Introduction
Lung cancer is one of the most common malignancies
worldwide, of which the incidence rate and mortality rank are the
highest amongst the different malignancies (1). The main histological types include
squamous cell carcinoma, adenocarcinoma, large-cell lung carcinoma
(LCLC) and small-cell lung carcinoma (SCLC). LCLC is a type of
undifferentiated carcinoma, that lacks the cell differentiation and
structural characteristics of small-cell lung cancer,
adenocarcinoma and squamous cell carcinoma. The incidence rate of
LCLC is significantly lower compared with the other types, with
LCLC accounting for ~9% of total lung cancer cases, as reported by
the World Health Organization (WHO) (2). However, in previous studies by
Hanagiri et al (3) and
Battafarano et al (4), the
incidence rate was reported as 5.8% (57/975) and 3.9% (82/2,099),
respectively. In addition, the incidence rate in China has been
reported to be between 1 and 2% (5,6). The
clinical manifestation of LCLC is not typical, and the early
diagnosis rate is low. LCLC is prone to regional lymph node and
distant metastasis, with poor prognosis. It is not sensitive to
chemotherapy and there is no standard treatment schedule. Due to
the high malignancy, LCLC has been increasingly studied in recent
years (7–11). The lower incidence of LCLC in China
has been hypothesized to be the result of geographic or ethnic
differences. However, with improvement in the diagnostic levels of
Chinese pathologists on the morphological features and
immunohistochemical characteristics of LCLC, the definite diagnosis
rate of LCLC has been significantly improved. In the present study,
the clinicopathological data from 174 patients with LCLC were
retrospectively analyzed, with the aim of summarizing the specific
clinicopathological features of LCLC and thereby improving the
definite diagnosis rate of LCLC.
Materials and methods
Patients and tumor specimens
A total of 174 patients with LCLC (male, 131;
female, 43; gender ratio, 3.05:1; mean age, 61.4 years; age range,
28–82 years), pathologically confirmed at the Department of
Pathology at the Affiliated Cancer Hospital of Tianjin Medical
University (Tianjin, China), were retrospectively reviewed between
2012 and 2013. The incidence of LCLC accounted for 5.7% of the
total lung cancer cases (n=3,053) during the corresponding time
period. A total of 125 patients (71.8%) had a history of smoking,
with an average smoking index of 670. This value is calculated by
the number of cigarettes smoked per day × years of smoking and
divided by 125 (12). The clinical
symptoms included a cough and expectoration (n=128, 73.6%), bloody
sputum or hemoptysis (n=88, 50.6%), chest pain (n=61, 35.1%),
dyspnea and short breath (n=34, 19.5%) and a fever (n=27, 15.5%).
Tumor, node and metastasis (TNM) classification of the cancer
tissues revealed 23 cases of stage Ia, 42 cases of stage Ib, 10
cases of stage IIa, 46 cases of stage IIb, seven cases of stage
IIIa, 37 cases of stage IIIb and nine cases of stage IV. All the
patients had intact clinicopathological data. The study was
conducted in accordance with the Declaration of Helsinki, and with
approval from the Ethics Committee of the Affiliated Cancer
Hospital of Tianjin Medical University. Written informed consent
was obtained from all the participants.
Imaging examination
All patients underwent a chest X-ray (DR-F; General
Electric Corp., Fairfield, CT, USA) or computed tomography (CT)
scan (Optima CT660; General Electric Corp.), which showed a single
lesion in each patient. The maximum diameter of the tumors varied
between 1.5 and 11.5 cm, with an average of 4.7 cm. The majority of
the tumors were of a peripheral type (89.1%, 155/174), with the
central type accounting for only 10.9% (19/174). In total, 101
cases were located in the right lung (superior lobe, 53; middle
lobe, 8; lower lobe, 29; near the hilum of the lung, 11) and 73
cases were located in the left lung (superior lobe, 47; lower lobe,
18; near the hilum of the lung, 8). The imaging examination
revealed lobulation in 105 cases (60.3%), a burr shadow in 53 cases
(30.5%), pleural effusion in 10 cases (5.7%), atelectasis in 12
cases (6.9%) and mediastinal lymphadenectasis in 34 cases
(19.5%).
