Introduction
Hyperlipidemia is defined as an abnormal lipid
metabolism or the presence of elevated levels of fats in the blood,
including total cholesterol (TC), triglycerides (TG), free fatty
acids, high-density lipoprotein (HDL), very low-density lipoprotein
(VLDL) and low-density lipoprotein (LDL). The increased levels of
blood-fats change the density and flow of the blood, which may
result in arteriosclerosis (1).
VLDL and LDL are easily oxidized, and oxidized VLDL and LDL (OxLDL)
promote the production of oxygen free radicals and reduce the mRNA
expression of nitric oxide synthase (2,3),
which are the causes for the accelerated adhesion of monocytes to
endothelial cells (4). OxLDL is
able to increase endothelial permeability, inhibit secretion and
lower metabolism, leading to vascular endothelial cell apoptosis
(5). The elevated levels of lipids
also lead to pathological changes in the tunica intima and a
disturbance in microcirculation. Diabetes and cardiovascular
disease have been shown to be associated with hyperlipidemia
(6). Therefore, lowering the
levels of blood lipids has important significance for the
prevention and treatment of diabetes and atherosclerosis.
Currently, lipid-lowering drugs, particularly
3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors
(statins), can effectively lower the levels of TC and LDL
cholesterol, reducing the incidence rate of cardiovascular events
and mortality (7). A consensus has
been reached that statins are the first-line agents for the
treatment of hypercholesterolemia (8,9).
However, the adverse reactions of statins, including myopathy and
rhabdomyolysis, should be considered (10,11).
Furthermore, the hepatotoxicity of statins has been reported in a
number of clinical cases (12).
Thus, there is a requirement for the development of novel
lipid-lowering drugs.
Ephedra has been used as a medicine in China for
thousands of years (13). Ephedra
plants, including Ephedra sinica Stapf, Ephedra
intermedia schrenk et C.A. Meyer and Ephedra equisetina
Bge, may be used to treat colds, hay fever, allergies, pneumonia,
asthma and bronchitis (14–16).
Since ephedra or ephedrine used alone or in combination with other
herbs or caffeine produces an average weight loss of 0.9 kg/month,
ephedra and ephedrine have been widely used as dietary supplements
to enable weight loss (17).
However, an increasing number of adverse reactions to ephedra have
been reported. Dietary supplements containing the ephedrine
alkaloid are associated with mortality as a result of adverse
reactions, including myocardial infarction, cardiac arrhythmia,
hypertension and stroke (18,19).
The U.S. Food and Drug Administration (FDA) have banned the use of
non-prescribed drugs containing ephedra or ephedra alkaloids, which
has lead to difficulties for the use and development of ephedra
drugs. To resolve this problem, novel pharmacological effects of
ephedra should be investigated. In addition to ephedrine alkaloids,
there are other substances in ephedra, such as polysaccharides,
organic acids, flavonoids and tannins (20–22).
These substances are anti-free radical and may lower blood pressure
and sugar to affect fat metabolism (23–25).
Therefore, the current study investigated the impact of ephedra
extractions, including ephedrine alkaloids, ephedra polysaccharides
and ephedra non-alkaloids, on hyperlipidemia and evaluated the
safety of the different extractions from Ephedra sinica
Stapf.
Materials and methods
Preparation of extractions from
Ephedra
Ephedra samples (batch no. 0909013; Xianning Kangjin
Chinese Herbal Medicine Co., Ltd., Xianning, China) were ground
into a coarse powder and fully dissolved in 1% sodium hydroxide
solution for 30 min, followed by reflux-extraction with
dichloromethane. Subsequently, the dichloromethane extracting
solution and residue were obtained. The dichloromethane extracting
solution was then further concentrated and extracted using an equal
volume of 2% hydrochloric acid, followed by separation of acidic
aqueous solution and dichloromethane solution. The acidic aqueous
solution was adjusted to neutral and concentrated, in order to
obtain ephedrine alkaloids by freeze drying. In addition, the
dichloromethane fraction was concentrated to obtain the lipophilic
non-alkaloid product. The residue was extracted with double
distilled water and precipitated with 95% alcohol to obtain ephedra
polysaccharide. The recovered alcohol was freeze-dried to obtain
ephedra non-alkaloid, which was then merged with the lipophilic
non-alkaloid product for later use as ephedra non-alkaloid. Sodium
hydroxide, dichloromethane and hydrochloric acid were all purchased
from the 3rd Branch of Tianjin Chemical Reagent Co., Ltd (Tianjin,
China).
Experimental animals and design
A total of 48 male Kunming mice, weighing 18–22 g,
were purchased from the Wuhan Institute of Biological Products
(Wuhan, China). All the animals were acclimatized to laboratory
conditions for seven days, during which they were fed a commercial
pellet diet and provided with water ad libitum. Experiments
were conducted under specific pathogen-free conditions. The animal
care and use procedures applied in the study were in accordance
with the guidelines established by the Animal Ethics Committee of
Wuhan Hospital of Traditional Chinese Medicine (Wuhan, China).
The 48 mice were randomly divided into six groups,
which included the normal control (G1; n=8), model control (G2;
n=8), positive control (G3; n=8), ephedrine alkaloid (G4; n=8),
ephedra polysaccharide (G5; n=8) and ephedra non-alkaloid (G6; n=8)
groups. Animals in the normal control group were fed a standard
basal diet, while the mice in the other five groups were fed a high
fat diet (78.8% basal diet, 10% egg yolk, 10% lard, 1% cholesterol,
0.2% bile salt) for three consecutive weeks to establish the
hyperlipidemic model. Successful model establishment was confirmed
by measurement of the lipid levels.
