Introduction
Diabetic retinopathy (DR) is the most common cause
of visual disorders leading to irreversible blindness.
Blood-retinal barrier (BRB) loss is crucial in the pathogenesis of
DR; however, the precise mechanisms leading to retinal vasculature
and tissue damage in DR have not been fully elucidated. The
integrity of the BRB is determined by a watertight apical
junctional complex that is composed of tight and adherens
junctions. Adherens junctions are predominantly formed by
homophilic interactions between proteins of the cadherin family,
which are transmembrane calcium-dependent adhesion proteins.
Vascular endothelial (VE)-cadherin is the best-characterized member
of this family of proteins (1).
VE-cadherin is an endothelial cell (EC)-specific adhesion molecule
that connects adjacent ECs (2).
While the barrier function of the endothelium is supported by
multiple cell-cell adhesion systems, the disruption of VE-cadherin
is sufficient to disrupt intercellular junctions (3). The association between VE-cadherin
and the actin cytoskeleton is necessary for strong mechanical
cell-cell interaction. This interaction is mediated via
VE-cadherin-bound β- and α-catenin, which, in turn, are associated
with actin filaments through actin-binding proteins, including
Epithelial Protein Lost In Neoplasm, vinculin, formin-1 or
α-actinin (4). The tyrosine (Tyr)
phosphorylation of VE-cadherin has been reported to cause a loss of
the ability of VE-cadherin to bind β-catenin (5), while the Tyr phosphorylation of
β-catenin has been demonstrated to decrease the affinity of
β-catenin for the cadherin and increase its turnover at junctions
(5). The assembly of the
VE-cadherin/catenin adhesion complex is strictly regulated; this
regulation involves a number of post-transcriptional processes,
including phosphorylation, dephosphorylation, protein interactions
and changes in protein stability (6). Ras-related C3 botulinum toxin
substrate 1 (Rac1), one of the Rho family members, has a key role
in this process, which has been previously reviewed by Hall
(7).
The active, guanosine triphosphate (GTP)-bound form
of Rac1 regulates the formation of submembraneous actin
cytoskeleton structures, which leads to the formation of
lamellipodia (7). Rac1
localization at membrane sites is a critical event in the
maturation of epithelial cell adherens junctions during cell
polarization (8). It has
previously been demonstrated that the Rac1-induced generation of
reactive oxygen species (ROS) disrupts VE-cadherin-based cell-cell
adhesion (9). Furthermore, the
interaction of active Rac1 with IQ motif containing GTPase
activating protein 1 (IQGAP1), which is associated with a
disassembly of E-cadherin-mediated adherens junctions, has been
shown to destabilize E-cadherin-mediated cell-cell adhesion in
pancreatic carcinoma cells. By contrast, the inhibition of Rac1
activity increased E-cadherin-mediated cellular adhesion (10). Rac and ROS are required for the
regulation of vascular endothelial growth factor (VEGF)-induced
microvascular permeability (11).
Furthermore, the nuclear accumulation of β-catenin in response to
Wnt requires Rac1 activation. A previous study demonstrated that
silencing of the Rac1 gene in the mouse embryonic limb bud ectoderm
disrupted canonical Wnt signaling and phenocopy deletion of
β-catenin, causing severe truncations of the limb (12). However, to date, no biological
function of Rac1 and β-catenin in DR has been demonstrated.
Materials and methods
Animal models
A total of 70 male Sprague Dawley rats with body
weights of 260±5.6 g were obtained from the Laboratory Animal
Center of the Fourth Military Medical University of the PLA (Xian,
China) for use in this study. Of these, 40 animals were housed
under a 12-h light/dark cycle, with four rats per cage, and were
fed standard rat chow and water ad libitum. Diabetes was
induced by a single intraperitoneal injection of streptozotocin
(STZ; Sigma, St. Louis, MO, USA) in 0.05 M citrate buffer (pH 4.5)
at a dose of 65 mg/kg body weight, and was defined as blood glucose
levels >15 mmol/l (270 mg/dl), seven days after STZ injection.
The other 30 animals received a single intraperitoneal injection of
0.05 M citrate buffer as a control group. Blood samples were
obtained from the rat tail vein and the glucose concentration was
determined with an automatic analyzer (Glucometer Elite XL; Bayer
Inc., Toronto, ON, Canada) using glucose oxidase/potassium
ferricyanide reagent strips. Body weights and blood glucose levels
were noted once a week after the induction of diabetes. This study
was carried out in strict accordance with the recommendations in
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee of
the Fourth Military Medical University of the PLA.
Measurement of retinal vascular
permeability
Retinal vascular permeability was quantified by
measuring albumin leakage from blood vessels into the retina using
the Evans blue method in accordance with the procedures described
in a previous study (13) with
minor modifications (14).
Tissue and retinal digest preparation for
immunohistochemistry
The animals were sacrificed with an intraperitoneal
overdose of pentobarbital. The eyes were enucleated, fixed in 4%
paraformaldehyde for 24 h and rinsed with water for 24 h. The
retinas and tissues were carefully removed. For retinal
immunohistochemistry, the fixed retinas were embedded in paraffin
and 5-μm histological sections were made.
For retinal digest preparation, the retinal
vasculatures of rats were isolated as described previously
(15). Briefly, each fixed retina
was cut into four parts and rinsed with 0.1 M phosphate-buffered
saline (PBS) for 24 h, prior to being placed into 3% pancreatin
solution dissolved by 0.1 mol/l Tris-HCl buffer fluid (pH 7.8) and
incubated for 3 h at 37°C. The solution was changed only when the
retina was not completely digested. The samples were then moved to
distilled water and agitated gently until the inner membrane and
remaining neurosensory retina were completely rinsed off and only a
thin layer of retinal vascular network was left. The retinal
vascular network layer was washed out and carried onto a slide,
prior to being unfolded and dried naturally. The samples were
stored at −20°C in preparation for immunohistochemistry.
Cell culture
RRECs were purified as previously described
(16). Contamination of the
microvessel preparations by neuronal tissue, assessed subsequent to
microscopic examination and western blotting with a monoclonal
antibody raised against rhodopsin (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), was typically <5% (6). The RRECs were isolated and cultured
as previously described (6). The
RRECs were grown in primary culture on dishes coated with collagen
IV/fibronectin (2 and 4 μg/cm2, respectively;
Sigma-Aldrich Corp., St. Louis, MO, USA) in Dulbecco’s modified
Eagle’s medium (DMEM; Vector Laboratories, Inc., Burlingame, CA,
USA) containing 15% human serum, 80 μg/ml heparin, 2 mm glutamine
and antibiotics (penicillin G potassium and streptomycin sulphate).
The purity of the RREC cultures was assessed, in accordance with a
previously described method (6),
to be >90%. The media were supplemented with 10 μM unesterified
docosahexaenoic acid (DHA) at a molar ratio over albumin in serum
of 1:10. This supplementation restored the DHA proportion of these
cells to the original value observed in the intact microvessels
(6). At confluence, the RRECs were
trypsinized and seeded in gelatin- and fibronectin-coated dishes
(Sigma-Aldrich Corp.). An identical batch of cells derived from one
primary culture was used to compare the effects of hyperglycemic
conditions. Glucose or mannitol (15, 25 or 30 mm) or bovine serum
albumin (control) was added to the RREC culture medium.
