To the best of our knowledge, this is the first
evidence demonstrating that adiponectin protects against
PQ-mediated lung injury by attenuating peroxynitrite-induced
protein nitration and DNA damage via a reduction in nitrative
stress.
In conclusion, the present study demonstrates that
gAd protects against PQ-induced lung injury in a BALB/c mouse
model, by attenuating oxidative/nitrative stress in a
dose-dependent manner. Adiponectin is therefore a potential
therapeutic agent against PQ-induced lung injury, for which no
efficacious treatment currently exists. Clarification of the
precise signaling mechanisms involved in the amelioration of
oxidative/nitrative stress by adiponectin requires further
investigation. In addition, further studies are required in order
to assess the effect of gAD given with and following PQ
administration, as the current study, which only examined the
effect of pre-treatment with gAd, does not accurately mimic true
clinical scenarios.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 30900493).
Abbreviations:
|
PQ
|
paraquat
|
|
gAd
|
globular domain adiponectin
|
|
3-NT
|
3-nitrotyrosine
|
|
MDA
|
malondialdehyde
|
|
NO
|
nitric oxide
|
|
8-OHdG
|
8-hydroxy-2-dydeoxy guanosine
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Suntres ZE: Role of antioxidants in
paraquat toxicity. Toxicology. 180:65–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bus JS, Aust SD and Gibson JE: Lipid
peroxidation: a possible mechanism for paraquat toxicity. Res
Commun Chem Pathol Pharmacol. 11:31–38. 1975.PubMed/NCBI
|
|
3
|
Yokota T, Meka CS, Medina KL, et al:
Paracrine regulation of fat cell formation in bone marrow cultures
via adiponectin and prostaglandins. J Clin Invest. 109:1303–1310.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corbetta S, Bulfamante G, Cortelazzi D, et
al: Adiponectin expression in human fetal tissues during mid- and
late gestation. J Clin Endocrinol Metab. 90:2397–2402. 2005.
View Article : Google Scholar
|
|
5
|
Piñeiro R, Iglesias MJ, Gallego R, et al:
Adiponectin is synthesized and secreted by human and murine
cardiomyocytes. FEBS Lett. 579:5163–5169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fruebis J, Tsao TS, Javorschi S, et al:
Proteolytic cleavage product of 30-kDa adipocyte complement-related
protein increases fatty acid oxidation in muscle and causes weight
loss in mice. Proc Natl Acad Sci USA. 98:2005–2010. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song W, Huo T, Guo F, et al: Globular
adiponectin elicits neuroprotection by inhibiting NADPH
oxidase-mediated oxidative damage in ischemic stroke. Neuroscience.
248C:136–144. 2013. View Article : Google Scholar
|
|
8
|
Tilg H and Moschen AR: Adipocytokines:
mediators linking adipose tissue, inflammation and immunity. Nat
Rev Immunol. 6:772–783. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldstein BJ, Scalia RG and Ma XL:
Protective vascular and myocardial effects of adiponectin. Nat Clin
Pract Cardiovasc Med. 6:27–35. 2009. View Article : Google Scholar :
|
|
10
|
Smith LL: Paraquat toxicity. Philos Trans
R Soc Lond B Biol Sci. 311:647–657. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bullivant CM: Accidental poisoning by
paraquat: Report of two cases in man. Br Med J. 1:1272–1273. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malone JD, Carmody M, Keogh B and O’Dwyer
WF: Paraquat poisoning; a review of nineteen cases. J Ir Med Assoc.
64:59–68. 1971.PubMed/NCBI
|
|
13
|
Copland GM, Kolín A and Shulman HS: Fatal
pulmonary intra-alveolar fibrosis after paraquat ingestion. N Engl
J Med. 291:290–292. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maeda N, Shimomura I, Kishida K, et al:
Diet-induced insulin resistance in mice lacking adiponectin/ACRP30.