Mice in the positive control group were administered
6.7 mg/kg simvastatin daily (batch no. 08048; Hangzhou MSD
Pharmaceutical Co., Ltd., Hanghzou, China) for four consecutive
weeks. Mice in the ephedrine alkaloid, ephedra polysaccharide and
non-alkaloid groups were orally administered 1.26 mg/g respective
extractions, once per day for four consecutive weeks. Mice in the
normal control and model control groups were administered an equal
volume of normal saline. The animals were weighed weekly. After
four weeks of administration, blood was collected from the eyeballs
of mice following fasting for 12 h, and the serum was separated by
centrifugation at 2,200 × g for 15 min at 4°C. The mice were
euthanized by cervical dislocation, without the use of anesthetic.
Organs, including the heart, liver, spleen, lung and kidney, were
excised and frozen until required for analysis. Organ coefficients
(ratio of organ to body weight) were calculated according to the
following formula: Organ coefficient = organ weight (mg)/body
weight (g).
Determination of the serum lipid
levels
Serum concentrations of TC, TG and HDL cholesterol
(HDL-C) were measured by TC Assay kit, TG Assay kit and HDL Assay
kit (Shanghai Mingdian Biological Engineering Co., Ltd., Shanghai,
China) respectively, according to the manufacturer’s instructions,
using an RT-9600 semi-automatic biochemical analyzer (Shenzhen
Leidu Life Science Co., Ltd. Nanning, China).
Evaluation of antioxidant capacity and
liver function
Activity levels of superoxide dismutase (SOD),
alanine aminotransferase (ALT) and aspartate aminotransferase
(AST), as well as the level of malondialdehyde (MDA) in the serum,
were evaluated by SOD Assay kit, ALT Assay kit, AST Assay kit and
MDA Assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) respectively, according to the manufacturer’s
instructions.
Observations of liver morphology
Liver morphologies in the animals from the six
groups were observed. The largest lobe of the liver was fixed with
neutral formalin, embedded in paraffin, divided into sections (4–5
μm) and stained by routine hematoxylin and eosin (H&E). The
morphological changes in the hepatic tissue were observed under a
light microscope (BX51; Olympus Corporation, Tokyo, Japan).
Acute toxicity
A total of 100 male Kunming mice (18–22 g of weight)
were used for the acute toxicity study, with 20 mice in each of the
ephedra polysaccharide and non-alkaloid groups, and 60 mice in the
ephedra alkaloid group (10 from each dose group are shown in
Table I). Initially, nine mice
were randomly divided into three groups and orally administered
ephedrine alkaloids, ephedra polysaccharides and ephedra
non-alkaloids, respectively. The mice were monitored to record any
clinical signs of toxicity, the time taken to the onset of these
symptoms and the time period until mortality. Results from the
initial exposure were used to select the subsequent dose, and the
up-and-down procedure was used to estimate the lethal dose
(26). The selected dosages of
ephedra alkaloids were 289, 413, 590, 843, 1,204 and 1,720 mg/kg,
the dosage of ephedra polysaccharides was 800 mg/kg and the dosage
of ephedra non-alkaloids was 4,168 mg/kg. The mean lethal dose
(LD50) was calculated using the modified Spearman-Karber
method (27). At 0 and 2 h after
oral administration, the mice were externally prewarmed for 5 min
at 39°C, and the systolic blood pressure (SBP) and heart rate (HR)
of the mice were measured using the tail-cuff method (BP98A;
Softron Co., Ltd., Tokyo, Japan).
 | Table IMortality rate of the mice treated
with graded doses of ephedra alkaloids. |
Table I
Mortality rate of the mice treated
with graded doses of ephedra alkaloids.
| Dose (mg/kg) | Route | Animals (n) | Mortality (n) |
|---|
| 289 | Orally | 10 | 0 |
| 413 | Orally | 10 | 3 |
| 590 | Orally | 10 | 6 |
| 843 | Orally | 10 | 7 |
| 1,204 | Orally | 10 | 8 |
| 1,720 | Orally | 10 | 10 |
Statistical analysis
Results are expressed as the mean ± standard
deviation, and statistical comparisons were performed using the
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference. All statistical tests were
performed using SPSS 16.0 software (SPSS., Inc., Chicago, IL,
USA).
Results
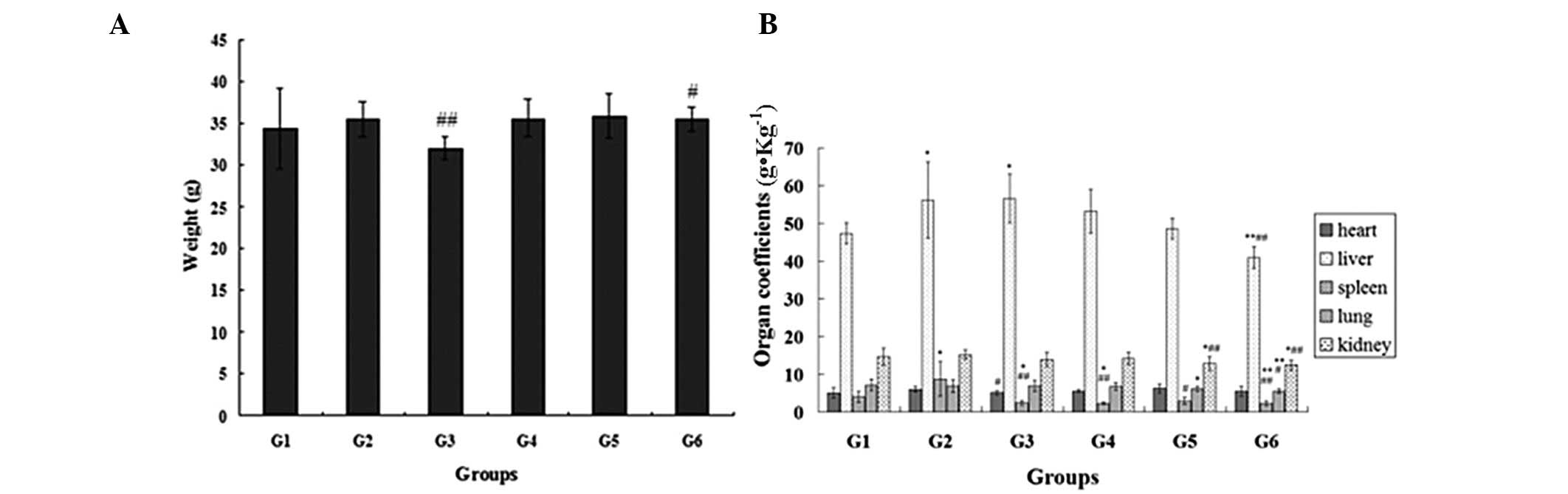
Effects of the extractions on the weight
and organ coefficients
No statistically significant differences were
observed in the body weight between the normal control group and
the other groups (P>0.05). Compared with the model control
group, the weight of the mice in the positive control (P<0.01)
and non-alkaloid groups (P<0.05) was significantly lower after
four weeks of drug administration (Fig. 1A).