Recombinant pSUPER-Rac1-shRNA
construction
The expression vector, pSUPER-GFP/Neo RNA
interference (RNAi) system (Oligoengine, Seattle, WA, USA) was used
for the expression of siRNA. The selection of the human and rat
homologous Rac1 gene siRNAs was based on the characterization of
siRNA by Elbashir et al (17). Among the different Rac1 target
sequences examined, the 19-nucleotide gene-specific sequence
spanning between nucleotides 367 and 385 downstream of the gene
transcription start site was selected to suppress Rac1 gene
expression. Following Basic Local Alignment Search Tool analysis
(www.ncbi.nlm.nih.gov/BLAST/), to ensure
that there was no significant sequence homology with other human
and rat genes, the selected sequence was AGACACGATCGAGAAACTG. The
short hairpin (sh)RNA insert template oligonucleotides consisted of
an RNA duplex containing a sense strand (5′-GAT
CCCCAGACACGATCGAGAAACTGTTCAAGAGACAGT TTCTCGATCGTGTCTTTTTTGGAAA-3′)
and an antisense strand (5′-AGCTTTTCCAAAAAAGACACGATCGAGAAA
CTGTCTCTTGAACAGTTTCTCGATCGTGTCTGGG-3′). The template
oligonucleotides were synthesized and purified by Sangon Biotech
Company (Shanghai, China), and then annealed and ligated into the
BglII and HindIII sites of the linearized
pSUPER-GFP/Neo RNAi system. A non-silencing control vector
(non-silencing shRNA; NS) was constructed using a 19-nucleotide
sequence (GCGCGCTTTGTAGGATTCG) with no significant homology to any
mammalian gene sequence. All inserted sequences were confirmed by
DNA sequencing.
siRNA transfection
RRECs were cultured at 37°C in a 5% CO2
atmosphere in DMEM supplemented with 10% fetal calf serum. Cells
were plated in 24-well plates at 2×105 cells per well,
cultured for 24 h and then transfected with pSUPER-Rac1-shRNA
according to the manufacturer’s instructions (Oligoengine).
Negative control cells were treated with NS consisting of a circle
plasmid encoding an siRNA whose sequence was not found in mouse,
human or rat genome databases. Lipofectamine 2000™ (Invitrogen Life
Technologies, Carlsbad, CA, USA) was used for the transfection
according to manufacturer’s instructions. Seventy-two hours after
transient transfection, silencing was examined using western
blotting and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis.
[3H]Sucrose permeability
assay
The [3H]sucrose permeability assay was
performed as described by Kim et al (18), with minor modifications. In brief,
RRECs (1×105 cells) with or without Rac1 gene siRNAs, NS
siRNA or siRNA targeting Rac1 were plated onto a Transwell™ filter.
At 60–70% confluency the complete medium was replaced with
endothelial basal medium-2 (EBM™-2; Lonza, Basel, Switzerland)
containing 25 mm D-glucose for 24 h. [3H]Sucrose, 50 μl
(0.8 μCi/ml) (1 μCi/μl; Amersham Pharmacia Biotech, Amersham, UK),
was added to the upper compartment. The amount of radioactivity
that diffused into the lower compartment was determined after 30
min by a liquid scintillation counter (Perkin Elmer/Wallac, Inc.,
Gaithersburg, MD, USA).
Total RNA extraction and qPCR
Total RNA was isolated from the RRECs and retinas at
passage 17 using Trizol™ reagent (Gibco-BRL, Gaithersburg, MD, USA)
according to the manufacturer’s instructions. A total of 2 μg
isolated RNA was reverse-transcribed into cDNA using a
high-capacity cDNA synthesis kit (Takara Bio, Inc., Shiga, Japan).
Quantitative analysis of gene expression was generated using a
sequence detection system (7300 Real-Time; Applied Biosystems,
Foster City, CA, USA) and a SYBR Green Real-Time PCR Master Mix kit
(Takara Bio, Inc). Semi-log amplification curves were evaluated by
the comparative quantification method (2−ΔΔCt), and
β-actin was used for data normalization. The primer sequences
(synthesized by Sangon Biotech Company) used in the present study
were as follows: Rac1 forward, 5′-GGACAAGAAGATTAT GACAG-3′ and
reverse, 5′-ATACCACTTTGCACGGACAT-3′; VE-cadherin forward,
5′-CCTACCAGCCCAAAGTGTGT-3′ and reverse, 5′-GACTTGGCATCCCATTGTCT-3′;
β-catenin forward, 5′-TGGGCAGTTTGCAATGACCAGA-3′ and reverse,
5′-ACGCATAATAGCATGGCGGGAA-3′; β-actin forward,
5′-GAGGGAAATCGTGCGTGAC-3′ and reverse,
5′-GAGTGACAGGTGGAAGGTC-3′.
Immunohistochemistry
The paraffin-embedded retinal tissue sections were
rehydrated through xylene and graded alcohols. These rehydrated
retinal tissue sections or slides containing the retinal
vasculature were treated with 3% hydrogen peroxide for 10 min. The
retinal tissue sections were made by microwaving in sodium citrate
for 20 min for antigen retrieval. Subsequent to three 5-min rinses
in PBS and incubation with normal blocking serum (Vector
Laboratories, Inc.) for 30 min, the retinal tissue sections and
slides containing the retinal vasculature were incubated with
primary antibodies in block solution overnight. The primary
antibodies were rabbit anti-GTP-Rac1 (diluted 1:50; Stressgen
Biotechnologies Corp., Victoria, BC, Canada), rabbit
anti-VE-cadherin (1:100; Santa Cruz Biotechnology, Inc.) and rabbit
anti-β-catenin polyclonal antibody (diluted 1:200; Abcam,
Cambridge, UK). Subsequent to a further three 5-min rinses in PBS,
the samples were incubated with a 1:2,000 dilution of biotinylated
goat anti-rabbit immunoglobulin G (IgG) antibody (Vector
Laboratories, Inc.) for 30 min and for 30 min with horseradish
peroxidase-conjugated avidin (Vector Laboratories, Inc.). The
antigens were detected with a diaminobenzidine kit (Sigma, St.
Louis, MO, USA); the brown/yellow reaction product was visualized
by light microscopy. Negative controls consisted of incubations in
5% isotype control serum without the primary antibody and did not
generate a reaction product. For image capture, a high-resolution
video camera (DXC-960MD; Sony Corp., Tokyo, Japan) mounted on a
BH-2 Olympus microscope (Olympus Corp., Melville, NY, USA) was
computer-linked and retinal images were selected at a distance of
~0.8 mm from the optic nerve head.
Western blot analysis
Western blotting was performed using standard
western blotting methods. The protein concentration was measured
using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL,
USA). Equal amounts of protein were separated by 5–10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
electrophoretically onto nitrocellulose membranes (Amersham
Pharmacia Biotech). The membranes were blocked for 30 min in 5%
skimmed milk. Following blocking, the membranes were incubated
overnight with anti-VE-cadherin (1:1,000; Santa Cruz Biotechnology,
Inc.), anti-GTP-Rac1 (1:1,000; Zymed Laboratories, San Francisco,
CA, USA) and anti-β-catenin (1:2,000; Zymed Laboratories)
antibodies at 4°C. Following washing with PBS with Tween 20
(PBS-T), the membranes were incubated for 1 h at room temperature
with horseradish peroxidase-conjugated anti-rabbit IgG or
anti-mouse IgG (1:10,000, Pierce) in PBS-T and 1% skimmed milk. To
ensure the equal loading of protein in each lane, the blots were
stripped and reprobed with an antibody against β-actin. Intensity
values were normalized relative to control values. The blots were
scanned using a flatbed scanner and the band intensity was analyzed
using the TINA software program (Raytest, Staubenhardt,
Germany).
