Introduction
Acute kidney injury (AKI) is a common and
significant complication (incidence, 17–95%) affecting the
prognosis of patients undergoing orthotopic liver transplantation
(OLT) (1,2). The deterioration of renal function is
due to various etiologies, including preoperative hepatorenal
pathological damage, multiple injury factors in the perioperative
period and nephrotoxic therapies following surgery (3). Although, various treatments targeting
AKI have been provided to patients following OLT, the results
remain unsatisfactory. Thus, developing an effective method for AKI
therapy following OLT is essential.
Ulinastatin, a glycoprotein derived from human
urine, has a molecular weight of 67 kDa and functions as an
inhibitor of a number of proteases, including trypsin,
chymotrypsin, elastase and various pancreatic enzymes (4). Several clinical studies and animal
experiments have reported that the application of ulinastatin can
play an important role in the protection against septic shock,
intensive pancreatitis, ischemia-reperfusion organ injury and
multi-organ dysfunction; it is believed that this protection is
associated with the anti-inflammatory effects of ulinastatin
(5–8). Although ulinastatin has been widely
used in clinical practice, studies investigating the effect of
ulinastatin on the renal protection of patients during the OLT
perioperative period are limited, and the underlying mechanisms
through which ulinastatin reduces pathological damage remain
unclear. Therefore, the present study investigated the influence of
ulinastatin on AKI, which is the most significant complication of
OLT.
Hemodynamic fluctuation during OLT is extremely
intensive and evident, leading to kidney hypoperfusion and hepatic
ischemia-reperfusion, and even resulting in renal secondary distant
pathological damage. Therefore, previous studies have hypothesized
that AKI mainly occurs during the perioperative period (2,9). In
the present study, ulinastatin was administered during surgery to
explore its kidney protective effect. The effect of ulinastatin
application on the incidence of AKI and patient prognosis were
investigated in clinical trails, while the underlying mechanism of
ulinastatin was examined using a rat autologous OLT (AOLT)
model.
Materials and methods
Patient data and allocation
In total, 60 patients (male, 57; female, 3) with
normal renal function and classified as American Society of
Anesthesiologists standard grade III-IV, undergoing an OLT at the
Third Affiliated Hospital of Sun Yat-sen University (Guangzhou,
China), were included in the study. Informed consent was obtained
from all the individuals enrolled in the study, and the
experimental protocol was approved by the Ethics Committee of the
Third Affiliated Hospital of Sun Yat-sen University (no.
chiCTR-DNRC-08000085). The median age of the patients was
43.72±8.63 years (age range, 28–68 years), and the Model for
End-Stage Liver Disease (MELD) score was calculated for each
patient (9). Patients with a
diagnosis of hepatorenal syndrome, septic shock, hypovolemic shock,
primary renal disease, diabetes, hypertension, heart disease and
retransplantation were excluded from the study.
All the patients undergoing an OLT were randomly
divided into two groups, the control group (C group; n=30) and
ulinastatin treatment group (U group; n=30). Anesthesia was induced
by intravenous (i.v.) injection of midazolam (0.05 mg/kg; Jiangsu
Nhwa Pharmaceutical Co., Ltd., Xuzhou, China), propofol (1 mg/kg;
AstraZeneca, Caponago, Italy), vecuronium bromide (0.1 mg/kg;
Xianju Pharmaceutical Co., Ltd., Hangzhou, China) and fentanyl (3
μg/kg; Humanwell Pharmaceutical Co., Ltd, Yichang, China), and
maintained with sevoflurane (0.5–3%; Hengrui Pharmaceutical Co.,
Ltd., Shanghai, China) and intermittent fentanyl and vecuronium
bromide (i.v.).
The patients received a modified piggyback liver
transplantation with venous reformation, but without the
performance of a venovenous bypass. The surgical procedures
regarding the first and second hepatic hilum were similar to a
classic OLT procedure; however, the most important difference was
the surgical management without short hepatic vein disposal. The
vena cava (VC) was interrupted when the first hepatic hilum was
disconnected from the back of the liver, using a Satinsky clamp.
After the VC of the second hepatic hilum was blocked, the liver was
removed. Next, the hepatic vein openings on the anterior wall of
the inferior VC (IVC) of the patient were connected, forming an
open inverted triangular cuff. The posterior wall of the donor IVC
was incised in order to form a wide-opened inverted triangular cuff
that matched the opening of the recipient IVC. Subsequently,
400–800 ml fresh frozen plasma was used to flush the graft, after
which the portal vein was anastomosed and the VC was ligated and
reperfused (10).
In the U group patients, ulinastatin (5,000–8,000
U/kg; Techpool Bio-Pharma Co., Ltd., Guangzhou, China) was
intravenously pumped for 30 min during the skin incisions.