Histological examination
Prior to surgery, the 174 patients underwent a
sputum exfoliative cytopathological examination. In addition, 69
cases received a fiber bronchoscope biopsy, 21 cases received a
CT-guided lung puncture biopsy and 37 patients received a
supraclavicular or cervical lymph node biopsy. All of the biopsy
tissues were sent for routine histopathological examination. They
were fixed in 10% neutral formalin and desiccated and embedded in
paraffin, and then were conventionally sectioned and stained with
hematoxylin and eosin. Following surgery, all of the tissues were
sent for routine histopathological examination and
immunohistochemical stains (13).
Specific antibodies including mouse anti human cytokeratin 5/6
(CK5/6), high molecular weight cytokeratin (HCK; 34βE12), P63
(4A4), thyroid transcription factor 1 (TTF 1; SPT24), cytokeratin 7
(CK7; OV-TL 12/30)monoclonal antibodies, and rabbit anti human
synaptophysin (Syn; SP11) and chromogranin A (CgA; SP12) were
purchased from Fuzhou Maixin Biological Technology Co., Ltd.
(Fuzhou, China). Polymeric horseradish peroxidase conjugated
anti-mouse/rabbit IgG (Fuzhou Maixin Biological Technology Co.,
Ltd.) was used as the secondary antibody.
Treatment
Of the 174 patients, 137 cases received surgical
treatment, including exploratory thoracotomy (n=9), regional wedge
excision (n=11), pulmonary lobectomy (n=98) and total pneumonectomy
(n=19). Among them, 73 patients also received postoperative
chemotherapy, 12 patients received postoperative radiotherapy and
13 patients received postoperative biotherapy. A total of 32
patients undertook palliative therapy, including simple
chemotherapy (n=19), radiotherapy (n=5) and biotherapy (n=8). In
total, 5 patients gave up treatment following confirmation of LCLC
by biopsy of the lymph nodes.
Statistical analysis
All data were analyzed using the SPSS 13.0 software
program (SPSS, Inc., Chicago, IL, USA). The two- or multi-sample
comparison of the survival rate was performed using chi-squared
test. For all statistical analysis, P<0.05 was considered to
indicate a statistically significant difference.
Results
Preoperative cellular and histological
examination
Sputum exfoliative cytological examinations of the
174 patients revealed only two cases with adenocarcinoma cells and
five cases with poorly differentiated cancer cells. Among the 69
patients that underwent a fiber bronchoscope biopsy, three cases
were diagnosed as LCLC, one case was diagnosed as combined SCLC,
two cases were diagnosed as squamous cell carcinoma and three cases
were diagnosed as poorly differentiated carcinoma (histological
type was uncertain). In addition, among the 21 cases that received
a simultaneous lung puncture biopsy, there were four cases of LCLC
