Introduction
Sepsis is a systemic inflammatory reaction syndrome
that is caused by infectious factors, and is one of the major
causes of mortality in critical patients. Deregulation of the body
during sepsis is considered to result in uncontrolled inflammation,
the release of large amounts of inflammatory mediators, the
development of inflammatory cascades and ultimately damage to
tissues and organs (1–4). Vascular endothelial cells (VECs)
serve as a vital interface between the blood and tissues. In the
presence of inflammatory stimulation, the cells are activated and
express adhesion molecules that play a critical role in leukocyte
aggregation (5). Previous studies
have shown that VECs are the major victim to pathogens and their
toxins in sepsis. For instance, endotoxin and other bacterial
components act on VECs to reduce vascular tension, widen the space
between the VECs, increase vascular permeability, promote the
release of inflammatory mediators and aggravate platelet
aggregation (6). As a result, the
inflammatory and coagulation systems become deregulated and
systemic inflammatory response syndrome and multiple organ
dysfunction syndrome develop (7,8). The
nuclear factor (NF)-κB signaling pathway plays an important
regulatory role in sepsis (9,10),
and blocking the NF-κB pathway is an important modality in the
treatment of sepsis (11,12).
microRNA (miRNA) is a small, single-stranded RNA
molecule that is ubiquitously present in eukaryotic organisms,
which is characterized by high conservation and tissue specificity.
miRNA binds to specific mRNA molecules to inhibit the expression of
target genes or degrade the mRNA, which subsequently contributes to
cell proliferation, differentiation, development, metabolism,
apoptosis and other physiological activities. Thus, miRNA exerts an
important regulatory function on eukaryotic genes (13–15).
miR-23b is a multifunctional miRNA that contributes
to the regulation of multiple signaling pathways, affecting cell
proliferation, differentiation, apoptosis and adhesion (16–24).
Moreover, the functions and underlying mechanisms are currently
under investigation. It has been reported that miR-23b prevents
multiple autoimmune diseases through the regulation of inflammatory
cytokine pathways, in which the molecule regulates a number of
inflammatory cytokines, such as NF-κB, tumor necrosis factor
(TNF)-α, interleukin (IL)-1β and IL-17 (25,26).
Therefore, it was hypothesized that miR-23b may act on sepsis
through the NF-κB pathway and IL-17; thus, regulating the
NF-κB-mediated activation of VECs.
In the present study, septic VECs were simulated
using bacterial lipopolysaccharide (LPS) to induce the activation
of human VECs, after which the cells were transfected with miR-23b
mimics and inhibitor sequences to observe the effect of
upregulating and inhibiting miR-23b on the expression levels of
inflammatory factors in septic VECs. The aim of the present study
was to investigate the potential of miR-23b as a therapeutic target
for sepsis treatment.
Materials and methods
Cell culture and miR-23b sequences
The 1D3 human VEC cell line (Shanghai Bioleaf
Biotech Co., Ltd., Shanghai, China) was preserved in liquid
nitrogen in the laboratory. The cells were routinely cultured in
modified RPMI-1640 medium containing 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare, Logan, UT, USA). The following sequences
were designed and synthesized by Shanghai GenePharma Co., Ltd
(Shanghai, China): miR-23b inhibitor sequence,
5′-GGUAAUCCCUGGCAAUGUGAU-3′; miR-23b inhibitor negative control
(NC) sequence, 5′-CAGUACUUUUGUGUAGUACAA-3′; miR-23b mimics
sequence, 5′-AUCACAUUGCCAGGGAUUACC-3′; miR-23b mimics NC sequence,
5′-UUCUCCGAACGUGUCACGUTT-3′. The sequences were labeled with
fluorescein amidite to observe fluorescence.
Transfection of miR-23b into the human
VECs
Using Lipofectamine 2000 transfection reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), the synthesized
sequences were transfected into the human VECs. Initially, the
mimics NC or inhibitor NC sequences were used to investigate the
optimum conditions for transfection. At day one prior to
transfection, 1×104–3×104 cells were
inoculated into 24-well plates, and 500 μl modified RPMI-1640
medium containing 10% FBS was added to each well. The cells were
cultured in an incubator containing 5% CO2 at 37°C until
the cells reached a confluence of 70–90%. Various doses of mimics
NC or inhibitor NC (6, 15, 20, 30, 50 or 100 pmol) were added to 50
μl serum-free Opti-MEM (Hyclone; GE Healthcare), which was followed
by gentle mixing. Lipofectamine 2000 (0.3 or 1 μl) was added to 50
μl serum-free Opti-MEM, mixed gently and rested at room temperature
for 5 min. The two solutions were subsequently mixed and added to
the plate wells containing the cells and 500 μl serum-free
RPMI-1640 medium, after which the plates were placed onto a swing
bed for gentle shaking. Following incubation for 5 h at 37°C, the
medium was replaced with 500 μl fresh modified RPMI-1640 medium
containing serum and the plates were swung for mixing. After a
further 24 h incubation at 37°C, the cells were observed and
photographed under fluorescence microscopy (CX41-32RFL; Olympus,
Tokyo, Japan). Pilot studies identified the optimal transfection
dose of the miR-23b sequence to be 50 pmol and the optimal dose of
Lipofectamine 2000 to be 1 μl, according to fluorescence intensity
measurements. Therefore, the dose of the miR-23b inhibitor, NC
inhibitor, miR-23b mimics and NC mimics sequences was 50 pmol, and
1 μl Lipofectamine 2000 was used for transfection. The procedure
for transfection was applied as aforementioned. In addition, a
blank control group was established by adding the same volume of
phosphate-buffered saline and Lipofectamine 2000.