The liver and spleen coefficients in the model
control group were significantly higher compared with those in the
normal control group (P<0.05). In addition, compared with the
model control group, the liver (P<0.01), spleen (P<0.01),
lung (P<0.05) and kidney (P<0.01) coefficients were markedly
reduced in the ephedra non-alkaloid group. In addition, the spleen
(P<0.05) and kidney (P<0.01) coefficients were notably
reduced in the ephedra polysaccharide group compared with the model
control group. The spleen (P<0.01) coefficient was also
significantly lower in the ephedrine alkaloid group when compared
with the model control group (Fig.
1B).
Effects of the extractions on the levels
of serum lipids
Statistical analysis of the differences in the
levels of serum lipids among the mice in the six groups was
performed (Fig. 2). The
administration of ephedrine alkaloids, ephedra polysaccharides and
non-alkaloids resulted in a significant decrease in the levels of
TC (P<0.01) and TG (P<0.01), and an increase in the level of
HDL-C (P<0.05), when compared with the model control group. The
same changes were observed in the levels of TC and HDL-C in the
positive control group. Compared with the normal control group, the
level of TC was significantly higher in the other five groups
(P<0.01), whereas the level of TG was significantly lower in the
ephedrine alkaloid, ephedra polysaccharide and non-alkaloid groups
(P<0.01). The level of HDL-C was markedly reduced in the model
control group compared with the normal control group
(P<0.01).
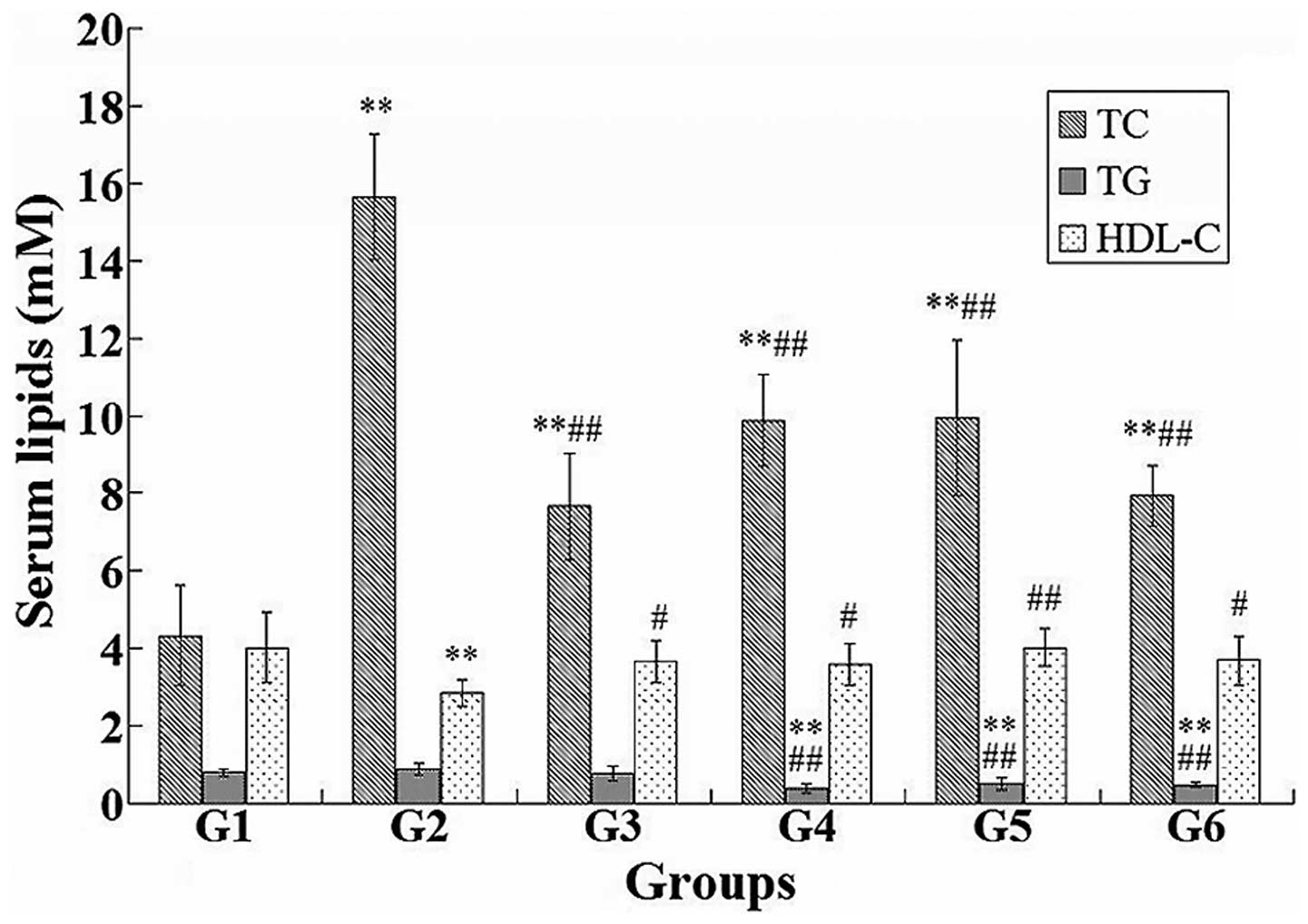 | Figure 2Differences in the serum lipids, TC,
TG and HDL-C, among the normal control (G1), model control (G2),
positive control (G3), ephedrine alkaloid (G4), ephedra
polysaccharide (G5) and ephedra non-alkaloid (G6) groups.