Subsequent administration of ulinastatin was applied 4 h after the
beginning of the surgery. The same volume of normal saline was
applied to the patients in the control group, in the same manner as
the administration of ulinastatin.
Patient demographic data, including the age, gender,
baseline serum creatinine (Cr) level and preoperative MELD scores,
were recorded prior to surgery. In addition, the surgery duration,
ischemia and reperfusion times were measured. Following the OLT,
AKI was diagnosed within three days according to the
recommendations of the Acute Kidney Injury Network (11). In addition, the AKI incidence
rates, serum levels of cystatin C, blood urea nitrogen (BUN) and
Cr, 30-day and one-year survival rates, intensive care unit (ICU)
time, ventilation time and hemodialysis rates were measured for all
the patients.
Animals
A total of 40 adult male Sprague-Dawley rats
(weight, 220–250 g) were purchased from the Experimental Animal
Center of Sun Yat-sen University (Guangzhou, China). All the
experiments were performed in accordance with the Animal Care and
Use Committee of Sun Yat-sen University, and followed the
university Guidelines of Animal Use and Protection, adopted from
the Guide for the Care and Use of Laboratory Animals (National
Institutes of Health, publication no. 80-23, revised in 1996). The
rats were divided randomly into three groups, namely the
sham-operation rat group (S-R group, n=10), the control rat group
(C-R group; n=10) that underwent AOLT and the ulinastatin rat
treatment group (U-R group; n=20). The U-R group was divided
further into two equal subgroups who received a medium (UM-R;
50,000 U/kg) or high dose (UH-R; 100,000 U/kg) of ulinastatin.
Animal surgical procedure
Rat AOLT models were established using a previously
reported method (12,13). All the rats were intraperitoneally
injected with 10% chloral hydrate (0.3 ml/100 g). During the
surgery, the rats were allowed to breathe oxygen on an electric
heating pad under a warming light. Prior to blocking the VC,
ulinastatin (50,000 U/kg or 100,000 U/kg for the medium and high
dose groups, respectively) dissolved in heparin (50 U/ml), or
saline, was injected into the caudal vein of the U-R and C-R group
rats, respectively. After opening the hepatic veins, the remaining
ulinastatin dissolved in protamine sulfate (0.5 mg/ml), or saline,
was injected into the caudal vein of the respective groups. By
contrast, the sham-operation group (S-R group) rats were subjected
to an abdominal incision and portal vein dissociation, followed by
suturing of the abdominal incision under anesthesia, without
blockade of the blood flow.
Histology and quantification of renal
injury
Kidney sections of the rats from the C-R or U-R
groups were obtained 8 h following reperfusion and were stained
with hematoxylin-eosin (H&E, Keygen Biotech Co., Ltd, Nanjing,
China) and periodic acid-Schiff. The samples were analyzed for
tubular cell necrosis, tubular dilation and intratubular detachment
(magnification, ×200; Eclipse E200; Nikon, Tokyo, Japan), and were
evaluated in a blinded manner by a nephrologist. Abnormalities were
graded using a semiquantitative score (range, 0–4+) defined as: 0,
no abnormalities; 1+, changes affecting <25% of the tubules; 2+,
25–50%; 3+, 50–75%; and 4+, >75% (14).
Biomarkers of inflammation and oxidative
stress
Renal cortexes were collected from all the rats. The
levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)
were measured using an enzyme-linked immunosorbent assay (ELISA)
kit (KeyGen Biotech. Co., Ltd.). In addition, the levels of
superoxide dismutase (SOD), hydrogen peroxide
(H2O2), malondialdehyde (MDA) and reactive
oxygen species (ROS) were determined using the equivalent assay
kits, according to the manufacturer’s instructions (KeyGen Biotech.
Co., Ltd.).
Biomarkers of renal injury
Blood samples were collected from the rats and
patients. Serum cystatin C levels were measured using an
immunonephelometric method (Dade Behring Marburg GmbH,, Marburg,
Germany), and a standard calibration formula was used to estimate
the glomerular filtration rate from the result. The serum levels of
BUN and Cr were measured using an ELISA kit (Rapidbio, Inc., West
Hills, CA, USA), according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA) and the data
are presented as the mean ± standard deviation. Data obtained from
the experimental groups were compared using a two-tailed unpaired
t-test. Statistical analysis was performed using analysis of
variance, followed by the Bonferonni correction to evaluate the
differences between pairs. The least significant difference test
was used to examine the homogeneity of variance. In addition, the
χ2 test was used to compare the differences between
rates (AKI incidence rate, AKI III cases, 30-day and one-year
survival rates and hemodialysis cases). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient demographics and outcomes
Table I shows the
demographic information and clinical characteristics of the C and U
patient groups. Following the OLT, 18 patients met the criteria for
AKI in the C group (60.00%), while the number of AKI patients in
the U group was 10 (33.3%). Although the incidence of AKI in the U
group was not found to be significantly less compared with the C
group (P>0.05), the number of patients developing severe AKI
(AKI III) (15) was much higher in
the C group compared with the U group (P=0.002). Additionally,
administration of ulinastatin improved the prognosis of the
patients. The 30-day and one-year survival rates showed no
statistically significant difference between the two groups;
however, the time the patients remained in ICU, the ventilation
time and the hemodialysis rates were evidently higher for patients
in the C group (P<0.05).