Rac1 activity assays
RRECs were cultured at 37°C in EBM-2 supplemented
with EGM™-2 SingleQuots™ (Lonza). RRECs were used between passages
four and eight. At 60–70% confluency the medium was changed to
EBM-2 (without supplements) containing 25 mM D-glucose for the
indicated time periods. Cells incubated with 5 mM D-glucose plus 20
mM D-mannitol or 25 mM L-glucose served as controls. Following
stimulation the cells were kept on ice, washed with ice-cold PBS
and assayed for Rac1 activation with glutathione
S-transferase-p21-activated kinase 1B (GST-PAK1B), as described by
Sander et al (19). The
beads were washed four times with lysis buffer and, after the final
wash, resuspended in sample buffer. Samples were then analyzed by
western blotting.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between two groups were conducted with an
independent-samples t-test. One-way analysis of variance was used
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Body weight change and blood glucose
level
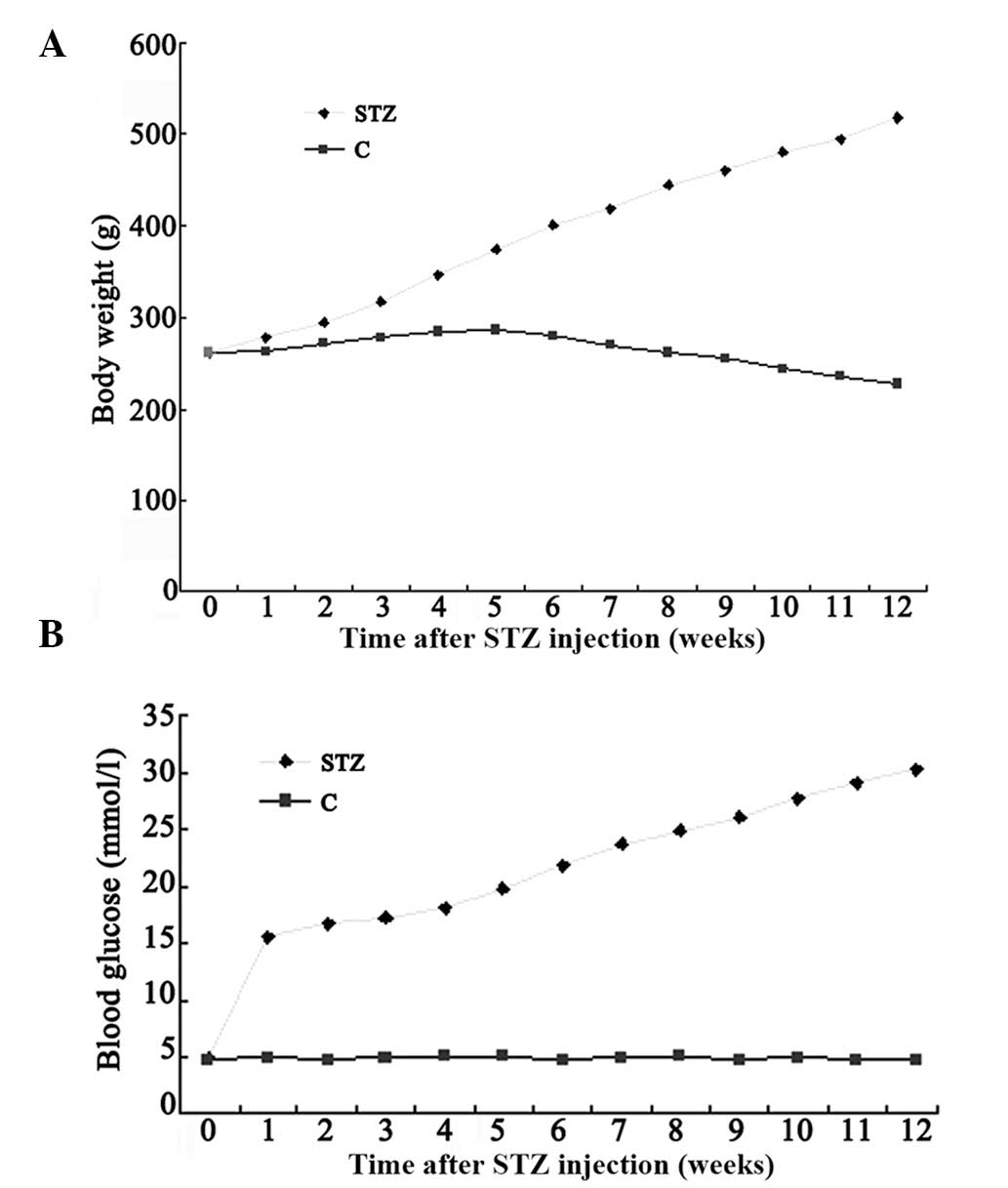
In the STZ-induced diabetic rats, body weight was
significantly lower than that in the age-matched controls. At 12
weeks after induction of diabetes, the body weight of the diabetic
rats was 236±14.1 g (n=40), which was significantly decreased
(P<0.001) from the body weight of the controls (496±10.2 g,
n=30) (Fig. 1A).
The diabetic rats showed significant increases in
blood glucose levels compared to the control rats. At 12 weeks
after induction of diabetes, the blood glucose level of the
diabetic rats was 30.2±4.8 mmol/l (n=40), which was significantly
different (P<0.001) from that of the controls (4.9±0.12 mmol/l,
n=30) (Fig. 1B).
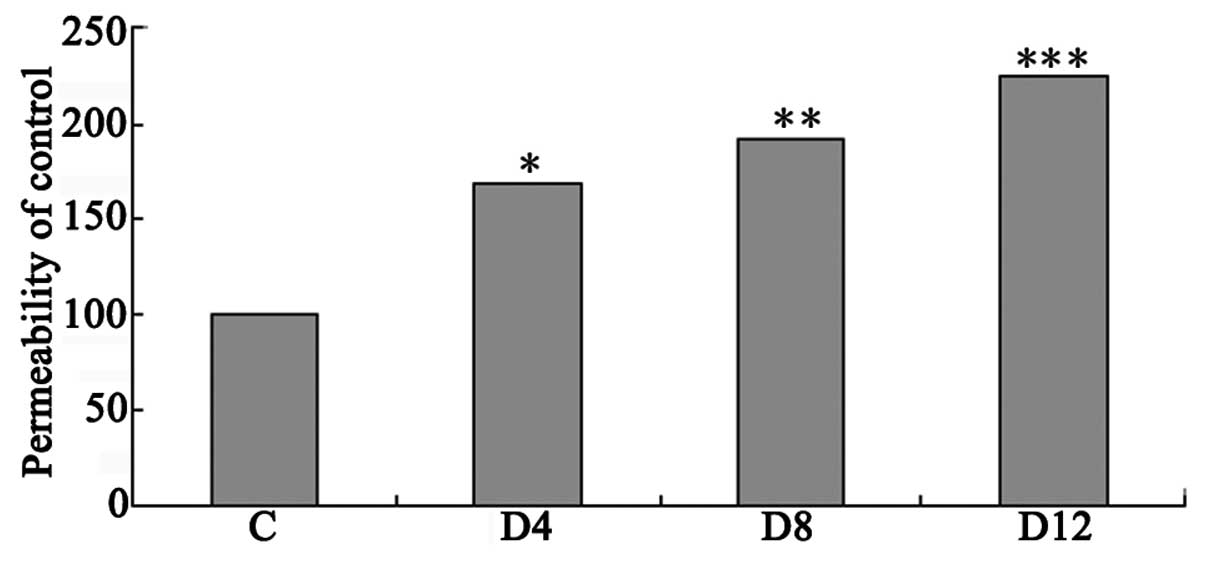
Alteration of retinal vascular
permeability in STZ-induced diabetic rats
Vascular permeability in the retina was measured
using the Evans blue method. Vascular permeability was increased by
68, 91 and 125%, respectively (each P<0.005), in the retinas at
four, eight and 12 weeks after the induction of diabetes compared
with that in the controls (Fig.