*P<0.05 and **P<0.01, vs. normal
control group; #P<0.05 and ##P<0.01,
vs. model control group. TC, total cholesterol; TG, triglycerides;
HDL-C, high-density lipoprotein cholesterol. |
Effects of the extractions on antioxidant
capacity and liver function
Changes in the MDA content, and the activity levels
of SOD, ALT and AST among the six groups are shown in Fig. 3. Compared with the model control
group, the ephedra polysaccharide and non-alkaloid groups revealed
a significantly increased activity of SOD and a reduced content of
MDA (P<0.05). Compared with the normal control group, the
activity of SOD was significantly enhanced and the content of MDA
was decreased in the ephedra polysaccharide and non-alkaloid groups
(P<0.05).
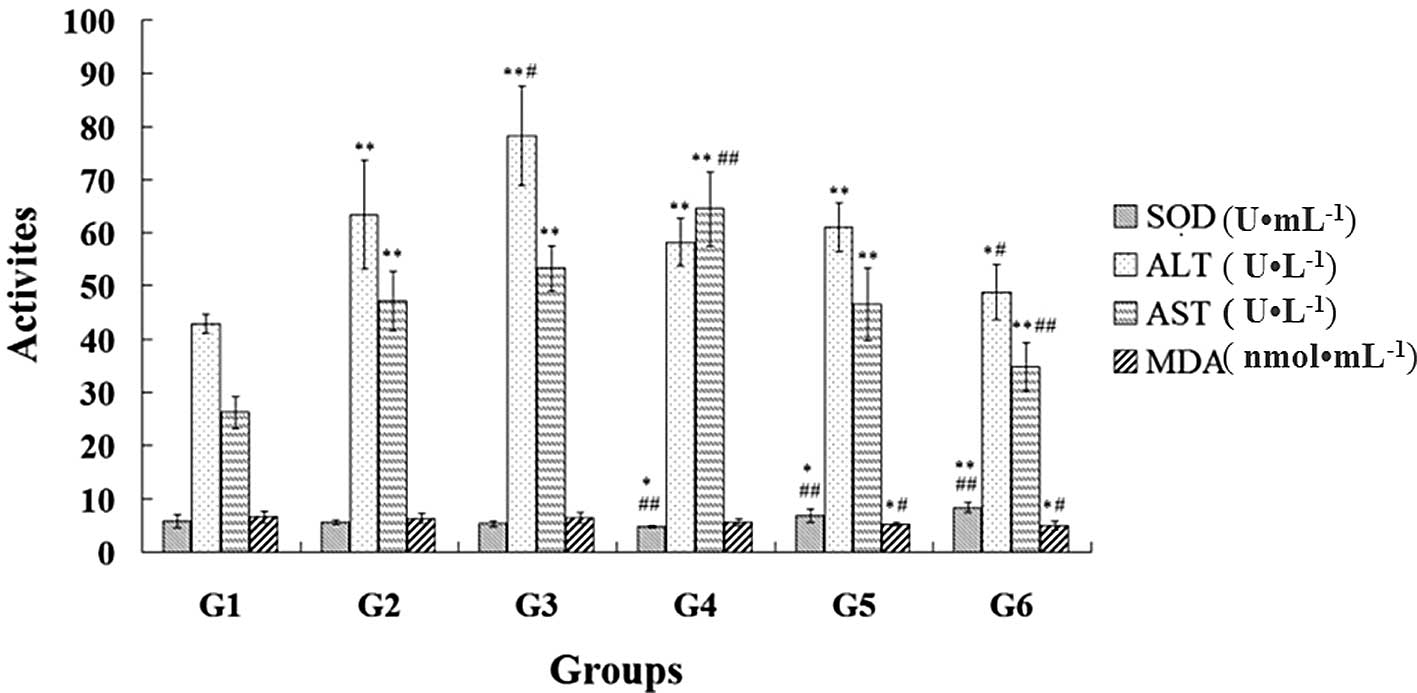 | Figure 3Effects of ephedra extractions on the
antioxidant capacity and liver function in the mice from the normal
control (G1), model control (G2), positive control (G3), ephedrine
alkaloid (G4), ephedra polysaccharide (G5) and ephedra non-alkaloid
(G6) groups. *P<0.05 and **P<0.01, vs.
normal control group; #P<0.05 and
##P<0.01, vs. model control group. SOD, superoxide
dismutase; ALT, alanine aminotransferase; AST, aspartate
aminotransferase; MDA, malondialdehyde. |
Compared with the model control group, the
non-alkaloid group demonstrated a decrease in the activity levels
of ALT (P<0.05) and AST (P<0.01), whereas the simvastatin
(positive control) and ephedrine alkaloids significantly increased
the activity levels of ALT (P<0.05) and AST (P<0.01),
respectively. The activity levels of ALT and AST were significantly
higher in the other five groups compared with those in the normal
control group (P<0.01).