 | Table IComparison of demographic information
between the two groups of patients undergoing liver
transplantation. |
Table I
Comparison of demographic information
between the two groups of patients undergoing liver
transplantation.
| Factor | U group (n=30) | C group (n=30) | P-value |
|---|
| Age, years | 42.9±10.7 | 44.5±8.8 | 0.521 |
| Female/male, n | 1/29 | 2/28 | 1.000 |
| Baseline serum
creatinine, μmol/l | 64.6±16.0 | 61.9±15.0 | 0.507 |
| Preoperative MELD
score ≥18, n | 10 | 11 | 0.787 |
| Duration of surgery,
min | 345.3±66.2 | 356.8±77.6 | 0.538 |
| Ischemic time,
min | 39.7±16.4 | 37.0±8.6 | 0.440 |
| Reperfusion time,
min | 215.5±50 | 226.3±69.7 | 0.513 |
| AKI incidence rate, n
(%) | 10/30 (33.3) | 18/30 (60) | 0.069 |
| AKI III cases, n
(% | ) 0/6 (0) | 6/6 (100) | 0.002 |
| 30-day survival rate,
n (%) | 29/30 (96.7) | 26/30 (86.7) | 0.353 |
| One-year survival
rate, n (%) | 27/30 (90) | 24/30 (80) | 0.472 |
| ICU time, h | 56.0±39.4 | 134.9±105.8 | 0.000 |
| Ventilation time,
h | 17.7±8.9 | 28.2±24.9 | 0.036 |
| Hemodialysis cases, n
(%) | 0/4 (0) | 4/4 (13.3) | 0.029 |
Notably, the serum cystatin C, β2
microglobulin (β2MG) and urinary β2MG levels
increased gradually during the perioperative period, and
particularly at 24 h following OLT, indicating that the renal
injuries had deteriorated. However, application of ulinastatin
decreased the levels of these bioactive substances. Although no
statistically significant difference in the serum Cr level was
identified between the two groups, a decreasing trend was apparent
in the U group (Fig. 1).
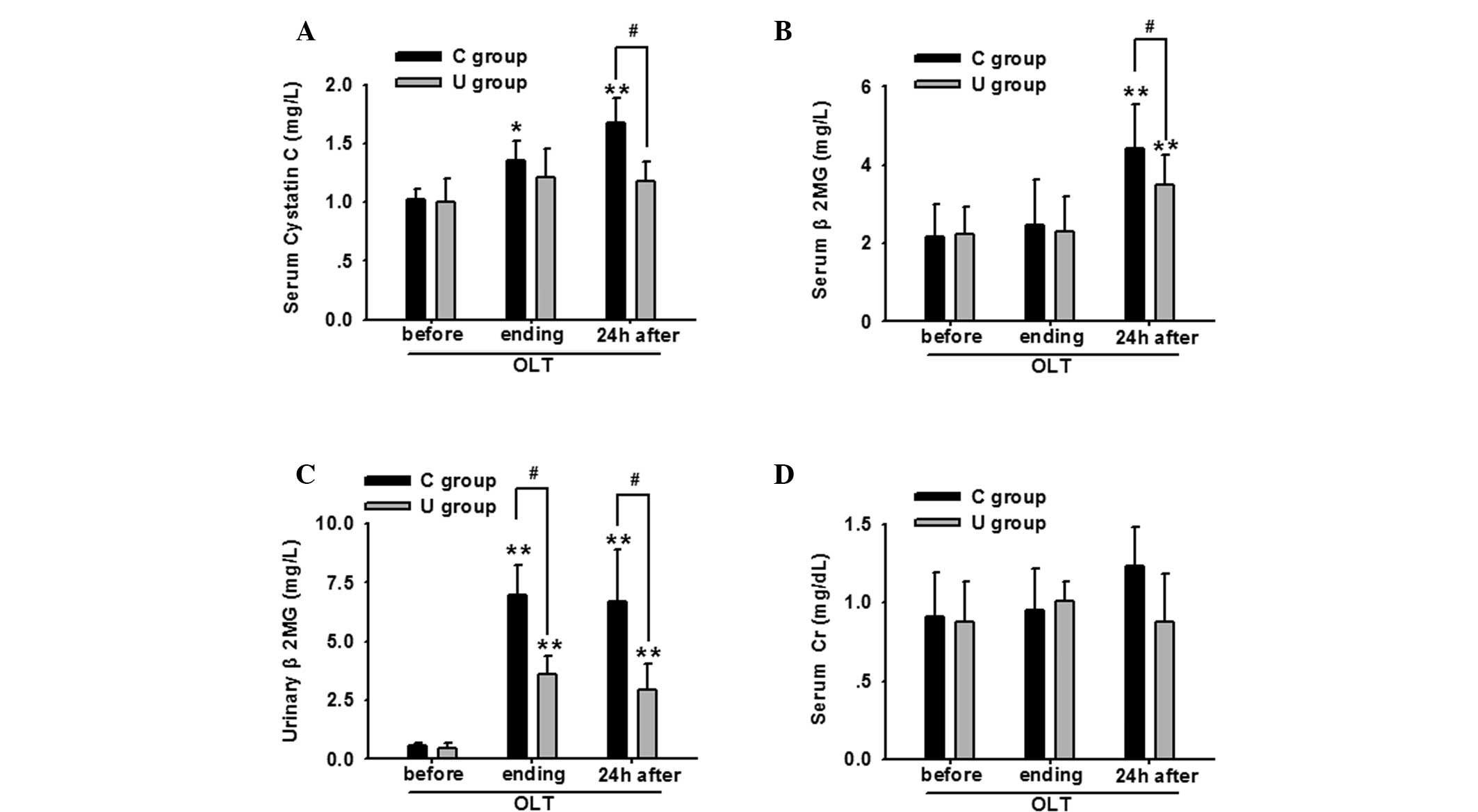 | Figure 1Ulinastatin application significantly
decreased the renal damage of OLT patients, as indicated by changes
in the serum levels of (A) cystatin C, (B) β2MG, (C)
urinary β2MG and (D) serum Cr at the various time
points, including before OLT, the surgery endpoint and 24 h after
OLT. *P<0.05 and **P<0.01, vs. before
OLT (n=30); #P<0.05, vs. C group at the same time
point (n=30). OLT, orthotopic liver transplantation;
β2MG, β2 microglobulin; Cr, creatinine; C