supported by immunohistochemistry, one case of neuroendocrine
carcinoma and three cases of poorly differentiated carcinoma
(histological type was uncertain).
Postoperative histological
examination
All the specimens were reviewed by two experienced
pathologists from the Affiliated Cancer Hospital of Tianjin Medical
University, according to the revised histological classification
standards of lung cancer for the diagnosis and classification of
LCLCs established by the WHO (third edition) (2). All the 174 patients (including 37
cases that underwent supraclavicular or cervical lymph node biopsy)
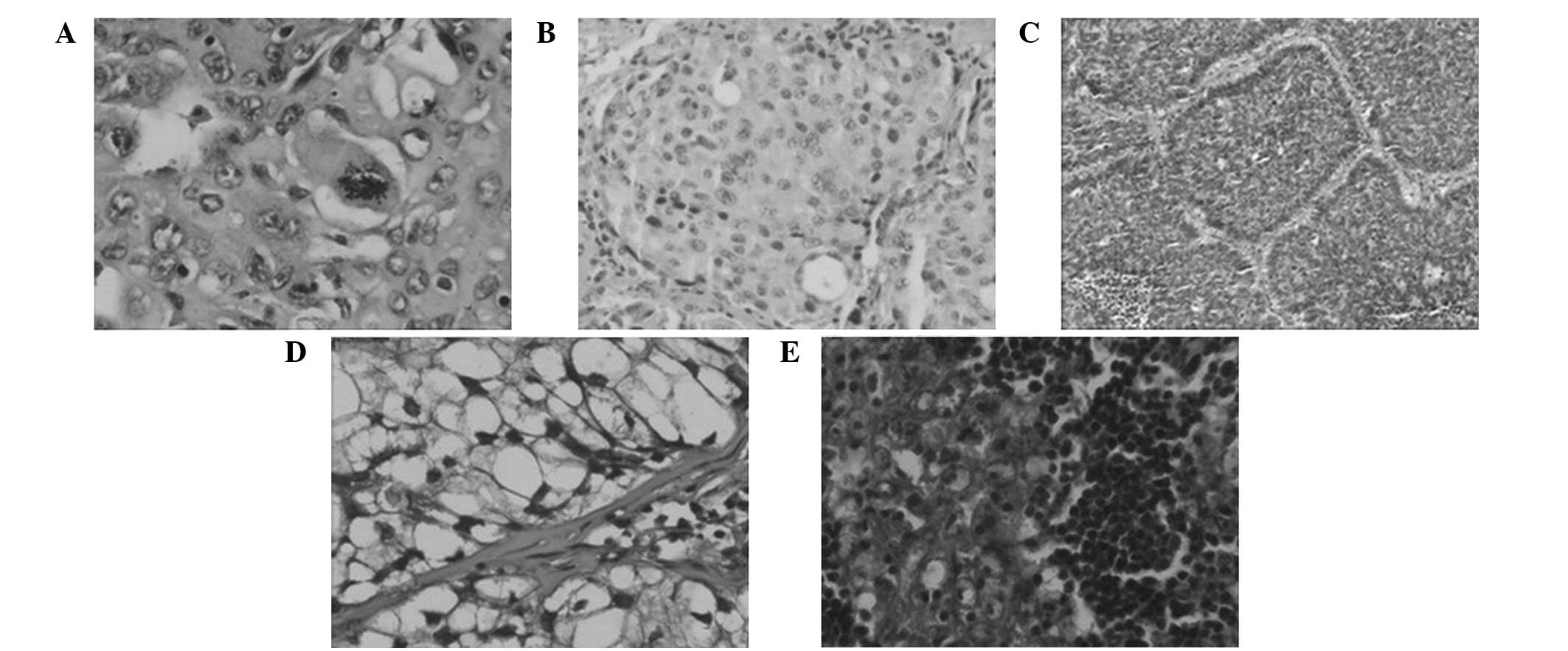
were diagnosed as LCLC. The cases were classified into classic LCLC
(46.0%, 80/174; Fig. 1A), large
cell neuroendocrine carcinoma (LCNEC; 36.8%, 64/174; Fig. 1B), complex LCNEC (3.4%, 6/174),
basaloid (10.9%, 19/174; Fig. 1C),
clear cell (1.7%, 3/174; Fig. 1D)
and lymphoepithelioma-like carcinomas (1.1%, 2/174; Fig. 1E). In addition, 96 cases were
confirmed pathologically to be lymph node positive and were located
at the hilum of the lung, parabronchus, supraclavicular, cervical
and mediastinal sections, accounting for 84.2% of the 114 patients
that underwent a lymph node biopsy.
Postoperative immunohistochemical
examination
Routine immunohistochemical analysis was performed
on the 174 specimens. In the 80 cases of classic LCLC, the positive
expression rates of 34βE12, CK5/6 and P63 were 100, 76.3 and 52.5%,
respectively; the positive expression rates of CK7 and TTF-1 were
100 and 51.3%, respectively; however, positive expression of Syn
and CgA was not observed. Among the 64 cases of LCNEC, the positive
expression rates of Syn and CgA were 85.9% (diffusely positive,
78.1%; focally positive, 7.8%) and 68.8% (diffusely positive,
46.9%; focally positive, 21.9%), respectively. Furthermore,
positive expression of Syn or CgA was observed in each patient
(100%), with 39 cases (60.9%) exhibiting positive expression for
both neuroendocrine markers. The positive expression rates of
34βE12, CK5/6 and P63 in the LCNEC tissues were 35.9, 45.3 and 25%,
respectively, with 54.7% of the LCNEC tissues positive for at least
one squamous epithelial marker. The positive expression rates of
CK7 and TTF-1 were 84.4 and 65.6%, respectively, and positive
expression of at least one glandular epithelial marker was observed
in 54 cases (84.4%). Within the 19 cases of basaloid carcinoma, the
positive expression rates of 34βE12, CK5/6 and P63 were 78.9, 68.4
and 52.6%, respectively. All the basaloid carcinoma tissues were
positive for at least one of these markers (100%). CK7 was
expressed in all the basaloid carcinoma tissues; however, TTF-1 or
CgA expression was not observed. Syn was weakly expressed in the
basaloid carcinoma tissues, accounting for 5.3% of cases.