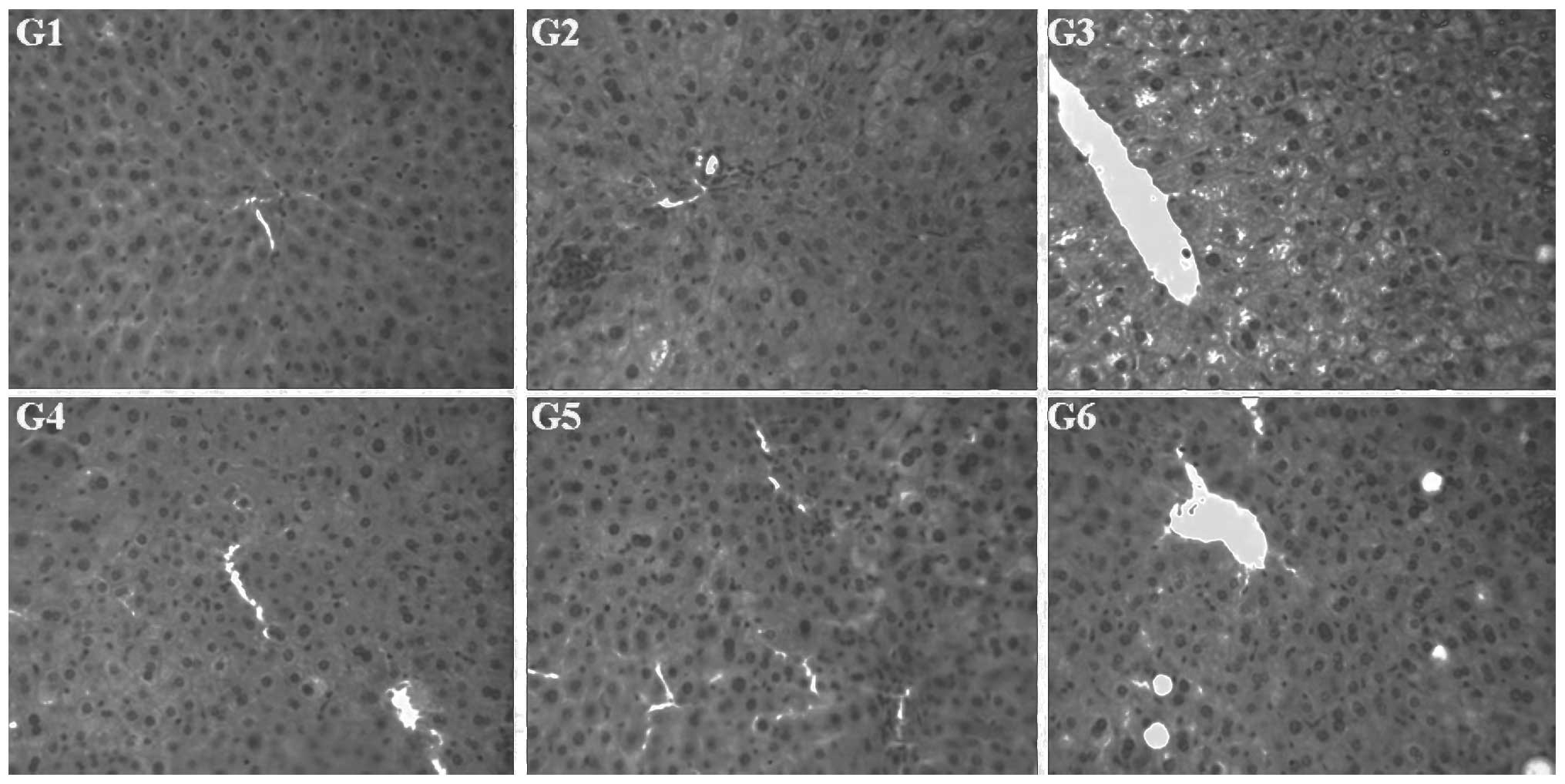
Morphology of the liver and biopsy of the
liver tissue
Macro-morphologies of the livers in the six groups
are shown in Fig. 4. The liver in
the normal control group was pinkish-brown, soft and elastic with a
sharp edge, smooth capsule and cut surface. In the model control
group, the livers of the mice showed a varying degree of fat
infiltration (liver appearance was cream-colored and greasy with a
blunt edge) and hepatomegaly. Compared with the model control
group, fatty degeneration of the liver in the other four groups was
improved to varying degrees, particularly in the ephedra alkaloid
and ephedra non-alkaloid groups.
The H&E staining results (Fig. 5) showed that the structure of the
hepatic lobule in the normal control group was clear and there were
no significantly degenerated cells, inflammatory cells or lipid
droplets present in the tissue. However, in the model control
group, the structure of the hepatic lobule was disordered and there
was marked swelling of the liver cells, focal necrosis and
widespread distribution of lipid droplets. In the other four
groups, there was no significant swelling of the liver cells,
enlargement of liver cell volume, fatty degeneration or necrosis.
Although sections of the lobular structures in the liver cells were
unclear, the livers in these four groups revealed less fatty
degeneration of the liver cells and smaller lipid droplets.