group, control group; U group, ulinastatin treatment group. |
Ulinastatin application significantly
decreases the renal damage of rats
Histological examination of the kidney was performed
8 h following reperfusion on the rat AOLT models. The results
demonstrated that the kidney damage of the rats that had undergone
an AOLT was extensive, and multifocal acute tubular injury was
observed through the loss of a brush border, the flattening and
loss of the tubular epithelium, hyaline casts, medullary congestion
and hemorrhage. However, ulinastatin application was shown to
improve the tubular injury and decrease the pathological scores
effectively, particularly at a high dose (Fig. 2A). Compared with the S-R group, the
serum levels of BUN, Cr and cystatin C in the C-R group were
significantly increased and peaked at 8 h following reperfusion
after AOLT, while ulinastatin application lowered the increase in
the serum levels, in accordance with the changes to the kidney
pathological injuries (Fig. 2B and
D).
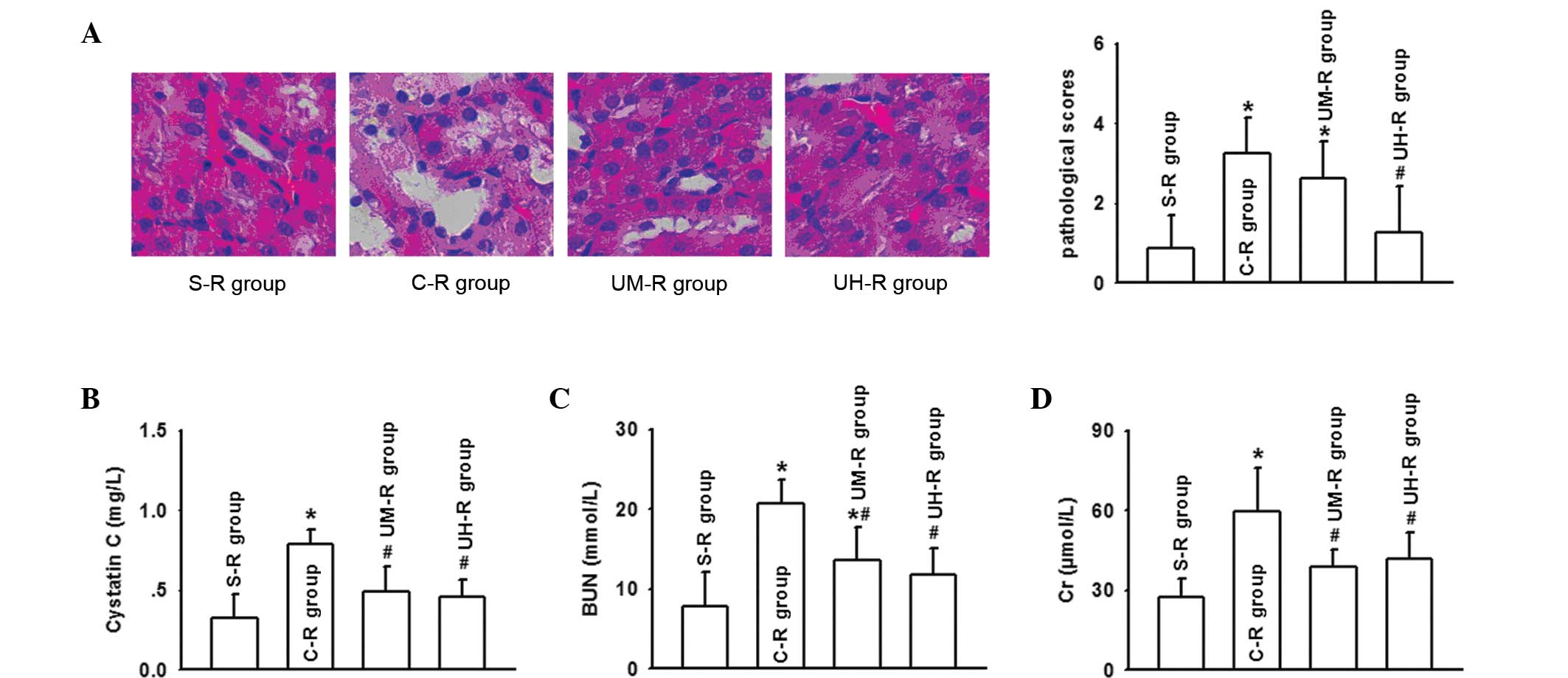 | Figure 2Ulinastatin application significantly
decreased the renal damage in rats, as indicated by (A) the
pathological damage (magnification, ×200) and kidney scores
(hematoxylin and eosin staining), and changes in the serum levels
of (B) cystatin C, (C) BUN and (D) Cr, determined 8 h following
autologous orthotopic liver transplantation (AOLT).
*P<0.05, vs. S-R group; #P<0.05, vs.
C-R group. BUN, blood urea nitrogen; Cr, creatinine; S-R group,
sham-operation rat group (n=10); C-R group, control rat group
(n=10; undergoing AOLT); UM-R group, rats receiving a medium dose
of ulinastatin (50,000 U/kg); UH-R group, rats receiving a high
dose of ulinastatin (100,000 U/kg). |
Ulinastatin application decreases renal
inflammatory reactions and oxidative stress
As aforementioned, ulinastatin application was shown
to protect against renal damage following AOLT; however, the
underlying mechanisms have yet to be reported. Severe kidney damage
was found to be accompanied with oxidative stress and inflammatory