Follow-up examination
Among the 174 patients with LCLC, 83 cases diagnosed
in 2012 were followed-up for one year; however, five cases did not
complete the follow-up (Table I).
The one-year survival rate of the cases was 57.8% (48/83). No
statistically significant association was identified between the
survival rate and the patient gender, age, tumor location and tumor
size. However, the survival rate of the patients was found to be
significantly correlated with the TNM stage, N stage, M stage,
occurrence of radical resection and pathological subtype. The N
stage was used to decide whether there is regional lymph node
metastasis and the metastasis degree; M stage was used to decide
whether there is distant metastasis. The one-year survival rate of
the patients classified with stage I was significantly higher
compared with the patients classified with a higher stage
(P<0.05). With regard to the N stage, the one-year survival rate
was significantly higher in patients with stage N0 than
in patients with other stages (P<0.05), and was also higher in
patients with stage N1 than in patients with stage
N3 (P<0.001). Patients classified as stage
M0 also had a significantly higher survival rate
compared with patients classified as stage M1 (P=0.016).
Patients that underwent a radical resection had a significantly
higher survival rate than patients who received palliative
treatment (P=0.018). In addition, the survival rate of the patients
with classic LCLC was significantly higher compared with the
patients with LCNEC (P=0.003). The additional 91 patients were
diagnosed in 2013 and were followed-up for less than one year. To
date, three cases have been lost and there have been 13 cases of
mortality.
 | Table IAssociation between the one-year
survival rate of 83 patients with LCC diagnosed in 2012 and their
clinicopathological features. |
Table I
Association between the one-year
survival rate of 83 patients with LCC diagnosed in 2012 and their
clinicopathological features.
| Variable | One-year survival
rate, % (n) |
|---|
| TNM stage |
| I | 82.9 (29/35) |
| II | 56.0 (14/25) |
| III | 26.3 (5/19) |
| IV | 0 (0/4) |
| N stage |
| N0 | 90.5 (19/21) |
| N1 | 63.4 (26/41) |
| N2 | 25 (1/4) |
| N3 | 11.8 (2/17) |
| M stage |
| M0 | 60.8 (48/79) |
| M1 | 0 (0/4) |
| Treatment |
| Radical
resection | 65.1 (41/63) |
| Palliative
treatment | 35.0 (7/20) |
| Pathological
subtype |
| Classic LCC | 74.4 (29/39) |
| LCNEC | 38.7 (12/31) |
| Basaloid
carcinoma | 50.0 (4/8) |
| Combined LCNEC | 50.0 (1/2) |
| Clear cell
carcinoma | 50.0 (1/2) |
|
Lymphoepithelioma-like carcinoma | 100 (1/1) |
Discussion
The incidence rate of LCLC is significantly lower
than other types of lung cancer, including squamous cell carcinoma,
adenocarcinoma and SCLC. With preliminary statistics, 174 patients
were diagnosed with LCLC at the Department of Pathology of the
Affiliated Cancer Hospital of Tianjin Medical University between
2012 and 2013, accounting for 5.7% of the total lung cancer cases
(3,053 cases) during the corresponding time period. Improvements to
the pathological diagnostic level of LCLC and the development of
immunohistochemical techniques have significantly increased the
definite diagnosis rate of LCLC (14–17).
In the present study, routine immunohistochemistry was performed on
the LCLC tissues, including three squamous cell markers (CK5/6,
34βE12 and P63), two glandular epithelial markers (TTF-1 and CK7)
and two neuroendocrine markers (Syn and CgA). Barbareschi et
al (18) diagnosed and
classified LCLC via the detection of the squamous markers, P63, CK5
and desmocollin 3, the glandular epithelial markers, TTF-1, Napsin
A and CK7, and the neuroendocrine markers, Syn, CgA and CD56.