2).
Activity and expression of Rac1 in the
retina of STZ-induced diabetic rats
To examine whether chronically elevated blood
glucose levels affected the expression of Rac1, RT-qPCR experiments
were performed using β-actin for normalization. The results showed
that the level of Rac1 mRNA expression was not increased in the
diabetic retinas at four, eight and 12 weeks after induction of
diabetes compared with that in the controls (Fig. 3A).
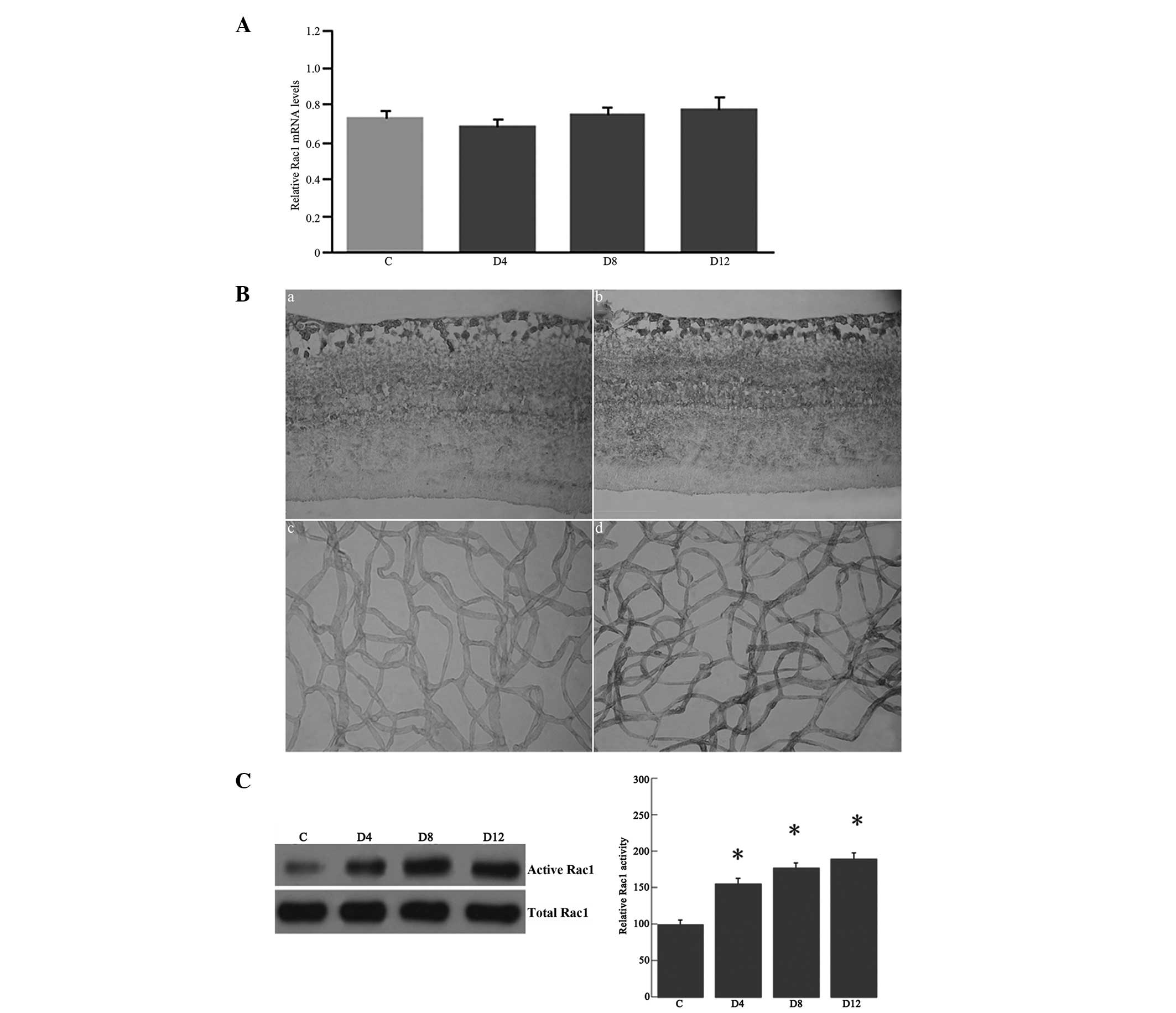 | Figure 3(A) Reverse transcription-quantitative
polymerase chain reaction analysis of Rac1 mRNA expression in the
retinas of streptozotocin-induced diabetic and control rats. The
retinal Rac1 mRNA expression was examined in the rat retinas at
four, eight and 12 weeks after induction of diabetes (D4, D8 and
D12, respectively), as well as in the controls, using β-actin as an
internal control. Levels of Rac1 mRNA expression remained unchanged
in the diabetic retinas at four, eight and 12 weeks after induction
of diabetes compared with those in the controls. (B)
Immunohistochemical analysis of Rac1 in the retinas and retinal
vasculature isolated by the trypsin digest technique: (a) Control
and (b) diabetic rat retinas; (c) control and (d) diabetic rat
retinal vasculature. The immunostaining of Rac1 was present in the
outer plexiform, inner plexiform and ganglion cell layers and in
the endothelial cells and pericytes. Rac1 immunoreactivity was
increased in the retinas and retinal vasculature of diabetic rats
at 12 weeks after the induction of diabetes (b and d) compared with
that of the controls (a and c). In the negative control staining,
no immunoreactivity for Rac1 was found in the retinas and the
retinal vasculature (figure not shown) (original magnification,
×400). (C) Rac1 activity from the retinal lysates of the rats.
Autoradiography depicting the Rac1 activity shows increased Rac1
activity in the retinas at four, eight and 12 weeks after the
induction of diabetes compared with that in the controls.
*P<0.05 vs. the control. C, control; Rac1,
Ras-related C3 botulinum toxin substrate 1. |
Immunohistochemical analysis using anti-Rac1
monoclonal antibody showed that the immunostaining of Rac1 occurred
in the retinal vasculature, including the ECs and pericytes, and in
the outer plexiform layer (OPL), the inner nuclear layer (INL), the
inner plexiform layer (IPL) and the ganglion cell layer (GCL) of
the rat retinas; the Rac1 immunoreactivity was significantly
increased in the retinas at 12 weeks after the induction of
diabetes compared with that in the controls (Fig. 3B).
The Rac1 activity in the retinas at various
time-points was then determined in the STZ-induced diabetic and
control rats using the CRIB-domain of PAK1B (GST-PAK) as an
activation-specific probe for activated Rac1, as described
previously (20). As shown in
Fig. 3C, Rac1 activation increased
by 55.6, 77.6 and 89.8%, respectively (each P<0.05), in the
retinas at four, eight and 12 weeks after the induction of diabetes
compared with that in the controls.
Level of β-catenin expression
To further evaluate the effects of hyperglycemia on
β-catenin in the retinas from the STZ-induced diabetic rats, its
immunoreactivity was determined using anti-β-catenin monoclonal
antibody. The results indicated that immunostaining of β-catenin
was present in the retinal vasculature and in the OPL, INL, IPL and
GCL of the retina in both the diabetic rats at 12 weeks after the
induction of diabetes and the controls; however, the β-catenin
immunoreactivity was significantly increased in the retinas at 12
weeks after the induction of diabetes compared with that in the
controls (Fig. 4A).