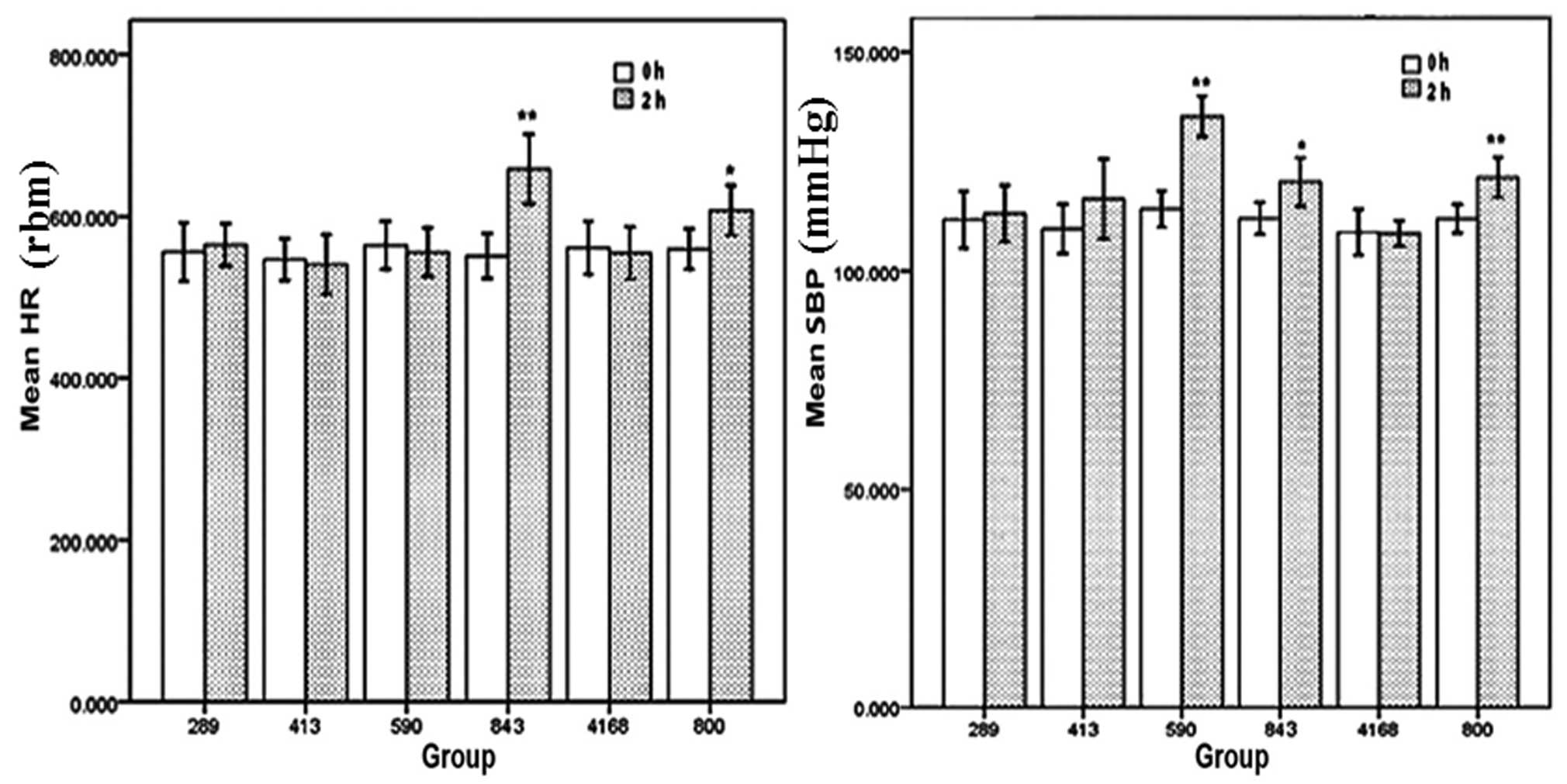
Acute toxicity
All the mice survived in the 800 mg/kg ephedra
polysaccharide group (n=20) and 4,168 mg/kg ephedra non-alkaloid
group (n=20), and no abnormal behavior was observed. The acute
toxicity of ephedra alkaloids to the mice was assessed by
determination of the seven-day LD50 value. The
calculated LD50 was 610 mg/kg with a 95% confidence
interval of 499–745 mg/kg (Table
I). No abnormal clinical symptoms were observed in the mice
administered 289 mg/kg ephedra alkaloids; however, one mouse
administered 413 mg/kg ephedra alkaloids died, and all mice exposed
to 843 mg/kg ephedra alkaloids showed hyperactive behavior. The HR
and SBP of the mice orally administered 289, 413, 590 and 843 mg/kg
ephedra alkaloids, 4,168 mg/kg ephedra non-alkaloids, or 800 mg/kg
ephedra polysaccharides at 0 and 2 h after administration are shown
in Fig. 6. A significant increase
in the HR was observed in the 843 mg/kg (P<0.01) ephedra
alkaloid and 800 mg/kg (P<0.05) ephedra polysaccharide groups at
2 h following administration. In addition, the SBP of the mice in
the 590 mg/kg (P<0.01), 843 mg/kg (P<0.05) ephedra alkaloid
and 800 mg/kg ephedra polysaccharide groups (P<0.01) were
significantly increased at 2 h following administration.
Discussion
Currently, there are various hypolipidemic drugs
available, including statins, fibrates and bile acid sequestrants;
however, they all exhibit numerous side effects. Therefore, there
is an urgent requirement for the development of hypolipidemic drugs
from natural resources. In the present study, ephedra extractions
were demonstrated to improve lipid metabolism in a diet-induced
hyperlipidemia mouse model.
Compared with the model control group, ephedra
extractions significantly reduced the levels of TC and TG, and
increased the level of HDL-C in the serum. Compared with the
positive control group, ephedra polysaccharides and non-alkaloids
had the advantage of lowering the TG level. A low level of HDL-C
has been documented as an indicator of high risk for cardiovascular
disease, and an increase in the level of HDL-C may potentially
contribute to anti-atherogenicity, inhibit LDL oxidation and
protect endothelial cells from the cytotoxic effects of OxLDL
(28,29). Therefore, the present results
indicate that ephedra non-alkaloids may protect against
cardiovascular disease by increasing the level of HDL-C.
The livers in the model control group revealed a
varying degree of fat infiltration and hepatomegaly; however, this
condition was improved in the other four groups, particularly the
ephedra alkaloid and non-alkaloid groups. The liver coefficient of
the non-alkaloid group was markedly lower compared with the other
groups, which indicated that the hepatomegaly had improved
significantly. The lipid droplets were smaller and less intensive
in the ephedrine alkaloid, ephedra polysaccharide and non-alkaloid
groups, which suggested that ephedra extractions lowered the lipid
levels. Ephedra polysaccharides and ephedra non-alkaloids may
scavenge lipids and reduce the absorption of fat to decrease the
levels of lipids.
In hypercholesteremia, the activity of SOD is
decreased and the levels of free radicals and MDA are increased.
Polyphenols and flavonoids may scavenge free radicals, including
hydroxyl and superoxide anions, to inhibit lipid peroxidation and
improve lipid profiles (30–33).