Since LCLCs are predominantly located in the
peripheral area of the lungs, there were fewer positive results
from preoperative sputum exfoliative cytology and bronchofiberscope
biopsy examinations. In addition, due to small specimen, tissue
extrusion and deformation it is difficult to be used for
immunohistochemistry. Therefore the preoperative diagnosis rate of
LCLC is far lower compared with that of squamous cell carcinoma,
SCLC and adenocarcinoma, whereas CT-guided lung puncture biopsy has
a relatively high positivity rate. In the present study, only seven
cases (4.0%) were confirmed or considered to be LCLC using
preoperative sputum exfoliative cytology, bronchofiberscope biopsy
or lung puncture biopsy. Comparatively, the positive ratio of the
lung puncture biopsy was superior to that of the other two methods,
which is consistent with the results reported by Watanabe et
al (19). Doddoli et al
(20) reported only one out of 20
cases of LCLC as definitely diagnosed using a preoperative
bronchofiberscope biopsy.
Clinically, LCLC is difficult to be differentiated
from other types of lung cancer. Diagnosis primarily depends on
histopathological examination, with the aim to exclude squamous
cell carcinoma, adenocarcinoma and SCLC. Currently, the
histopathological diagnosis of classic LCLC is based on the
following evidence (2). Firstly,
the tumor cells are bulky and polygonal. Secondly, tumor cells have
a moderate amount of cytoplasm. Thirdly, the tumor cells present
large nuclei, vesicular nuclei and prominent nucleoli. Fourthly,
the tumor cells are nests. Fifthly, the ultrastructure of the tumor
cells exhibits a small amount of adenoid or squamous
differentiation. In the present study, classic LCLC accounted for
46.0% of the LCLC cases (80/174). Classic LCLC should be identified
from poorly differentiated squamous cell carcinoma and the solid
invasive adenocarcinoma. Generally, a small amount of cell
keratinization or intercellular bridges can be observed in poorly
differentiated squamous cell carcinoma, while mucus droplets in the
cytoplasm can be observed in the solid invasive adenocarcinoma. As
detected using an immunohistochemical method, LCLCs express varying
degrees of squamous and glandular epithelial markers, but no
neuroendocrine markers. All the 80 cases of classic LCLC were in
compliance with the aforementioned morphological and
immunohistochemical characteristics.
According to the third edition of the histological
classification criteria for lung cancer established by the WHO
(2), in addition to classical
LCLC, LCLC also can be classified into the following six subtypes:
LCNEC, complex LCNEC, basaloid carcinoma, clear cell carcinoma,
lymphoepithelioma-like carcinoma and LCLC with striated muscle-like
phenotype. LCNEC is the most common type, and can be diagnosed
based on the following histological features (2,21,22):
i) Large tumor cell volume; ii) moderate or rich amount of
cytoplasm; iii) prominent nucleoli and mitotic count: >11
mitoses per 10 high-power fields (HPFs); iv) tumor cells exhibiting
morphological features of neuroendocrine differentiation, including
organ-like nests, trabecular, rosette and palisade arrangements; v)
large areas of necrosis are commonly observed; and vi)
immunohistochemical positive staining for at least one
neuroendocrine marker. A total of 64 cases of LCNEC (36.8%) were
identified in the current study, with Syn and CgA expression rates
of 85.9 and 68.8%, respectively. Additionally, the expression rate,
intensity and scope were higher for Syn than CgA, indicating that
Syn is a more valuable individual neuroendocrine marker for the
diagnosis of LCNEC. All the LCNEC cases exhibited positive
expression for Syn or CgA, with 42 cases expressing Syn and CgA, 25
cases expressing Syn but no CgA, and 14 cases expressing CgA but no
Syn, indicating that the expression of these two markers does not
cover each other. Thus, a combination of these two neuroendocrine
markers or more may improve the definite diagnosis rate of LCNEC.
These observations were consistent with the results of Tanaka et
al (23). The diagnosis of
LCNEC should be differentiated from atypical carcinoids, as these
are two types of neuroendocrine tumor that exhibit organ-like,
trabecular, rosette and palisading morphological characteristics,
as well as necrosis. However, atypical carcinoids exhibit
relatively few mitotic figures (2–10/10 HPF) and LCNECs commonly
show broader necrosis than atypical carcinoids. In addition, LCNEC
cases should be differentiated from basaloid carcinoma.