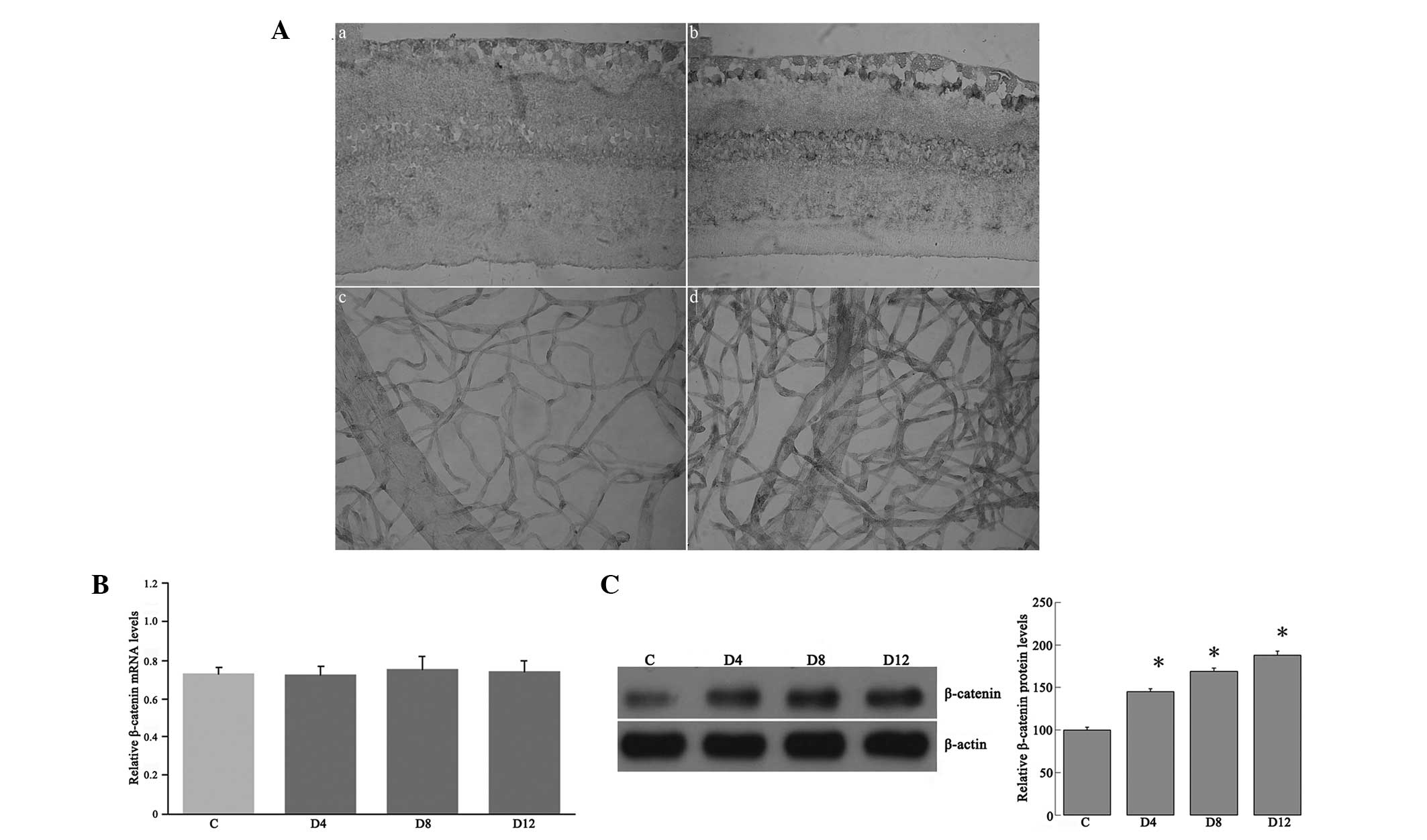 | Figure 4(A) Representative immunohistochemical
analysis of β-catenin in the retinas and retinal vasculature.
β-catenin immunoreactivity was observed in the retinal vasculature
and in the outer plexiform, inner nuclear, inner plexiform and
ganglion cell layers of retinas from (a and c) control rats and (b
and d) diabetic rats 12 weeks after the induction of diabetes;
immunoreactivity was increased in the retinas of the diabetic rats.
In the negative control staining, no immunoreactivity for β-catenin
was found in the retinas and retinal vasculature (figure not shown)
(original magnification, ×400). (B) Reverse
transcription-quantitative polymerase chain reaction analysis of
β-catenin mRNA expression in the retinas of streptozotocin-induced
diabetic and control rats. The retinal β-catenin mRNA expression
was examined in the rat retinas at four, eight and 12 weeks after
the induction of diabetes (D4, D8 and D12, respectively), as well
as in the controls, using β-actin as an internal control. (C)
Western blot analysis for β-catenin from the retinal lysates of the
rats. Autoradiography depicting the β-catenin shows the β-catenin
expression increased in the retinas at four, eight and 12 weeks
after the induction of diabetes compared with that in the controls.
*P<0.05 vs. the control. |
To examine the effects of hyperglycemia on the
signaling molecules of β-catenin, the activity and expression of
β-catenin was determined by RT-qPCR and western blot analysis. The
RT-qPCR showed that levels of β-catenin mRNA expression were not
changed in the retinas of the diabetic rats at four, eight or 12
weeks after the induction of diabetes compared with that in the
controls (Fig. 4B); however,
western blotting showed that β-catenin protein levels were
increased by 45.2, 68.8 and 88.1%, respectively (each P<0.05),
in the retinas at four, eight and 12 weeks after the induction of
diabetes compared with that in the controls (Fig. 4C).
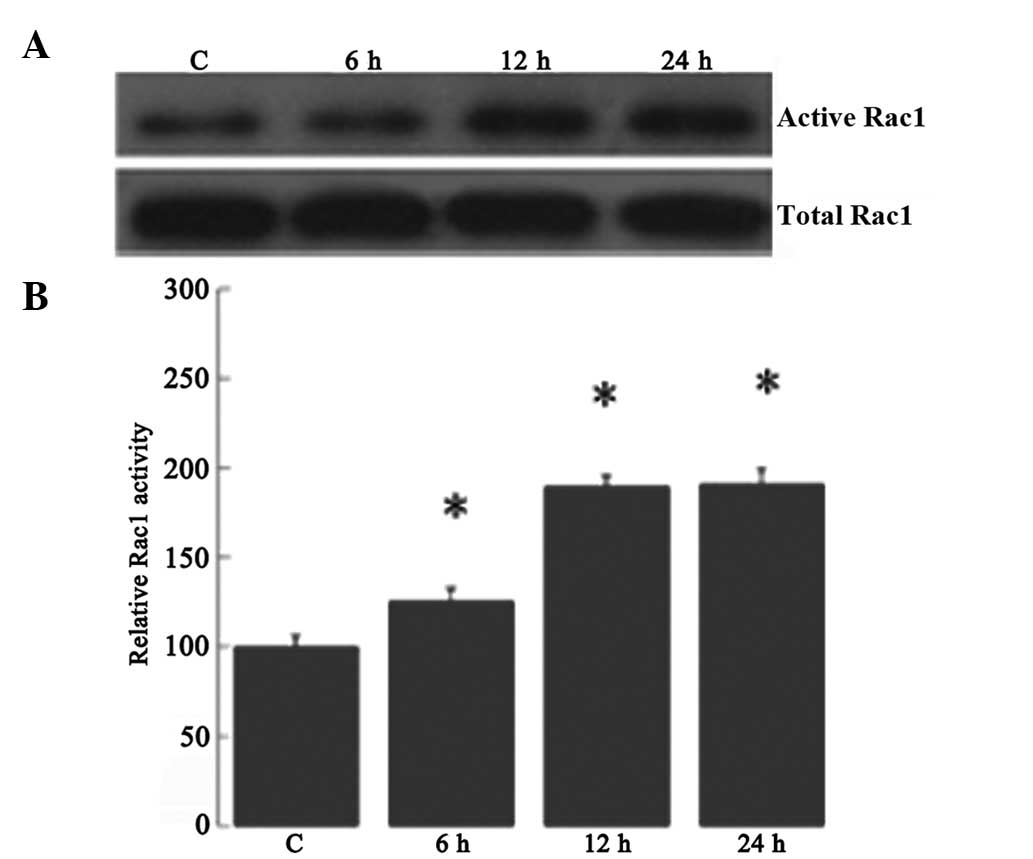
High glucose-induced increased Rac1
activity in RRECs
It was next investigated whether high glucose
regulates Rac1 activity in RRECs. The Rac1 activity in high
glucose-induced RRECs at various time-points was determined using
the CRIB-domain of PAK1B (GST-PAK) as an activation-specific probe
for activated Rac1, as described previously (19). Rac1 activity was increased by 25.6,
89.8 and 90.8%, respectively, in the high glucose-induced RRECs at
six, 12 and 24 h compared with that in the controls (Fig. 5).