These drugs are also known to stimulate catalase and SOD gene
transcription, and decrease the MDA concentration (34,35).
In the current study, ephedra polysaccharides and non-alkaloids
significantly increased the activity of SOD and decreased the level
of MDA, which indicated that ephedra polysaccharides and
non-alkaloids were able to remove free radicals. Lipid
peroxidation, in the form of increased MDA production, has been
observed in previous studies, and serum levels of MDA have been
shown to correlate with the severity of chronic hepatitis,
indicating increased oxidative stress in patients with nonalcoholic
steatohepatitis (36,37). Not only the cell membrane lipids,
but also the cell membrane proteins, can be oxidized by free
radicals (38), which are
responsible for the damage of hepatic cell structure and function
and lipid metabolism disorder in the liver. SOD is the first line
of defense against oxygen-derived free radicals, converting
superoxide anions into H2O2 and reducing the
destruction of hydrogen and lipid hydroperoxides (39). The results of the present study
revealed that ephedra polysaccharides and ephedra non-alkaloids are
able to remove free radicals to protect the liver. In the ephedra
polysaccharide and non-alkaloid groups, there were no significantly
swollen liver cells and fatty degeneration of the hepatic cells. In
addition, the lobular structure of the liver cells was clearer
compared with that of the model control and positive control
groups. Therefore, ephedra non-alkaloids may exhibit protective
effects on the liver.
The liver plays a critical role in the normal
metabolism of energy substrates, particularly lipid metabolism. AST
and ALT are the main indicators for evaluating liver function and
the response to liver injury (40). Compared with the normal and model
control groups, simvastatin and ephedrine alkaloids significantly
increased the activity levels of ALT and AST (P<0.05),
indicating that these compounds had side effects on the liver.
However, ephedra non-alkaloids were shown to decrease the activity
levels of ALT (P<0.05) and AST (P<0.01), which indicated that
ephedra non-alkaloids may restore liver function.
Since the administration of ephedra non-alkaloids is
limited by the solubility and dose volume, the LD50 was
unable to be measured, indicating that the toxicity of ephedra
non-alkaloids is extremely low. In the acute toxicity study, the
maximum tolerated dose of oral ephedra non-alkaloids in the mice
was 367.5-fold larger than the maximal dosage in humans, as
specified in the Pharmacopoeia of the People’s Republic of China
(41) (9 g crude drug). In
addition, the SBP and HR of the mice did not notably change and no
abnormal behavior was observed. However, the SBP and HR of the mice
administered high dosages of ephedra polysaccharides and alkaloids
were significantly increased. These results demonstrated that
ephedra non-alkaloids may not induce cardiovascular side effects
and are safe for use.
In conclusion, ephedra non-alkaloids are relatively
safe and have the potential to improve hyperlipidemia. The
protective effects of ephedra non-alkaloids may be due to the
prevention of free radical generation, as well as recuperation of
liver function during liver damage.
Acknowledgements
The authors thank FengHe (ShangHai) Information
Technology Co., Ltd for their advice and support during the
study.
References
|
1
|
Lind L and Lithell H: Decreased peripheral
blood flow in the pathogenesis of the metabolic syndrome comprising
hypertension, hyperlipidemia, and hyperinsulinemia. Am Heart J.
125:1494–1497. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colomé C, Martínez-González J, Vidal F, de
Castellarnau C and Badimon L: Small oxidative changes in
atherogenic LDL concentrations irreversibly regulate adhesiveness
of human endothelial cells: effect of the lazaroid U74500A.
Atherosclerosis. 149:295–302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinberg D: Lewis A. Conner Memorial
Lecture. Oxidative modification of LDL and atherogenesis.
Circulation. 95:1062–1071. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cockerill GW, Saklatvala J, Ridley SH, et
al: High-density lipoproteins differentially modulate
cytokine-induced expression of E-selectin and cyclooxygenase-2.
Arterioscler Thromb Vasc Biol. 19:910–917. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khan BV, Harrison DG, Olbrych MT,
Alexander RW and Medford RM: Nitric oxide regulates vascular cell
adhesion molecule 1 gene expression and redox-sensitive
transcriptional events in human vascular endothelial cells. Proc
Natl Acad Sci USA. 93:9114–9119. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Assmann G and Schulte H: The Prospective
Cardiovascular Münster (PROCAM) study: prevalence of hyperlipidemia
in persons with hypertension and/or diabetes mellitus and the
relationship to coronary heart disease. Am Heart J. 116:1713–1724.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiu JH, Abdelhadi RH, Chung MK, et al:
Effect of statin therapy on risk of ventricular arrhythmia among
patients with coronary artery disease and an implantable
cardioverter-defibrillator. Am J Cardiol. 95:490–491. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maron DJ, Fazio S and Linton MF: Current
perspectives on statins. Circulation. 101:207–213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohli P, Desai NR, Giugliano RP, et al:
Design and rationale of the LAPLACE-TIMI 57 trial: a phase II,
double-blind, placebo-controlled study of the efficacy and
tolerability of a monoclonal antibody inhibitor of PCSK9 in
subjects with hypercholesterolemia on background statin therapy.
Clin Cardiol. 35:385–391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Armitage J: The safety of statins in
clinical practice. Lancet. 370:1781–1790. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neuvonen PJ, Niemi M and Backman JT: Drug
interactions with lipid-lowering drugs: mechanisms and clinical
relevance. Clin Pharmacol Ther. 80:565–581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conforti A, Magro L, Moretti U, et al:
Fluvastatin and hepatic reactions: a signal from spontaneous
reporting in Italy. Drug Saf. 29:1163–1172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abourashed EA, El-Alfy AT, Khan IA and
Walker L: Ephedra in perspective - a current review. Phytother Res.
17:703–712. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soni MG, Carabin IG, Griffiths JC and
Burdock GA: Safety of ephedra: lessons learned. Toxicol Lett.