The histopathological characteristics of basaloid
carcinomas are as follows (1,10):
i) Tumor cells are relatively small with cube to fusiform shape,
appearing monomorphic; ii) decreased levels of cytoplasm; iii)
moderate nuclear chromatin, finely granular, a lack of nucleoli or
little punctiform nucleoli, and mitotic figures of ≥15/10 HPF; iv)
tumor cells presenting as solid nests or anastomotic beam-like
ordering, around which is a palisade arrangement, with rosettes
found in 1/3 of cases; v) comedonecrosis is commonly observed; vi)
ultrastructure lacks squamous differentiation; and vii)
immunohistochemical analyses are often positive for hemopoietic
cell kinase (HCK) and negative for TTF-1 and neuroendocrine
indicators. The 19 cases of basaloid carcinoma in the present study
accounted for 10.9% of the total LCLC cases. Basaloid carcinomas
should firstly be differentiated from LCNECs, since these two
tumors exhibit necrosis, a palisade arrangement and occasionally
rosettes. However, the majority of LCNECs present with large
extensive necrosis, while basaloid carcinomas often exhibit
comedo-like necrosis. Immunohistochemical staining is of great
importance for the identification of these two types. All the
LCNECs diffusely expressed at least one of the neuroendocrine
indicators; however, the majority of basaloid carcinomas did not
express neuroendocrine indicators, with only 10% focally expressing
one neuroendocrine indicator. In addition, HCK was not expressed in
LCNEC cases, but often expressed in basaloid carcinoma.
Approximately 50% of the LCNECs expressed TTF-1, but no basaloid
carcinoma expressed TTF-1. Basaloid carcinoma should be also
differentiated from the basal-like subtype of squamous cell
carcinoma and SCLC.
Combined LCNEC refers to tumors with morphological
and immunohistochemical characteristics of LCNEC, as well as
characteristics of well-defined non-small cell lung cancer,
including squamous cell carcinoma, adenocarcinoma, giant cell
carcinoma and spindle cell carcinoma. LCNEC combined with SCLC has
been classified into the SCLC category. In the present study, there
were only six cases of combined LCNEC, five of which were combined
with invasive adenocarcinoma and one that was combined with
squamous cell carcinoma. Morbini and Inghilleri (24) reported a novel type of combined
LCNEC, in which sections of the tumor exhibited morphological and
immunohistochemical characteristics of LCNEC, while other sections
were characteristic of basaloid carcinoma.
Histologically, clear cell carcinomas have large
tumor cells in a polygonal shape, abundant cytoplasm and watery
transparency or foam. The tumor cells exhibit diffuse and lamellar
growth, and the ultrastructure lacks adenoid or squamous
differentiation. Clinically, metastatic clear cell carcinomas from
kidney, thyroid and the salivary gland should be excluded from the
diagnosis of primary lung clear cell carcinoma. In the present
study, there were only 3 cases of lung clear cell carcinoma, which
were clinically excluded from the metastatic carcinomas from other
tissues metastasis. In addition, only two cases of
lymphoepithelioma-like carcinoma were identified, exhibiting a
large volume of tumor cells, syncytium-like, large nuclei,
vesicular nuclei and evident red nucleoli. The tumor cells
exhibited diffuse and lamellar growth with a clear pushing
boundary. Lymphatic infiltration was also observed. Diagnosis of
LCLC combined with a skeletal muscle-like phenotype requires ≥10%
of the tumor cells to be composed of muscle-like cells, whose
cytoplasm contains an eosinophilic body. This subtype is very rare,
and no cases were observed in the present study.
Currently, the treatment of LCLC is a comprehensive,
primarily with surgical excision (3,6,25).
In the present study, the radical resection rate of LCLC was 73.5%
(128/174), of which the majority underwent a lobectomy. Whether
chemotherapy is required following surgery remains controversial
(26). Although biological
treatment has achieved good results in the treatment of a variety
of solid tumors (27,28), there have been no specific studies
on the treatment efficacy of biological therapy in patients with
LCLC. The 83 patients diagnosed with LCLC in 2012 were followed-up
for one year, and the one-year survival rate of patients treated
with radical resection (65.1%) was significantly higher compared
with that of patients treated with palliative treatment (35.0%). No
statistically significant difference was observed in the one-year
survival rate of patients receiving postoperative radiochemotherapy
and patients that were treated with surgery alone. Hanagiri et
al (3) reported that the
postoperative five-year survival rate of Japanese patients with
LCLC of the lung receiving surgery was 60.5%, which was
significantly higher compared with that of non-surgical patients.
In addition, Saji et al (29) demonstrated that the five-year
survival rate of patients with LCLC receiving comprehensive
treatment was superior to that of patients treated with surgery
alone. However, this may have resulted from the short follow-up
period in this study.