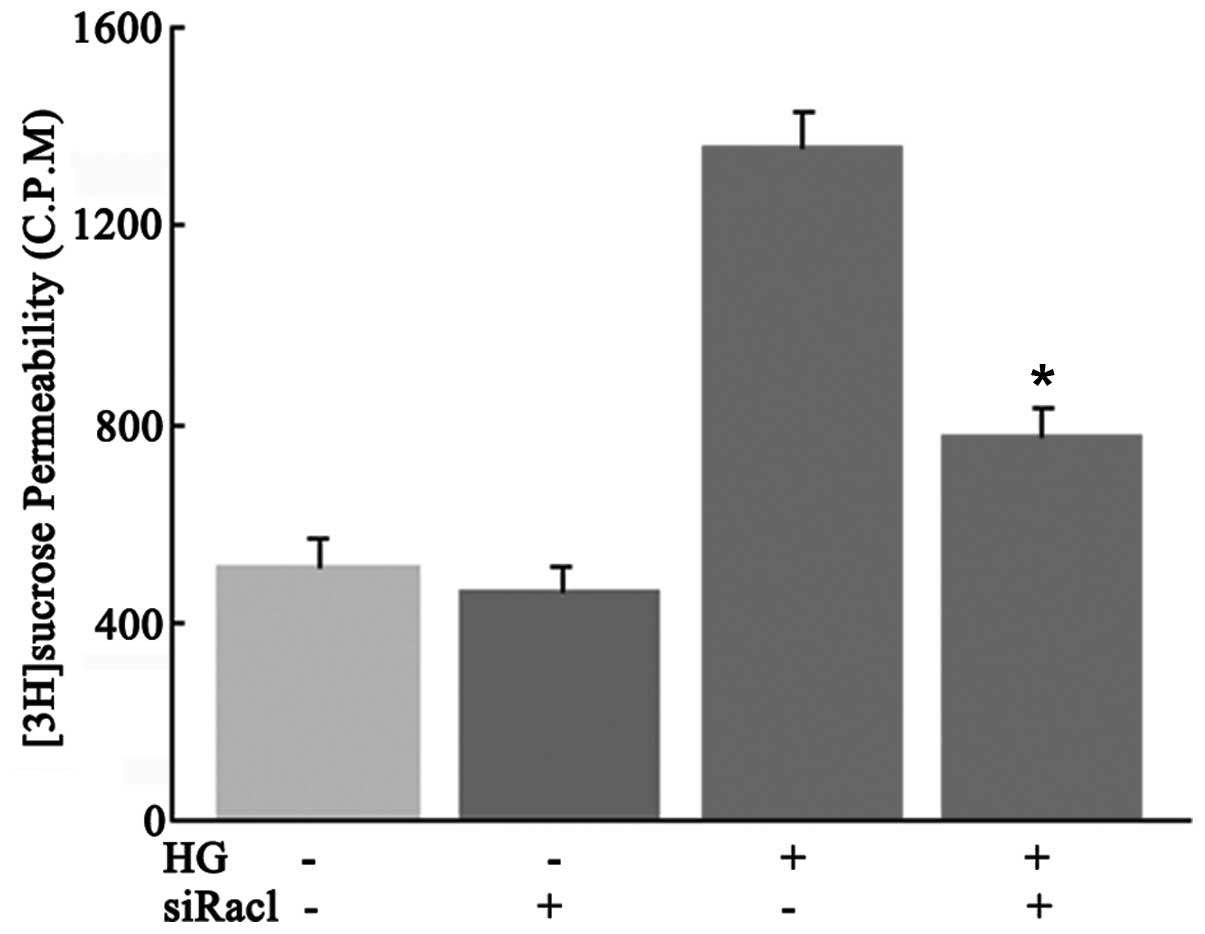
Effect of Rac1 activity on the
permeability of high glucose-induced RRECs
To investigate the effect of Rac1 inhibition by
Rac1-siRNA on high glucose-induced hyperpermeability in RRECs, a
[3H]sucrose permeability assay was performed. As shown
in Fig. 6, Rac1 inhibition by
Rac1-siRNA transfection effectively prevented hyperpermeability in
high glucose-induced RRECs (P<0.05).
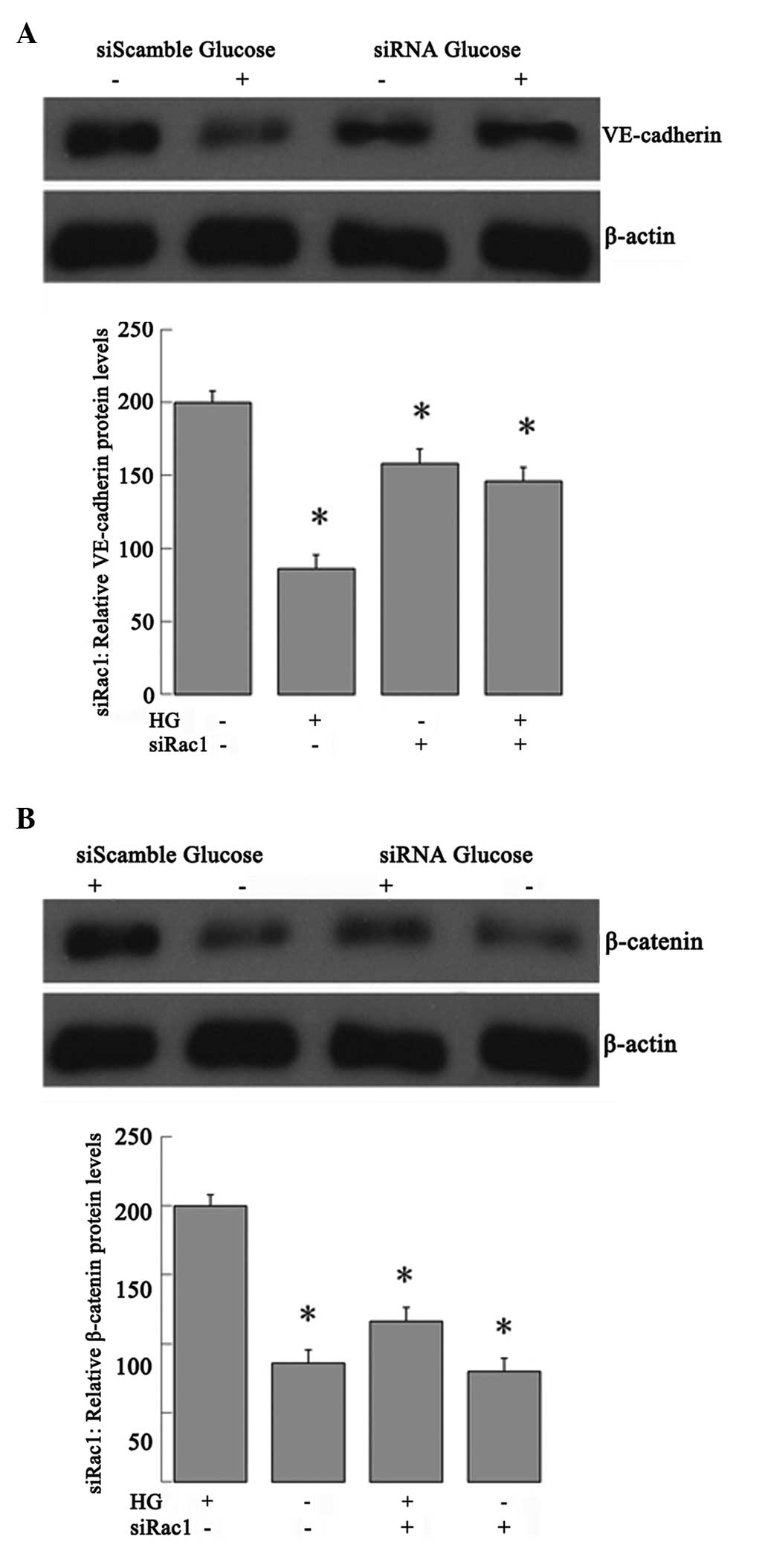
Effect of Rac1 activity on VE-cadherin
and β-catenin protein expression
To further evaluate the effects of Rac1 activity on
VE-cadherin and β-catenin protein expression in the high
glucose-induced RRECs, the RRECs were transfected with Rac1-siRNA
or NS siRNA for 12 h and then treated with 25 mmol/ml glucose.