LPS-stimulated human VECs
In total, 2×104–5×104 cells
were successfully transfected with miR-23b and inoculated into
12-well plates. Into each well, 500 μl modified RPMI-1640 medium,
containing 10% FBS, was added. The cells were cultured for 24 h at
37°C in an incubator containing 5% CO2. Upon reaching a
confluence of 70–90%, LPS was added to a final concentration of 1
μg/ml. The cells were cultured for 4 and 8 h, after which the total
RNA was extracted for quantitative polymerase chain reaction
(PCR).
Quantitative PCR
Total RNA extraction was performed according to the
instructions of the TRIzol reagent kit (Invitrogen Life
Technologies). Reverse transcription of the mRNA was conducted
using a RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Waltham, MA, USA). The reaction system included 2 μl
RNA and 1 μl oligo (dT)18 primer, and the final volume was adjusted
to 12 μl with RNase-free deionized water. The solution was
incubated at 70°C for 5 min on a PikoReal PCR amplifier (Thermo
Fisher Scientific), and placed on ice for quick cooling. Next, 4 μl
5X buffer, 2 μl dNTP (10 mM), 1 μl RNA inhibitor and 1 μl reverse
transcriptase (Thermo Fisher Scientific) were added to the
solution, which was followed by mixing using a pipette. The
solution was incubated at 42°C for 60 min on a PCR amplifier,
followed by 5 min incubation at 70°C to inactivate the reverse
transcriptase. The products of the reverse transcription reaction
were quantitatively analyzed using THUNDERBIRD™ SYBR®
qPCR Mix kit (Toyobo Co., Ltd., Osaka, Japan). A 0.2-ml PCR tube
was used, which contained 12.5 μl 2X qPCR Mix, 2.0 μl gene primer
or internal standard primer (2.5 μM), 2.0 μl reverse transcripts
and 8.5 μl ddH2O. The conditions for amplification were
as follows: Initial denaturation at 95°C for 3 min, followed by 40
cycles of 95°C for 15 sec, 59°C for 30 sec and 72°C for 25 sec. The
quantitative PCR assay of miR-23b was performed using a custom
reverse transcription and quantitative PCR kit (Changzhou Chutian
Biotechnology Co., Ltd., Changzhou, China), where U6 was used as an
internal standard, according to the instructions of the kit.
Table I shows the sequences of the
primers used for quantitative PCR.
 | Table IQuantitative polymerase chain
reaction primers. |
Table I
Quantitative polymerase chain
reaction primers.
| Gene | Primers
(5′→3′) | Length (bp) |
|---|
| NF-κB (NM_003998,
NM_001165412) | Sense,
TGAGTCCTGCTCCTTCCAA
Antisense, GAGAGGTGGTCTTCACTGGG | 150 |
| IL-6
(NM_000600) | Sense,
AAGCAGCAAAGAGGCACTG
Antisense, TTTCACCAGGCAAGTCTCCT | 106 |
| TNF-α
(NM_000594) | Sense,
GTGCTGGCAACCACTAAGAAT
Antisense, GCCTAAGGTCCACTTGTGTCA | 170 |
|
VCAM-1(NM_001078) | Sense,
GCTGCTCAGATTGGAGACTCA
Antisense, CGCTCAGAGGGCTGTCTATC | 100 |
| E-selectin
(NM_000450) | Sense,
AATCCAGCCAATGGGTTCG
Antisense, GCTCCCATTAGTTCAAATCCTTCT | 104 |
| ICAM-1
(NM_000201) | Sense,
GCTCAAGTGTCTAAAGGATGGC
Antisense, CATTATGACTGCGGCTGCTA | 196 |
Western blot analysis
Cells were harvested by centrifugation at 2,000 × g
for 5 min at 37°C, and 106 cells were added to 250 μl
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) to extract the total protein. In
total, 50 μg protein was subjected to SDS-PAGE, after which the
protein was transferred onto a 0.45-μm polyvinylidene fluoride
membrane (Millipore Corporation, Billerica, MA, USA). The membrane
was incubated with mouse monoclonal NF-κB P65 (1:1,000; cat. no.