In addition to radical resection, the present study
found that the TNM, N and M stages and histological types were
significantly correlated with the one-year survival rate of the 83
patients with LCLC confirmed in 2012. The one-year survival rate of
the patients with stage I was significantly higher compared with
the patients with stages II, III and IV. The survival rate was also
significantly higher in patients with stage II when compared with
patients with stages III and IV. Similarly, the one-year survival
rate of patients with stage N0 was significantly higher
than that of patients with stages N1, N2 and
N3, and was also significantly higher in patients with
stage N1 when compared with patients with stage
N3. Furthermore, the one-year survival rate of the
patients with stage M0 was significantly higher compared
with the patients with stage M1. Among the various
subtypes of LCLC, the patients with classic LCLC had a
significantly higher one-year survival rate (74.4%) than patients
with LCNEC (38.7%). Shimada et al (30) and Sun et al (11) also reported that among the subtypes
of LCLC, LCNEC is more aggressive with a higher degree of
malignancy, and clinical features that are closer to SCLC. Among
the 91 patients diagnosed with LCLC in 2013, 13 patients succumbed
during the follow-up period, indicating that LCLC of the lung is a
highly malignant and aggressive tumor.
Future studies should expand the sample size and
follow-up time period in order to summarize the morphological and
immunohistochemical features of LCLC more accurately, analyze the
impacting factors on patient prognosis more comprehensively and
compare the pros and cons of the treatment programs in more detail.
Only by following the diagnostic criteria of LCLC strictly and
fully combining immunohistochemical analyses with electron
microscopy can the diagnosis rate of LCLC and its subtypes be
improved. Subsequently, a basis for the clinical standardization of
the comprehensive treatment of LCLC may be established.
Acknowledgements
The study was funded by a grant from the National
Science Foundation of China (no. 30560053).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA-Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis WD, Brambilla E, Muller-Hermelink
HK and Harris CC: World Health Organization Classification of
Tumours. Pathology and Genetics of Tumours of the Lung, Pleura,
Thymus and Heart. IARC Press; Lyon: 2004
|
|
3
|
Hanagiri T, Oka S, Takenaka S, et al:
Results of surgical resection for patients with large cell
carcinoma of the lung. Int J Surg. 8:391–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Battafarano RJ, Fernandez FG, Ritter J, et
al: Large cell neuroendocrine carcinoma: an aggressive form of
non-small cell lung cancer. J Thorac Cardiovasc Surg. 130:166–172.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Yu JP, Yu WW, et al: Relationship
between VEGF expression and dendritic cells and regulatory T cell
distribution in large cell lung cancer. Zhongguo Zhong Liu Lin
Chuang. 39:1501–1504. 2012.(In Chinese).
|
|
6
|
Zhu R, Liu JM and Wang L: Clinical
analysis of 109 cases of large cell lung cancer. Linchuang
Zhongliuxue Zazhi. 17:912–914. 2012.
|
|
7
|
Kobold S, Völk S, Clauditz T, et al:
Interleukin-22 is frequently expressed in small- and large-cell
lung cancer and promotes growth in chemotherapy-resistant cancer
cells. J Thorac Oncol. 8:1032–1042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khaghanzadeh N, Mojtahedi Z, Ramezani M,
Erfani N and Ghaderi A: Umbelliprenin is cytotoxic against QU-DB
large cell lung cancer cell line but anti-proliferative against
A549 adenocarcinoma cells. Daru. 20:692012. View Article : Google Scholar
|
|
9
|
Yousefi Z, Sarvari J, Nakamura K, et al:
Secretomic analysis of large cell lung cancer cell lines using
two-dimensional gel electrophoresis coupled to mass spectrometry.
Folia Histochem Cytobiol. 50:368–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sabater-Marco V, García-García JA and
Roig-Vila JV: Basaloid large cell lung carcinoma presenting as
cutaneous metastasis at the colostomy site after abdominoperineal
resection for rectal carcinoma. J Cutan Pathol. 40:758–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun JM, Ahn MJ, Ahn JS, et al:
Chemotherapy for pulmonary large cell neuroendocrine carcinoma:
similar to that for small cell lung cancer or non-small cell lung
cancer? Lung Cancer. 77:365–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lubin JH and Caporaso NE: Cigarette
smoking and lung cancer: modeling total exposure and intensity.