Western blot analysis showed that Rac1 was required for the high
glucose-induced VE-cadherin expression decrease (Fig. 7A) and also for high glucose-induced
β-catenin expression (Fig. 7B).
Cells transfected with the NS siRNA were able to decrease
VE-cadherin expression and enhance β-catenin expression upon
high-glucose treatment.
Discussion
Retinopathy is one of the most disabling diabetic
complications, characterized by functional abnormalities of the
retinal microvasculature. In the eyes, the BRB has an important
role in retinal homeostasis; breakdown of the BRB in pathological
conditions such as diabetes may lead to retinopathy and blindness.
In this process, hyperglycemia is an underlying contributing
factor; however, the mechanisms that mediate the BRB breakdown are
not yet fully understood in DR. The present results demonstrated
for the first time, to the best of our knowledge, that Rac1
activity and β-catenin expression were increased in the early
stages of DR. Furthermore, Rac1 inhibition decreased β-catenin
expression and prevented the decrease in VE-cadherin expression in
high glucose-induced RRECs. These findings may enhance the
understanding of the pathogenesis of DR.
The BRB breakdown eight days after the induction of
diabetes by STZ occurs predominantly at the level of the retinal
blood vessels (20). In agreement
with this, the present results showed that retinal permeability
increased gradually in the diabetic retinas with the progression of
DR. Similarly, western blot analysis showed that Rac1 activity
increased gradually in the diabetic retinas with the progression of
DR. This suggests that increased Rac1 activity may be involved in
the retinal control and development of vascular permeability in
STZ-induced DR. The present results demonstrated that increased
Rac1 activity occurred in the retina at four, eight and 12 weeks
after the induction of diabetes by STZ, although Rac1 mRNA levels
remained unchanged. The results also revealed that the
immunoreactivity of Rac1 was increased in the retinas of the
STZ-induced diabetic rats. Immunostaining of Rac1 was localized to
the cells in the OPL, the INL, the IPL, the GCL and the
microvessels of the rat retinas. It has been suggested that Rac1
reorganizes the VEGF-induced actin cytoskeleton in ECs by
regulating nicotinamide adenine dinucleotide
phosphate-oxidase-derived ROS during EC migration (21). The activation of Rac1 in choroidal
ECs is essential for their migration across a monolayer of retinal
pigment epithelium towards a VEGF gradient (22). These results suggest that Rac1 and
VEGF interact reciprocally through an ROS-dependent signaling
pathway.
Appropriate levels of active Rac are required for
the formation and maintenance of adherens junctions, and either too
high or too low activities promote disassembly. Both constitutively
active and dominant negative Rac increase endothelial permeability,
consistent with the necessity of maintaining levels of Rac within
strict limits for optimal junctional integrity (23). In addition, several studies have
demonstrated that the activation of Rac1 downstream of VEGF, and
other growth factors, promotes junction disassembly and increases
permeability (24,25). VEGF has been observed to regulate
EC permeability through PAK, a direct downstream effector of Rac
(26); however, this association
is more complex than simply high Rac activation leading to
increased permeability. That active Rac can both increase and
decrease permeability depending on the stimulus leads to the
hypothesis that the route of activation may be critical and that
different scaffolding proteins may direct Rac signaling pathways in
different directions. A previous study showed that Rac1
gene-targeting shRNA successfully inhibited hypoxia-induced retinal
neovascularization and VEGF expression in a mouse model of
oxygen-induced retinopathy (27).
In a different study, it was demonstrated that VEGF regulated
microvascular permeability through the activation of Rac1 and the
production of ROS. These molecules, in turn, regulated the Tyr
phosphorylation of adherens junction proteins VE-cadherin and
β-catenin, ultimately regulating junctional integrity (11). The present results showed that Rac1
inhibition by Rac1-siRNA transfection effectively prevented
hyperpermeability, the decrease in VE-cadherin expression and the
increase in β-catenin protein levels in high glucose-induced
RRECs.
The association of VE-cadherin with the actin
cytoskeleton is necessary for strong mechanical cell-cell
interaction, which is mediated via E-cadherin-bound β-catenin. The
assembly of the VE-cadherin/catenin adhesion complex is under tight
control, which involves different post-transcriptional processes,
including phosphorylation and dephosphorylation, protein
interactions and the alteration of protein stability (6). Published data have shown that the
VE-cadherin expression decreases in rats after two weeks of
diabetes (28). The enhanced
phosphorylation of VE-cadherin at Tyr-731 changes the binding
affinity for β-catenin and likely decreases cytoskeletal attachment
(5). The loss of binding affinity
for VE-cadherin and β-catenin, which may change the phosphorylation
of β-catenin, may affect the levels of β-catenin protein. The
present results indicated that the immunostaining of β-catenin was
present in the retinal vasculature and in the OPL, INL, IPL and GCL
of the retina in both the diabetic rats at 12 weeks after the
induction of diabetes and the controls, but the β-catenin
immunoreactivity was significantly increased in the retinas of the
rats at 12 weeks after the induction of diabetes. Although the data
showed that levels of β-catenin mRNA were not increased in the
diabetic retinas at four, eight and 12 weeks after the induction of
diabetes, western blot analysis showed that β-catenin protein
levels were increased in the retinas following the induction of
diabetes. In a previous study, it was shown that retinal levels and
nuclear translocation of β-catenin were increased in humans with DR
and in three DR models. The high glucose-induced activation of
β-catenin was attenuated by aminoguanidine, suggesting that
oxidative stress is a direct cause for the Wnt pathway activation
in diabetes (29). Following its
translocation into the nucleus, β-catenin acts as a transcription
factor. Under normal physiological conditions in ECs, cytosolic
β-catenin binds to a protein complex, such as glycogen synthase
kinase 3β (GSK3β) (31). GSK3β-dependent phosphorylation of
β-catenin leads to β-catenin ubiquitination and degradation. When
GSK3β is phosphorylated it loses its activity; thus, β-catenin
escapes ubiquitination, accumulates in the cytosol and translocates
into the nucleus to induce gene transcription.