13346S, Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
monoclonal TNF-α (1:1,000; cat. no. ab53450), rabbit monoclonal
IL-6 (1:1,000; cat. no. ab32530), rabbit monoclonal VCAM-1
(1:1,000; cat. no. ab134047, Abcam, Cambridge, UK), rabbit
polyclonal ICAM-1 (1:1,000; cat. no. sc-7891), rabbit polyclonal
E-selectin (1:1,000; cat. no. sc-14011) and rabbit polyclonal
β-actin antibodies (1:1,000; cat. no. sc-130656, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), and shaken overnight at
4°C. Following discoloration, a horseradish peroxidase-conjugated
goat anti-rabbit or goat anti-mouse secondary antibody (1:5,000;
KPL, Inc., Gaithersburg, MD, USA) was added and incubated at room
temperature for 30 min to wash out the unbound antibodies. Color
development was performed by adding an enhanced chemiluminescence
developer (Pierce Biotechnology, Inc., Rockford, IL, USA) and
waiting for 1–2 min. Thereafter, the remnant liquid was discarded,
and the membrane was embedded with a fresh membrane and subjected
to X-ray exposure. The exposure condition was adjusted according to
the light intensity. Following development and fixation, the images
were analyzed for grayscale using BandScan 5.0 software (Glyko,
Novato, CA, USA).
Statistical analysis
All experiments were performed three times, and data
are expressed as the mean ± standard deviation. A χ2 or
two-sided t-test was performed for statistical analysis using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Transfection of the miR-23b inhibitor
sequence inhibits miR-23b expression, while transfection of the
miR-23b mimics sequence increases miR-23b expression in human
VECs
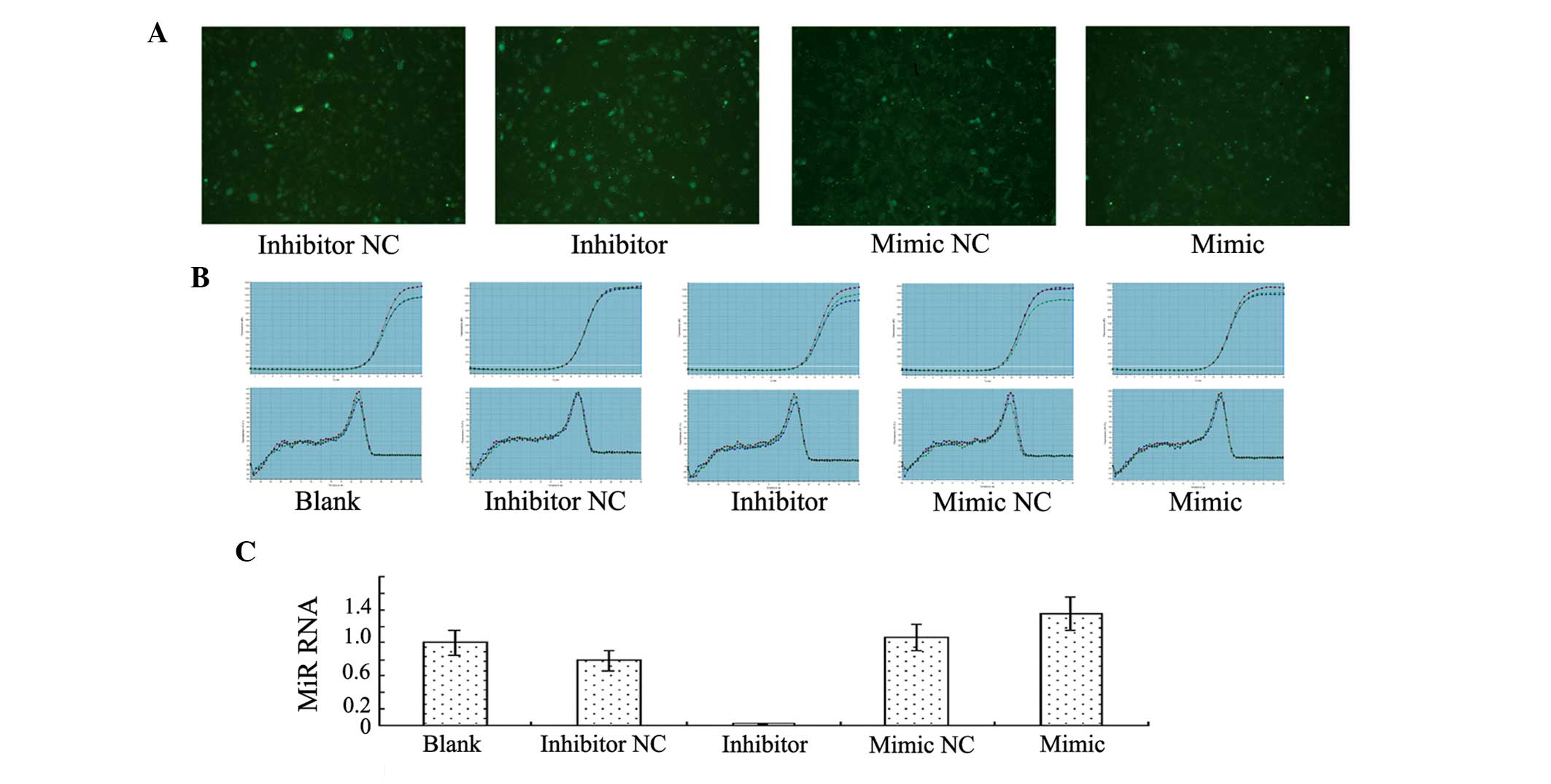