150:97–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuang H, Yonggang X, Yang B, Wang Q and
Wang Y: Screening and comparison of the immunosuppressive
activities of polysaccharides from the stems of Ephedra sinica
Stapf. Carbohydrate Polymers. 83:787–795. 2011. View Article : Google Scholar
|
|
16
|
Xia Y, Kuang H, Yang B, Wang Q, et al:
Optimum extraction of acidic polysaccharides from the stems of
Ephedra sinica Stapf by Box-Behnken statistical design and its
anti-complement activity. Carbohydrate Polymers. 84:282–291. 2011.
View Article : Google Scholar
|
|
17
|
Shekelle PG, Hardy ML, Morton SC, et al:
Efficacy and safety of ephedra and ephedrine for weight loss and
athletic performance: a meta-analysis. JAMA. 289:1537–1545.
2003.PubMed/NCBI
|
|
18
|
Kalman D, Incledon T, Gaunaurd I, Schwartz
H and Krieger D: An acute clinical trial evaluating the
cardiovascular effects of an herbal ephedra-caffeine weight loss
product in healthy overweight adults. Int J Obes Relat Metab
Disord. 26:1363–1366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haller CA and Benowitz NL: Adverse
cardiovascular and central nervous system events associated with
dietary supplements containing ephedra alkaloids. N Engl J Med.
343:1833–1838. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
al-Khalil S, Alkofahi A, el-Eisawi D and
al-Shibib A: Transtorine, a new quinoline alkaloid from Ephedra
transitoria. J Nat Prod. 61:262–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Purev O, Pospísil F and Motl O: FPaOM:
Flavonoids from Ephedra sinica Stapf. Collect Czech Chem Commun.
53:3193–3196. 1988. View Article : Google Scholar
|
|
22
|
Starratt AN and Caveney S:
Quinoline-2-carboxylic acids from Ephedra species. Phytochemistry.
42:1477–1478. 1996. View Article : Google Scholar
|
|
23
|
Konno C, Mizuno T and Hikino H: Isolation
and hypoglycemic activity of ephedrans A, B, C, D and E, glycans of
Ephedra distachya herbs. Planta Med. 162–163. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chumbalov TK, Chekmeneva LN and Polyakov
VV: Phenolic acids of Ephedra equisetina. Chemistry of Natural
Compounds. 13:238–239. 1977. View Article : Google Scholar
|
|
25
|
Zhang L, Zou G and Yang T: Studies on
extraction of water-soluble polysaccharides and the function of
cleaning oxygen free-radical function of ephedra. Amino Acids and
Biotic Resources. 22:24–26. 2000.
|
|
26
|
OECD (Organisation for Economic
Co-operation and Development). OECD Guidelines for the Testing of
Chemicals. Guideline 425: Acute Oral Toxicity-Up-and-Down
Procedure. 1932001.
|
|
27
|
Karber G: Determination of LD50. Arch Exp
Pathol Pharma. 162:4801931.
|
|
28
|
Wilson PW, Abbott RD and Castelli WP: High
density lipoprotein cholesterol and mortality. The Framingham Heart
Study Arteriosclerosis. 8:737–741. 1988.
|
|
29
|
Assmann G and Nofer JR: Atheroprotective
effects of high-density lipoproteins. Annu Rev Med. 54:321–341.
2003. View Article : Google Scholar
|
|
30
|
Rice-Evans CA, Miller NJ, Bolwell PG,
Bramley PM and Pridham JB: The relative antioxidant activities of
plant-derived polyphenolic flavonoids. Free Radic Res. 22:375–383.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tripathi YB, Singh BK, Pandey RS and Kumar
M: BHUx: a patent polyherbal formulation to prevent
atherosclerosis. Evid Based Complement Alternat Med. 2:217–221.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ljubuncic P, Dakwar S, Portnaya I, et al:
Aqueous extracts of Teucrium polium possess remarkable antioxidant
activity in vitro. Evid Based Complement Alternat Med. 3:329–338.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Punitha IS, Rajendran K and Shirwaikar A
and Shirwaikar A: Alcoholic stem extract of Coscinium fenestratum
regulates carbohydrate metabolism and improves antioxidant status
in streptozotocin-nicotinamide induced diabetic rats. Evid Based
Complement Alternat Med. 2:375–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toyokuni S, Tanaka T, Kawaguchi W, et al:
Effects of the phenolic contents of Mauritian endemic plant
extracts on promoter activities of antioxidant enzymes. Free Radic
Res. 37:1215–1224. 2003. View Article : Google Scholar
|
|
35
|
Ralay Ranaivo H, Rakotoarison O, Tesse A,
et al: Cedrelopsis grevei induced hypotension and improved
endothelial vasodilatation through an increase of Cu/Zn SOD protein
expression. Am J Physiol Heart Circ Physiol. 286:H775–H781. 2004.
View Article : Google Scholar
|
|
36
|
Paradis V, Mathurin P, Kollinger M, et al:
In situ detection of lipid peroxidation in chronic hepatitis C:
correlation with pathological features. J Clin Pathol. 50:401–406.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yadav D, Hertan HI, Schweitzer P, Norkus
EP and Pitchumoni CS: Serum and liver micronutrient antioxidants
and serum oxidative stress in patients with chronic hepatitis C. Am
J Gastroenterol. 97:2634–2639. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kako KJ: Free radical effects on membrane
protein in myocardial ischemia/reperfusion injury. J Mol Cell
Cardiol. 19:209–211. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harris ED: Regulation of antioxidant
enzymes. FASEB J. 6:2675–2683. 1992.PubMed/NCBI
|
|
40
|
Limdi JK and Hyde GM: Evaluation of
abnormal liver function tests. Postgrad Med J. 79:307–312. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
State Pharmacopoeia Commission of the
People’s Republic of China. Pharmacopoeia of the People’s Republic
of China. 8th edition. People’s Medical Publishing House; Beijing:
Part 1. pp. 2242005
|