reaction activation. The levels of TNF-α and IL-6 increased
markedly, in accordance with changes in the levels of MDA,
H2O2 and ROS during the perioperative period,
while the SOD levels decreased. However, the application of
ulinastatin effectively balanced the oxidative stress and
inflammatory reaction, particularly at a high dose. The levels of
the inflammatory factors, TNF-α and IL-6, were decreased, as well
as the levels of MDA, H2O2 and ROS, while the
level of SOD increased (Fig. 3).
Therefore, the protective function of ulinastatin was considered to
be associated with its anti-inflammatory and antioxidant
effects.
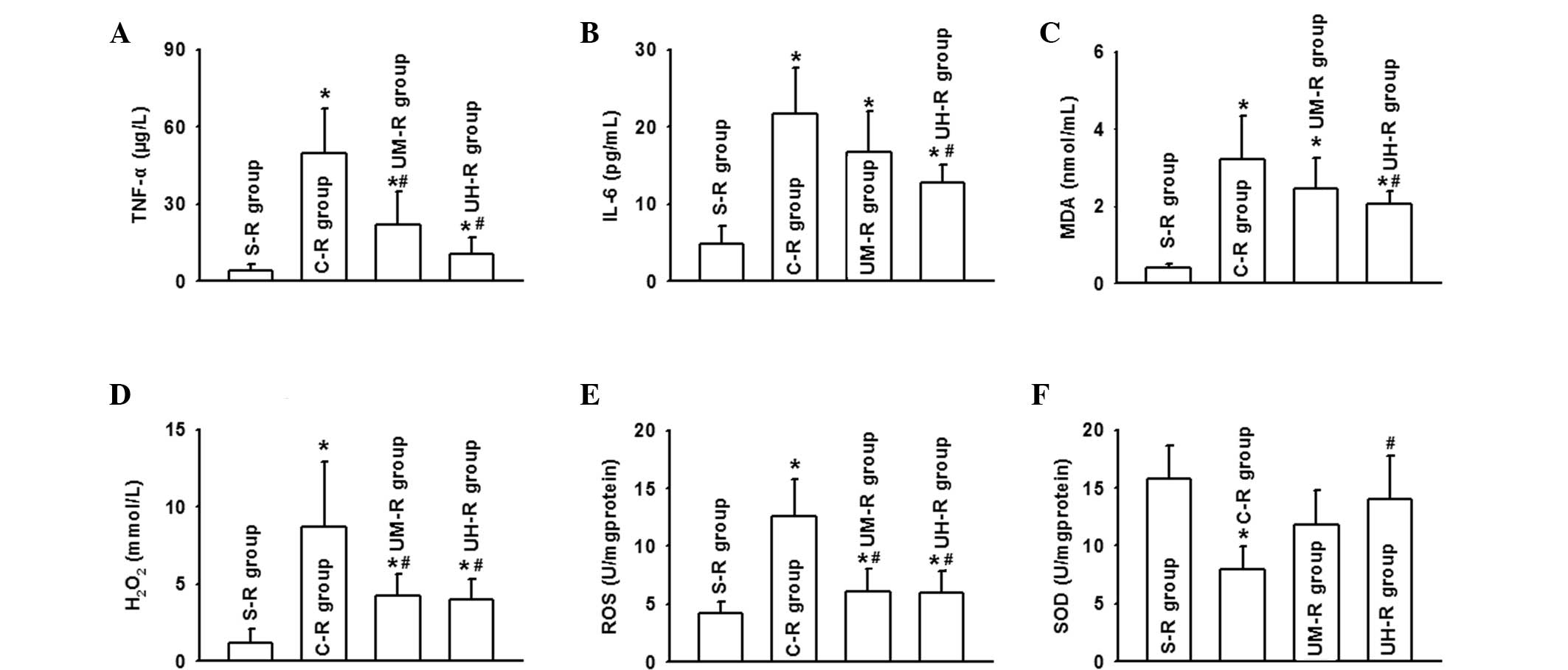 | Figure 3Ulinastatin application attenuated the
oxidative stress and inflammatory reaction, particularly in the
UH-R group, by reducing the serum levels of (A) TNF-α, (B) IL-6,
(C) MDA, (D) H2O2 and (E) ROS, and (F)
increasing the serum level of SOD, determined 8 h following
autologous orthotopic liver transplantation (AOLT).
*P<0.05, vs. S-R group; #P<0.05, vs.
C-R group. TNF, tumor necrosis factor; IL, interleukin; MDA,
malondialdehyde; H2O2, hydrogen peroxide;
ROS, reactive oxygen species; SOD, superoxide dismutase; S-R group,
sham-operation rat group (n=10); C-R group, control rat group
(n=10; undergoing AOLT); UM-R group, rats receiving a medium dose
of ulinastatin (50,000 U/kg); UH-R group, rats receiving a high
dose of ulinastatin (100,000 U/kg). |
Discussion
Renal damage has been demonstrated to be closely
associated with the prognosis of patients suffering from OLT. Thus,
the American Society of Nephrology has indicated that the diagnosis
of renal failure may be replaced by AKI, which may aid the earlier
prognosis of renal failure and provide effective prevention for the
further deterioration of renal function in patients. AKI is a
common and significant complication of liver transplantation, and
is responsible for the poor prognosis of OLT patients (16–18).
However, no effective methods for the prevention of AKI are
currently available. In the present study, the effects of a