Human VECs transfected with miR-23b emitted green
fluorescence under fluorescence microscopy (Fig. 1A). The quantitative PCR results
indicated that miR-23b expression decreased significantly in the
group transfected with the miR-23b inhibitor sequence when compared
with the blank control group and the group transfected with the
inhibitor NC sequence, demonstrating that transfection with the
miR-23b inhibitor inhibited miR-23b expression effectively. By
contrast, miR-23b expression increased significantly in the group
transfected with the miR-23b mimics sequence when compared with the
blank control group and the mimics NC group, indicating that
transfection with miR-23b mimics promoted miR-23b expression
(Fig. 1B and C).
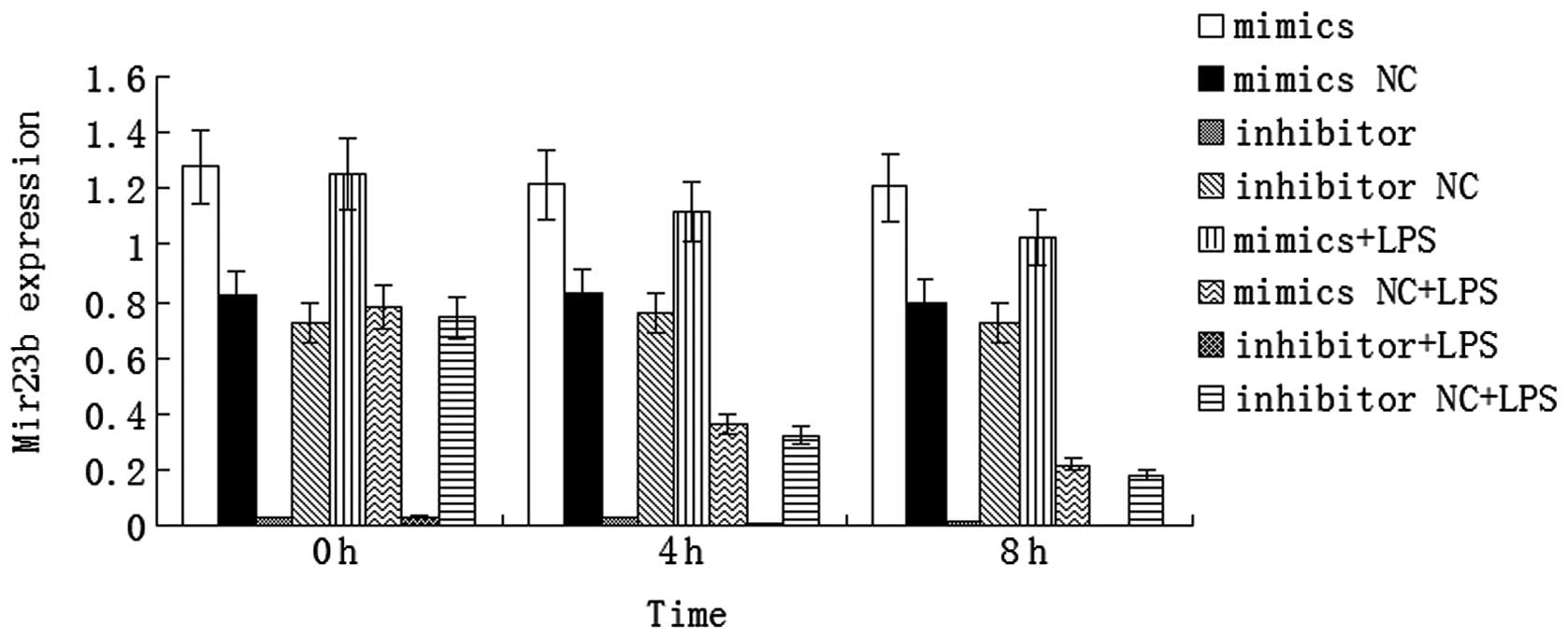
LPS downregulates miR-23b expression in
human VECs
VECs in sepsis were simulated using LPS-stimulated
human VECs. The results demonstrated that miR-23b expression
decreased significantly in the cells transfected with the mimics NC
or inhibitor NC sequences at 4 or 8 h after LPS stimulation when
compared with the cells that did not undergo LPS stimulation
(P<0.01). In addition, the results revealed that miR-23b
expression decreased in the LPS-stimulated human VECs; thus, VEC
activation in sepsis was accompanied by the inhibition of miR-23b
expression. By contrast, miR-23b expression decreased slightly in
the cells transfected with the mimics sequence at 4 or 8 h after
LPS stimulation when compared with the unstimulated cells, but
remained at a high level. miR-23b expression remained low in the
cells transfected with the inhibitor sequence following LPS
stimulation (Fig. 2).