The important role of the Rho family member Rac1 in
the VE-cadherin/catenin adhesion complex, which is tightly
controlled and requires the regulated assembly of actin filaments
at sites of cell-cell contacts, has been reviewed by Hall (7). Data show that the enhanced expression
of active Rac1 reduces cell-cell adhesion and increases directed
cell motility and migration through the extracellular matrix.
Furthermore, activated Rac1 binds to IQGAP1, which is linked to a
reduced association of IQGAP1 with β-catenin. These alterations
have been shown to be associated with a disassembly of the
E-cadherin/catenin adhesion complex, resulting in inhibited
cellular aggregation and an elevated migratory capacity of
pancreatic carcinoma cells (10).
The present results showed that Rac1 activation inhibited
VE-cadherin protein expression and increased the expression of
β-catenin protein levels in high glucose-induced RRECs. In
addition, Rac1 inhibition by Rac1-siRNA transfection effectively
prevented the decrease in VE-cadherin expression and increase in
β-catenin protein levels in high glucose-induced RRECs. The present
data provide novel insight that Rac1 activation is involved in BRB
breakdown in diabetes.
In conclusion, in the present study a pathway by
which Rac1 activation increased endothelial permeability was
identified, demonstrating that Rac1 inhibits VE-cadherin protein
expression and increases β-catenin protein expression. Rac1
activation has been implicated in numerous pathological situations
where vascular permeability is altered.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (no. 30973254).
References
|
1
|
Nelson WJ: Adaptation of core mechanisms
to generate cell polarity. Nature. 422:766–774. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corada M, Mariotti M, Thurston G, et al:
Vascular endothelial-cadherin is an important determinant of
microvascular integrity in vivo. Proc Natl Acad Sci USA.
96:9815–9820. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
May C, Doody JF, Abdullah R, et al:
Identification of a transiently exposed VE-cadherin epitope that
allows for specific targeting of an antibody to the tumor
neovasculature. Blood. 105:4337–4344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weis WI and Nelson WJ: Re-solving the
cadherin-catenin-actin conundrum. J Biol Chem. 281:35593–35597.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Potter MD, Barbero S and Cheresh DA:
Tyrosine phosphorylation of VE-cadherin prevents binding of p120-
and beta-catenin and maintains the cellular mesenchymal state. J
Biol Chem. 280:31906–31912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gumbiner BM: Regulation of
cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol.
6:622–634. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hall A: Rho GTPases and the control of
cell behaviour. Biochem Soc Trans. 33:891–895. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ehrlich JS, Hansen MD and Nelson WJ:
Spatio-temporal regulation of Rac1 localization and lamellipodia
dynamics during epithelial cell-cell adhesion. Dev Cell. 3:259–270.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Wetering S, van Buul JD, Quik S, et
al: Reactive oxygen species mediate Rac-induced loss of cell-cell
adhesion in primary human endothelial cells. J Cell Sci.
115:1837–1846. 2002.PubMed/NCBI
|
|
10
|
Hage B, Meinel K, Baum I, Giehl K and
Menke A: Rac1 activation inhibits E-cadherin-mediated adherens
junctions via binding to IQGAP1 in pancreatic carcinoma cells. Cell
Commun Signal. 7:232009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monaghan-Benson E and Burridge K: The
regulation of vascular endothelial growth factor-induced
microvascular permeability requires Rac and reactive oxygen
species. J Biol Chem. 284:25602–25611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu X, Tu X, Joeng KS, et al: Rac1
activation controls nuclear localization of beta-catenin during
canonical Wnt signaling. Cell. 133:340–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Q, Qaum T and Adamis AP: Sensitive
blood-retinal barrier breakdown quantitation using Evans blue.
Invest Ophthalmol Vis Sci. 42:789–794. 2001.PubMed/NCBI
|
|
14
|
Gao G, Shao C, Zhang SX, et al:
Kallikrein-binding protein inhibits retinal neovascularization and
decreases vascular leakage. Diabetologia. 46:689–698.
2003.PubMed/NCBI
|
|
15
|
Boeri D, Cagliero E, Podestá F and Lorenzi
M: Vascular wall von Willebrand factor in human diabetic
retinopathy. Invest Ophthalmol Vis Sci. 35:600–607. 1994.PubMed/NCBI
|
|
16
|
Lecomte M, Paget C, Ruggiero D,
Wiernsperger N and Lagarde M: Docosahexaenoic acid is a major n-3
polyunsaturated fatty acid in bovine retinal microvessels. J
Neurochem. 66:2160–2167. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elbashir SM, Harborth J, Lendeckel W, et
al: Duplexes of 21-nucleotide RNAs mediate RNA interference in
cultured mammalian cells. Nature. 411:494–498. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JH, Kim JH, Jun HO, Yu YS and Kim KW:
Inhibition of protein kinase C delta attenuates blood-retinal
barrier breakdown in diabetic retinopathy. Am J Pathol.
176:1517–1524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sander EE, van Delft S, ten Klooster JP,
et al: Matrix-dependent Tiam1/Rac signaling in epithelial cells
promotes either cell-cell adhesion or cell migration and is
regulated by phosphatidylinositol 3-kinase. J Cell Biol.
143:1385–1398. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Do carmo A, Ramos P, Reis A, Proença R and
Cunha-vaz JG: Breakdown of the inner and outer blood retinal
barrier in streptozotocin-induced diabetes. Exp Eye Res.
67:569–575. 1998. View Article : Google Scholar
|
|
21
|
Moldovan L, Moldovan NI, Sohn RH, Parikh
SA and Goldschmidt-Clermont PJ: Redox changes of cultured
endothelial cells and actin dynamics. Circ Res. 86:549–557. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peterson LJ, Wittchen ES, Geisen P,
Burridge K and Hartnett ME: Heterotypic RPE-choroidal endothelial
cell contact increases choroidal endothelial cell transmigration
via PI 3-kinase and Rac1. Exp Eye Res. 84:737–744. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wójciak-Stothard B, Potempa S, Eichholtz T
and Ridley AJ: Rho and Rac but not Cdc42 regulate endothelial cell
permeability. J Cell Sci. 114:1343–1355. 2001.PubMed/NCBI
|
|
24
|
Braga VM, Del Maschio A, Machesky L and
Dejana E: Regulation of cadherin function by Rho and Rac:
modulation by junction maturation and cellular context. Mol Biol
Cell. 10:9–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gavard J and Gutkind JS: VEGF controls
endothelial-cell permeability by promoting the
beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol.
8:1223–1234. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stockton RA, Schaefer E and Schwartz MA:
p21-activated kinase regulates endothelial permeability through
modulation of contractility. J Biol Chem. 279:46621–46630. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang XZ, Huang X, Qiao JH, Zhang JJ and
Zhang MX: Inhibition of hypoxia-induced retinal neovascularization
in mice with short hairpin RNA targeting Rac1, possibly via
blockading redox signaling. Exp Eye Res. 92:473–481. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Navaratna D, McGuire PG, Menicucci G and
Das A: Proteolytic degradation of VE-cadherin alters the
blood-retinal barrier in diabetes. Diabetes. 56:2380–2387. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Hu Y, Zhou T, et al: Activation of
the Wnt pathway plays a pathogenic role in diabetic retinopathy in
humans and animal models. Am J Pathol. 175:2676–2685. 2009.
View Article : Google Scholar : PubMed/NCBI
|