broad-spectrum protease inhibitor, ulinastatin, were examined.
Clinical trial results revealed that ulinastatin application
decreased the AKI incidence following OLT, particularly for severe
AKI, while improving the prognosis of patients. In a rat AOLT
model, ulinastatin application was shown to protect against AKI
following surgery and regulate oxidative stress and the
inflammatory reaction.
Liver transplantation can result in complicated
pathophysiological changes, including hemorrhage, intensive cycle
fluctuations induced by vascular occlusion and opening, intestinal
congestion and damage and liver ischemia-reperfusion injury. Trauma
caused by these changes activates the systemic oxidative stress and
inflammatory reaction, leading to multiple organ complications,
ultimately affecting the prognosis of patients undergoing OLT
(19–21).
A previous study using a rabbit model indicated that
ulinastatin application attenuated lung injury by inhibiting the
release of inflammatory mediators (22). Yang et al further
demonstrated that ulinastatin protected liver function and improved
the clinical outcomes of patients undergoing a hepatectomy,
possibly through the inhibition of inflammation and oxidation at an
earlier stage (23). Other studies
have also reported that the protective effects of ulinastatin may
be associated with the SOD level increase (24) and membrane stabilization in rat
models (25). These observations
demonstrate that ulinastatin is a promising drug for organ
protection. In addition, the clinical trial results of the current
study revealed that ulinastatin application was beneficial for
patients undergoing OLT. Ulinastatin decreased the incidence of AKI
and was particularly effective in severe AKI cases. Although no
statistically significant differences were observed between the
30-day and one-year survival rates, ulinastatin application reduced
the ICU and ventilation times and hemodialysis rate. Thus,
ulinastatin is recommended as a protective strategy used during the
perioperative period of OLT to improve patient prognosis.