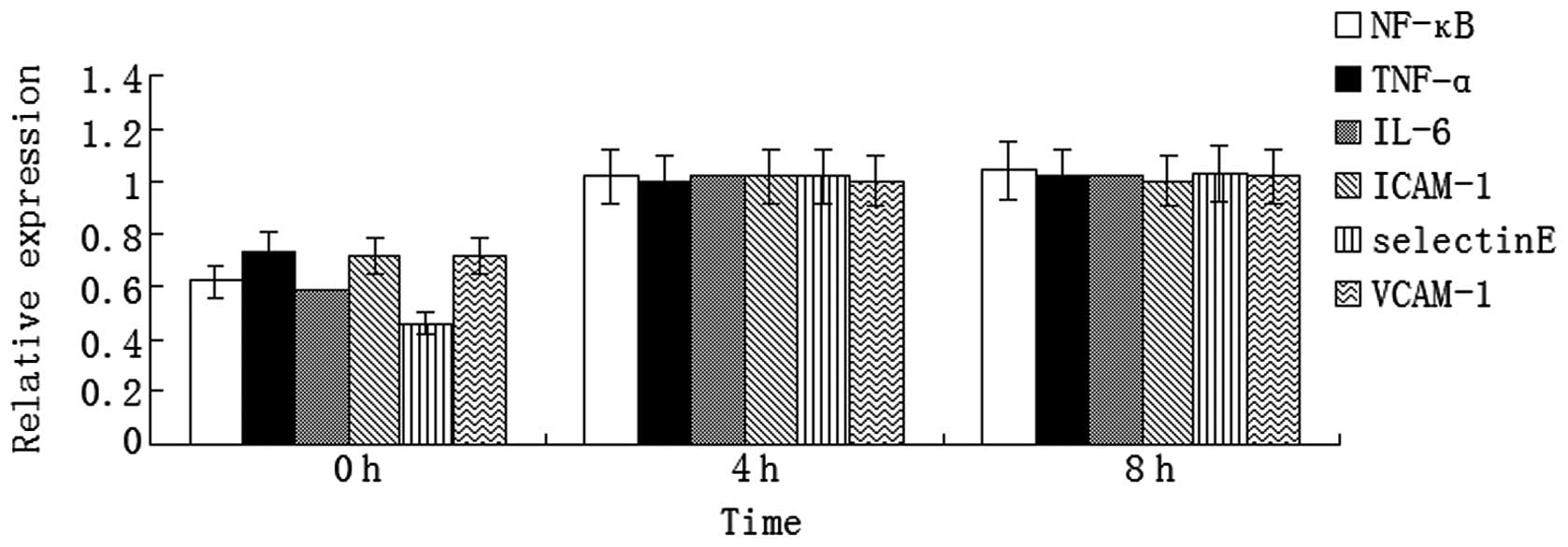
LPS promotes inflammatory cytokine
expression in human VECs
Expression levels of inflammatory cytokines,
including NF-κB, TNF-α, IL-6, ICAM-1, E-selectin and VCAM-1,
increased significantly at 4 and 8 h after LPS stimulation in the
human VECs (P<0.05, Fig. 3).
These results demonstrated that LPS stimulated the human VECs to
express inflammatory cytokines; thus, contributing to the
inflammatory reaction in sepsis.
miR-23b mimics inhibits the
LPS-stimulated expression of inflammatory factors
The mRNA expression levels of NF-κB, TNF-α, IL-6,
ICAM-1, E-selectin and VCAM-1 increased significantly at 4 and 8 h
after LPS stimulation in the human VECs transfected with the mimics
NC sequence (P<0.05); however, the expression levels decreased
significantly in the cells transfected with the mimics sequence
(P<0.05; Fig. 4A). Western blot
analysis revealed that the protein expression levels of NF-κB,
TNF-α, IL-6, ICAM-1 and E-selectin were significantly lower in the
mimics group when compared with the mimics NC group after 4 h of
LPS stimulation (P<0.05), while the expression level of VCAM-1
was significantly lower in the mimics group when compared with the
mimics NC group following 8 h of LPS stimulation (P<0.05;
Fig. 4B).