Nat Med. 8:731–737. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bråkenhielm E, Veitonmäki N, Cao R, et al:
Adiponectin-induced antiangiogenesis and antitumor activity involve
caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci
USA. 101:2476–2481. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumada M, Kihara S, Sumitsuji S, et al:
Association of hypoadiponectinemia with coronary artery disease in
men. Arterioscler Throm Vasc Biol. 23:85–89. 2003. View Article : Google Scholar
|
|
17
|
Shore SA, Terry RD, Flynt L, Xu A and Hug
C: Adiponectin attenuates allergen-induced airway inflammation and
hyperresponsiveness in mice. J Allergy Clin Immunol. 118:389–395.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miller M, Pham A, Cho JY, Rosenthal P and
Broide DH: Adiponectin-deficient mice are protected against
tobacco-induced inflammation and increased emphysema. Am J Physiol
Lung Cell Mol Physiol. 299:L834–L842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dinis-Oliveira RJ, Duarte JA,
Sánchez-Navarro A, Remião F, Bastos ML and Carvalho F: Paraquat
poisonings: mechanisms of lung toxicity, clinical features, and
treatment. Crit Rev Toxicol. 38:13–71. 2008. View Article : Google Scholar
|
|
20
|
Rannels DE, Kameji R, Pegg AE and Rannels
SR: Spermidine uptake by type II pneumocytes: interactions of amine
uptake pathways. Am J Physiol. 257:L346–L353. 1989.PubMed/NCBI
|
|
21
|
Jo YH, Kim K, Rhee JE, et al: Therapeutic
hypothermia attenuates acute lung injury in paraquat intoxication
in rats. Resuscitation. 82:487–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
LeBel CP, Ischiropoulos H and Bondy SC:
Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of
reactive oxygen species formation and oxidative stress. Chem Res
Toxicol. 5:227–231. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaweł S, Wardas M, Niedworok E and Wardas
P: Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek.
57:453–455. 2004.(In Polish).
|
|
24
|
Kuloglu M, Ustundag B, Atmaca M, Canatan
H, Tezcan AE and Cinkilinc N: Lipid peroxidation and antioxidant
enzyme levels in patients with schizophrenia and bipolar disorder.
Cell Biochem Funct. 20:171–175. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Candan F and Alagözlü H: Captopril
inhibits the pulmonary toxicity of paraquat in rats. Hum Exp
Toxicol. 20:637–641. 2001. View Article : Google Scholar
|
|
26
|
Jung TW, Lee JY, Shim WS, et al:
Adiponectin protects human neuroblastoma SH-SY5Y cells against
MPP+-induced cytotoxicity. Biochem Biophys Res Commun.
343:564–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Li MR and Guo ZX: Effects of
adiponectin on oxidative stress and apoptosis in human cardiac
myocytes cultured with high glucose. Chin Med J (Engl).
125:4209–4213. 2012.
|
|
28
|
Peake PW, Kriketos AD, Campbell LV, Shen Y
and Charlesworth JA: The metabolism of isoforms of human
adiponectin: studies in human subjects and in experimental animals.
Eur J Endocrinol. 153:409–417. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akuta T, Zaki MH, Yoshitake J, Okamoto T
and Akaike T: Nitrative stress through formation of
8-nitroguanosine: insights into microbial pathogenesis. Nitric
Oxide. 14:101–108. 2006. View Article : Google Scholar
|
|
30
|
Berisha HI, Pakbaz H, Absood A and Said
SI: Nitric oxide as a mediator of oxidant lung injury due to
paraquat. Proc Natl Acad Sci USA. 91:7445–7449. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morán JM, Ortiz-Ortiz MA, Ruiz-Mesa LM and
Fuentes JM: Nitric oxide in paraquat-mediated toxicity: A review. J
Biochem Mol Toxicol. 24:402–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahmad I, Kumar A, Shukla S, Pandey Pandey
H and Singh C: The involvement of nitric oxide in maneb- and
paraquat-induced oxidative stress in rat polymorphonuclear
leukocytes. Free Radical Res. 42:849–862. 2008. View Article : Google Scholar
|
|
33
|
Tao L, Gao E, Jiao X, et al: Adiponectin
cardioprotection after myocardial ischemia/reperfusion involves the
reduction of oxidative/nitrative stress. Circulation.
115:1408–1416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cao Y, Tao L, Yuan Y, et al: Endothelial
dysfunction in adiponectin deficiency and its mechanisms involved.
J Mol Cell Cardiol. 46:413–419. 2009. View Article : Google Scholar :
|
|
35
|
Estévez AG and Jordán J: Nitric oxide and
superoxide, a deadly cocktail. Ann NY Acad Sci. 962:207–211. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferdinandy P, Danial H, Ambrus I, Rothery
RA and Schulz R: Peroxynitrite is a major contributor to
cytokine-induced myocardial contractile failure. Circ Res.
87:241–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beckman JS and Koppenol WH: Nitric oxide,
superoxide, and peroxynitrite: the good, the bad, and ugly. Am J
Physiol. 271:C1424–C1437. 1996.PubMed/NCBI
|
|
38
|
Halliwell B: Can oxidative DNA damage be
used as a biomarker of cancer risk in humans? Problems, resolutions
and preliminary results from nutritional supplementation studies.
Free Radical Res. 29:469–486. 1998. View Article : Google Scholar
|
|
39
|
Denicola A and Radi R: Peroxynitrite and
drug-dependent toxicity. Toxicology. 208:273–288. 2005. View Article : Google Scholar : PubMed/NCBI
|