Oxidative stress and inflammatory reactions induced
by the trauma caused by OLT are considered to be the main
mechanisms for the formation of AKI resulting from ischemia and
toxins (26,27). Ischemia-reperfusion injury,
intestinal endotoxemia and the disruption of the internal
environmental balance prompt the deterioration of AKI. Therefore,
anti-inflammatory and antioxidant processes during the early stages
of OLT may be significant in the reduction of AKI incidence
(28,29). In the present study, the levels of
TNF-α and IL-6 were found to evidently increase in the rat AOLT
model, reflecting the degree of the inflammatory reaction (30,31).
In addition, the levels of ROS and H2O2
increased, signifying the activation of oxidative damage, while the
increase in the level of MDA indicated the degree of lipid
peroxidation (32). These
oxidative and inflammatory mediators have been demonstrated to
participate in kidney damage induced by various pathogenies,
including AKI following AOLT (33,34).
In the present study, ulinastatin application was found to be
beneficial in the protection against AKI. In order to investigate
the possible mechanisms underlying the protective effects of
ulinastatin, a rat AOLT model was established and ulinastatin was
found to decrease renal pathological damage by inhibiting oxidative
stress and inflammatory reactions. These results provide direct
evidence of the effect of ulinastatin on kidney protection during
OLT.
In summary, ulinastatin was found to attenuate AKI
following OLT, partly by inhibiting the inflammatory process and
oxidative stress. Therefore, ulinastatin may be a valuable clinical
candidate for application during OLT. However, limitations exist in
the current study, such as the limited number of clinical samples.
Thus, in future studies, an increased sample size should be used
and further oxidative and inflammatory mediators should be
detected, in order further the understanding of the protective
mechanism of ulinastatin. From these studies, a novel strategy for
kidney protection in OLT patients may be established.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81401628 and 81170449),
the key project of the Natural Science Foundation of Guangdong (no.
S2011020002780) and the Medical Research Foundation of Guangdong
Province (no. B2014141).
References
|
1
|
Barri YM, Sanchez EQ, Jennings LW, et al:
Acute kidney injury following liver transplantation: definition and
outcome. Liver Transpl. 15:475–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sirota JC, Walcher A, Faubel S, et al:
Urine IL-18, NGAL, IL-8 and serum IL-8 are biomarkers of acute
kidney injury following liver transplantation. BMC Nephrol.
14:172013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lima EQ, Zanetta DM, Castro I, et al: Risk
factors for development of acute renal failure after liver
transplantation. Ren Fail. 25:553–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Umeadi C, Kandeel F and Al-Abdullah IH:
Ulinastatin is a novel protease inhibitor and neutral protease
activator. Transplant Proc. 40:387–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu JB and Yao SL: Protective effects of
hemin pretreatment combined with ulinastatin on septic shock in
rats. Chin Med J (Engl). 121:49–55. 2008.
|
|
6
|
Liu R, Qi H, Wang J, et al: Ulinastatin
activates the renin-angiotensin system to ameliorate the
pathophysiology of severe acute pancreatitis. J Gastroenterol
Hepatol. 29:1328–1337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao J, Zhu X, Ji G, et al: Ulinastatin
protects cardiomyocytes against ischemia-reperfusion injury by
regulating autophagy through mTOR activation. Mol Med Rep.
10:1949–1953. 2014.PubMed/NCBI
|
|
8
|
Yang Q, Liu X, Liu M, Zhang L and Guan Y:
Ulinastatin-mediated protection against zymosan-induced multiple
organ dysfunction in rats. Biologicals. 38:552–556. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romano TG, Schmidtbauer I, Silva FM,
Pompilio CE, D’Albuquerque LA and Macedo E: Role of MELD score and
serum creatinine as prognostic tools for the development of acute
kidney injury after liver transplantation. PloS One. 8:e640892013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Xia L, He ZS, Ouyang W, Z H and
Xie CH: Modulation of matrix metalloproteinase-9 and tissue
inhibitor of metalloproteinase-1 in RAW264.7 cells by irradiation.
Mol Med Rep. 3:809–813. 2010.
|
|
11
|
Mehta RL, Kellum JA, Shah SV, et al; Acute
Kidney Injury Network. Acute Kidney Injury Network: report of an
initiative to improve outcomes in acute kidney injury. Crit Care.