Cancer Epidemiol Biomarkers Prev. 15:517–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia Y, Li B, Gao N, et al: Expression of
tumor-associated calcium signal transducer 2 in patients with
salivary adenoid cystic carcinoma: Correlation with
clinicopathological features and prognosis. Oncol Lett.
8:1670–1674. 2014.PubMed/NCBI
|
|
14
|
Rekhtman N, Tafe LJ, Chaft JE, et al:
Distinct profile of driver mutations and clinical features in
immunomarker-defined subsets of pulmonary large-cell carcinoma. Mod
Pathol. 26:511–522. 2013. View Article : Google Scholar :
|
|
15
|
Buendia AJ, Sánchez J, Martinez CM and
Navarro JA: Immunohistochemical characterization of a pulmonary
large-cell carcinoma in a dog. Vet Pathol. 45:484–488. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stojsic J, Stevic R, Kontic M, et al:
Large cell lung carcinoma with unusual imaging feature,
immunophenotype and genetic finding. Pathol Oncol Res. 17:175–179.
2011. View Article : Google Scholar
|
|
17
|
Pardo J, Martinez-Peñuela AM, Sola JJ, et
al: Large cell carcinoma of the lung: an endangered species? Appl
Immunohistochem Mol Morphol. 17:383–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barbareschi M, Cantaloni C, Del Vescovo V,
et al: Heterogeneity of large cell carcinoma of the lung: an
immunophenotypic and miRNA-based analysis. Am J Clin Pathol.
136:773–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe R, Ito I, Kenmotsu H, et al:
Large cell neuroendocrine carcinoma of the lung: is it possible to
diagnose from biopsy specimens? Jpn J Clin Oncol. 43:294–304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doddoli C, Barlesi F, Chetaille B, et al:
Large cell neuroendocrine carcinoma of the lung: an aggressive
disease potentially treatable with surgery. Ann Thorac Surg.
77:1168–1172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mimae T, Yamashita M, Maeda A, et al:
Large cell neuroendocrine carcinoma of the lung. Kyobu Geka.
62:442–445. 2009.PubMed/NCBI
|
|
22
|
Gollard R, Jhatakia S, Elliott M and Kosty
M: Large cell/neuroendocrine carcinoma. Lung Cancer. 69:13–18.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka Y, Ogawa H, Uchino K, et al:
Immunohistochemical studies of pulmonary large cell neuroendocrine
carcinoma: a possible association between staining patterns with
neuroendocrine markers and tumor response to chemotherapy. J Thorac
Cardiovasc Surg. 145:839–846. 2013. View Article : Google Scholar
|
|
24
|
Morbini P and Inghilleri S: Large-cell
lung carcinoma with basaloid architecture and neuroendocrine
differentiation: a new type of combined large-cell neuroendocrine
carcinoma. Int J Surg Pathol. 19:252–258. 2011. View Article : Google Scholar
|
|
25
|
Fournel L, Falcoz PE, Alifano M, et al:
Surgical management of pulmonary large cell neuroendocrine
carcinomas: a 10-year experience. Eur J Cardiothorac Surg.
43:111–114. 2013. View Article : Google Scholar
|
|
26
|
Rossi G, Cavazza A, Marchioni A, et al:
Role of chemotherapy and the receptor tyrosine kinases KIT,
PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine
carcinoma of the lung. J Clin Oncol. 23:8774–8785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Wang C, Yu J, et al: Dendritic
cell-activated cytokine-induced killer cells enhance the anti-tumor
effect of chemotherapy on non-small cell lung cancer in patients
after surgery. Cytotherapy. 11:1076–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Q, Zhang H, Li Y, et al: Anti-tumor
effects of CIK combined with oxaliplatin in human
oxaliplatin-resistant gastric cancer cells in vivo and in vitro. J
Exp Clin Cancer Res. 29:1182010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saji H, Tsuboi M, Matsubayashi J, et al:
Clinical response of large cell neuroendocrine carcinoma of the
lung to perioperative adjuvant chemotherapy. Anticancer Drugs.
21:89–93. 2010. View Article : Google Scholar
|
|
30
|
Shimada Y, Niho S, Ishii G, et al:
Clinical features of unresectable high-grade lung neuroendocrine
carcinoma diagnosed using biopsy specimens. Lung Cancer.
75:368–373. 2012. View Article : Google Scholar
|