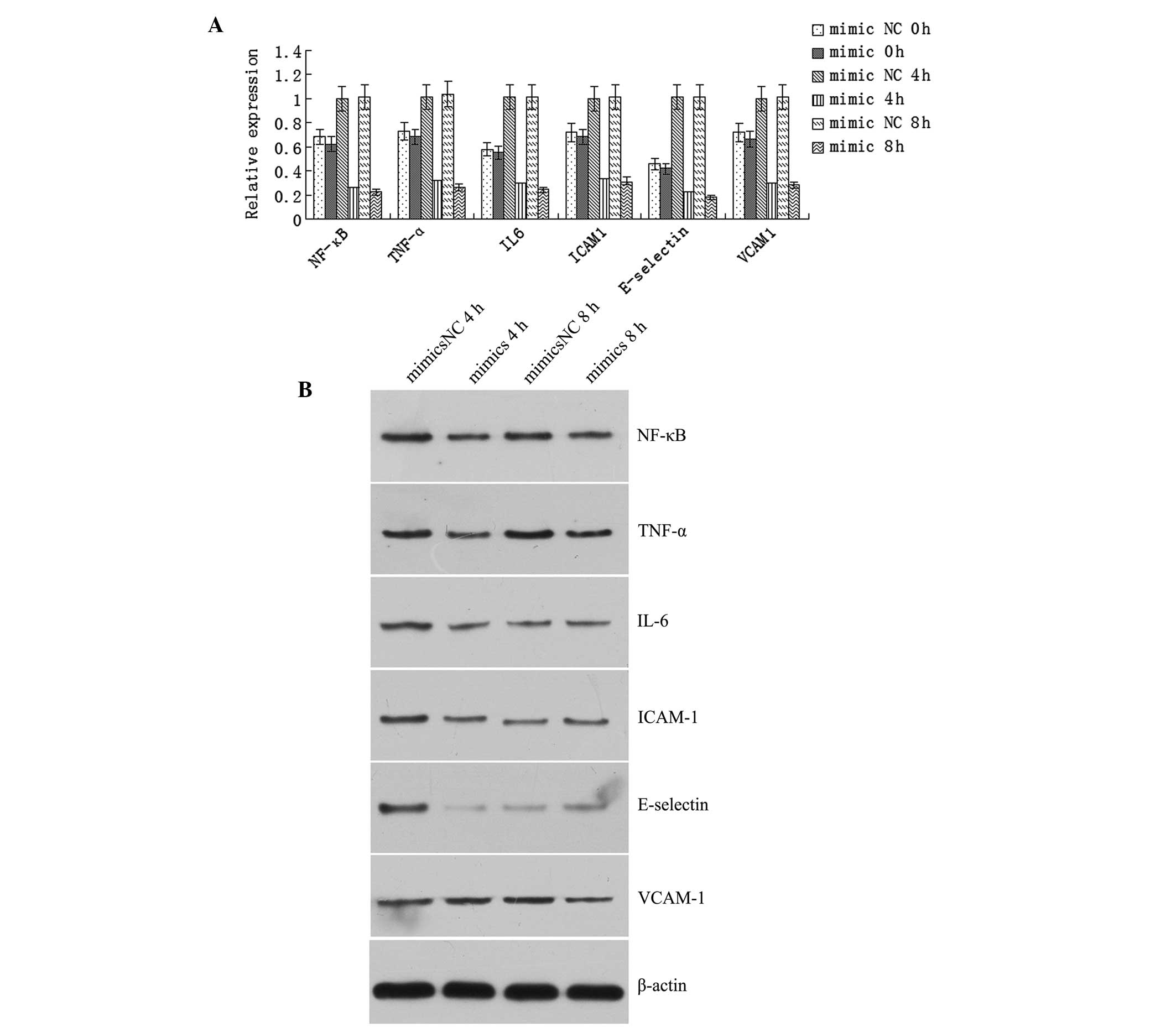 | Figure 4Transfection with the miR-23b mimics
sequence inhibited the lipopolysaccharide (LPS)-stimulated
expression of inflammatory factors. (A) Quantitative polymerase
chain reaction revealed that mRNA expression levels of NF-κB,
TNF-α, IL-6, ICAM-1, E-selectin and VCAM-1 increased significantly
in the human vascular endothelial cells transfected with the mimics
NC after 4 and 8 h of LPS stimulation, and decreased significantly
in the cells transfected with the mimics sequence (P<0.05). (B)
Western blot analysis revealed that the protein expression levels
of NF-κB, TNF-α, IL-6, ICAM-1 and E-selectin were significantly
lower in the mimics group when compared with the mimics NC group
after 4 h of LPS stimulation (P<0.05). Furthermore, the protein
expression level of VCAM-1 was significantly lower in the mimics
group when compared with the mimics NC group after 8 h of LPS
stimulation (P<0.05). NC, negative control; NF, nuclear factor;
TNF, tumor necrosis factor; IL, interleukin; ICAM, intercellular
adhesion molecule; VCAM, vascular cell adhesion molecule. |
Effect of the miR-23b inhibitor sequence
on the expression of inflammatory factors in LPS-stimulated
cells
The mRNA expression levels of NF-κB, TNF-α, IL-6,
ICAM-1, E-selectin and VCAM-1 increased significantly in the human
VECs transfected with the inhibitor NC sequence following LPS
stimulation for 4 and 8 h when compared with the prestimulation
levels (P<0.05). In addition, the expression levels of the
inflammatory factors increased significantly in the cells
transfected with the inhibitor sequence following LPS stimulation,
as compared with the prestimulation levels (P<0.05).