11:R312007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chi X, Zhang A, Luo G, et al: Knockdown of
myeloid differentiation protein-2 reduces acute lung injury
following orthotopic autologous liver transplantation in a rat
model. Pulm Pharmacol Ther. 26:380–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ge M, Chi X, Zhang A, et al: Intestinal
NF-E2-related factor-2 expression and antioxidant activity changes
in rats undergoing orthotopic liver autotransplantation. Oncol
Lett. 6:1307–1312. 2013.PubMed/NCBI
|
|
14
|
Kong HY, Chen F, He Y, et al: Intrarenal
resistance index for the assessment of acute renal injury in a rat
liver transplantation model. BMC Nephrol. 14:552013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ostermann M and Chang R; Riyadh ICU
Program Users Group. Correlation between the AKI classification and
outcome. Crit Care. 12:R1442008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ricci Z, Cruz DN and Ronco C:
Classification and staging of acute kidney injury: beyond the RIFLE
and AKIN criteria. Nat Rev Nephrol. 7:201–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klaus F, Keitel da Silva C, Meinerz G, et
al: Acute kidney injury after liver transplantation: incidence and
mortality. Transplant Proc. 46:1819–1821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Narciso RC, Ferraz LR, Mies S, et al:
Impact of acute kidney injury exposure period among liver
transplantation patients. BMC Nephrol. 14:432013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walsh TS, Hopton P, Garden OJ and Lee A:
Effect of graft reperfusion on haemodynamics and gas exchange
during liver transplantation. Br J Anaesth. 81:311–316. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Yang L, Tao K, et al: Protective
effects of hydrogen enriched saline on liver ischemia reperfusion
injury by reducing oxidative stress and HMGB1 release. BMC
Gastroenterol. 14:122014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iasi M, Favero SG, Soler WV, et al:
Oxidative stress in liver transplantation with special reference to
Santa Casa-SP solution: a preclinical study. Transplant Proc.
35:1134–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song Z, Chen G, Lin G, Jia C, Cao J and Ao
G: The ultra-early protective effect of ulinastatin on rabbit acute
lung injury induced by paraquat. BMC Emerg Med. 13(Suppl 1):
S72013.PubMed/NCBI
|
|
23
|
Yang H, Mao Y, Lu X, et al: The effects of
urinary trypsin inhibitor on liver function and inflammatory
factors in patients undergoing hepatectomy: a prospective,
randomized, controlled clinical study. Am J Surg. 202:151–157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao C, Huan J, Li W and Tang J: Protective
effects of ulinastatin on pancreatic and renal damage in rats
following early scald injury. Burns. 35:547–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen CC, Liu ZM, Wang HH, He W, Wang Y and
Wu WD: Effects of ulinastatin on renal ischemia-reperfusion injury
in rats. Acta Pharmacol Sin. 25:1334–1340. 2004.PubMed/NCBI
|
|
26
|
Hori T, Uemoto S, Chen F, et al: Oxidative
stress and extracellular matrices after hepatectomy and liver
transplantation in rats. World J Hepatol. 6:72–84. 2014.PubMed/NCBI
|
|
27
|
Hori T, Uemoto S, Walden LB, et al:
Pretreatment of small-for-size grafts in vivo by γ-aminobutyric
acid receptor regulation against oxidative stress-induced injury in
rat split orthotopic liver transplantation. Int J Hepatol.
2013:1491232013.
|
|
28
|
Kadkhodaee M, Mikaeili S, Zahmatkesh M, et
al: Alteration of renal functional, oxidative stress and
inflammatory indices following hepatic ischemia-reperfusion. Gen
Physiol Biophys. 31:195–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palsamy P and Subramanian S: Resveratrol
protects diabetic kidney by attenuating hyperglycemia-mediated
oxidative stress and renal inflammatory cytokines via Nrf2-Keap1
signaling. Biochim Biophys Acta. 1812:719–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kyriakidis I and Papa A: Serum TNF-α,
sTNFR1, IL-6, IL-8 and IL-10 levels in hemorrhagic fever with renal
syndrome. Virus Res. 175:91–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiong L and Yang L: Effects of alkaloid
sinomenine on levels of IFN-γ, IL-1β, TNF-α and IL-6 in a rat renal
allograft model. Immunotherapy. 4:785–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hamed HA, Das SK, Sokhi UK, et al:
Combining histone deacetylase inhibitors with MDA-7/IL-24 enhances
killing of renal carcinoma cells. Cancer Biol Ther. 14:1039–1049.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al-Rasheed NM, Faddah LM, Mohamed AM,
Abdel Baky NA, Al-Rasheed NM and Mohammad RA: Potential impact of
quercetin and idebenone against immuno- inflammatory and oxidative
renal damage induced in rats by titanium dioxide nanoparticles
toxicity. J Oleo Sci. 62:961–971. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kermanizadeh A, Vranic S, Boland S, et al:
An in vitro assessment of panel of engineered nanomaterials using a
human renal cell line: cytotoxicity, pro-inflammatory response,
oxidative stress and genotoxicity. BMC Nephrol. 14:962013.
View Article : Google Scholar : PubMed/NCBI
|

