Furthermore, the levels of the inflammatory factors increased
significantly after 4 or 8 h of LPS stimulation when compared with
the levels in the cells transfected with the inhibitor NC sequence
(Fig. 5A). Western blot analysis
revealed that the protein expression levels of NF-κB, TNF-α, IL-6,
ICAM-1, E-selectin and VCAM-1 increased significantly in the cells
transfected with the inhibitor sequence when compared with the
cells transfected with the inhibitor NC sequence after 4 or 8 h of
LPS stimulation (P<0.05; Fig.
5B).
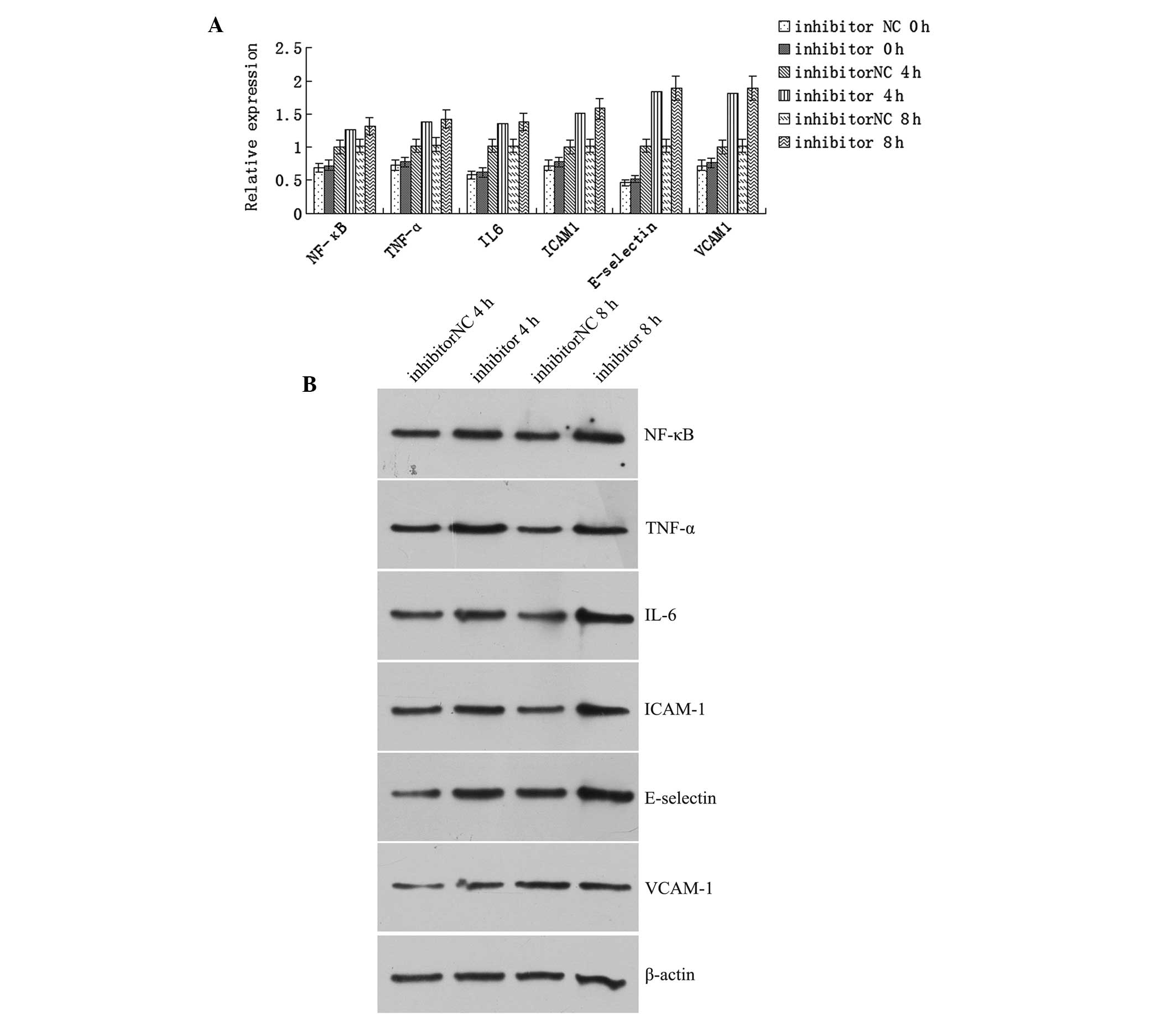 | Figure 5Transfection with the miR-23b
inhibitor sequence promoted the expression of the inflammatory
factors in lipopolysaccharide (LPS)-stimulated cells. (A) mRNA
expression levels of NF-κB, TNF-α, IL-6, ICAM-1, E-selectin and
VCAM-1 increased significantly in the human vascular endothelial
cells transfected with the inhibitor NC after 4 and 8 h of LPS
stimulation when compared with the prestimulation levels
(P<0.05). In addition, expression levels of these factors
increased significantly in the cells transfected with the miR-23b
inhibitor sequence following LPS stimulation when compared with the
prestimulation levels (P<0.05). The expression levels of these
factors were greater in the cells transfected with miR-23b
inhibiter as compared with those transfected with the inhibitor NC
after 4 or 8 h of LPS stimulation. (B) Western blot analysis
revealed that the protein expression levels of NF-κB, TNF-α, IL-6,
ICAM-1, E-selectin and VCAM-1 increased significantly after 4 or 8
h of LPS stimulation in the cells transfected with the inhibitor
sequence when compared with the cells transfected with the
inhibitor NC (P<0.05). NC, negative control; NF, nuclear factor;
TNF, tumor necrosis factor; IL, interleukin; ICAM, intercellular
adhesion molecule; VCAM, vascular cell adhesion molecule. |
Discussion
Sepsis is a systemic inflammatory reaction syndrome
caused by infection, which may lead to shock and multiple organ
dysfunction (27). The
pathogenesis of sepsis is extremely complex (28). The invasion of pathogens triggers
the release of inflammatory factors, which results in systemic
inflammatory reactions. Currently, the role of miRNA in sepsis has
become a focus of research. miRNA, a type of endogenous,
non-encoding, single-stranded RNA with a length of ~22 nucleotides,
can degrade mRNA or inhibit translation, subsequently regulating
gene expression at the post-transcriptional level (29). There are a number of target genes
of miRNA, with evidence demonstrating that miRNA regulates the
expression of ≥30% of human genes (30,31).
Upon invasion of pathogenic microorganisms, host cells have been
demonstrated to produce miRNA quickly, promoting the release of
inflammatory factors to cause immune hyperactivity, and inducing
apoptosis or degrading the inflammatory factors to cause
immunosuppression (32–37). Therefore, miRNA plays a critical
role in regulating inflammatory reactions in sepsis (38). VECs are single-layer squamous cells
that cover the vascular lining. The cells serve as a barrier, and
are involved in substance exchange, vascular tension regulation,
coagulation and the inflammatory reaction. VEC damage is closely
associated with sepsis. Pathogenic microorganisms and their
products may impair VECs and lead to microcirculation disturbance
(39) and the amplification of
inflammatory reactions (40,41).
In the present study, LPS-stimulated human VECs were used to
simulate VECs in sepsis. LPS is the major cell wall component of
Gram-negative bacilli; thus, LPS is a major pathogenic substance
that causes toxic reactions. Therefore, LPS is often used to
reproduce sepsis models (42). The
results demonstrated that LPS promoted the human VECs to express
various inflammatory factors, including NF-κB, TNF-α, IL-6, ICAM-1,
E-selectin and VCAM-1 (Fig.
2).
miR-23b is a newly identified miRNA. The molecule
has been shown to exert a similar function to an oncogene in
glioma, urinary system tumors (kidney cancer and prostatic
carcinoma) and breast cancer (43–48).
miR-23b regulates cytoskeletal reconstruction, cell invasion and
metastasis (49). In addition,
miR-23b has been shown to inhibit inflammatory reactions in
autoimmune diseases; thus, can exert protection against autoimmune
diseases (50,51). However, to the best of our
knowledge, there have been no previous studies investigating the
role of miR-23b in sepsis. The present study demonstrated that
miR-23b expression decreased in LPS-stimulated human VECs. In order
to investigate the regulatory mechanisms of miR-23b on inflammatory
factor expression in VECs during sepsis, miR-23b expression in
human VECs was upregulated and downregulated through transfection
with miR-23b mimics and inhibitor sequences, as well as the
respective NC sequences. The results demonstrated that upregulation
of miR-23b inhibited the expression of NF-κB, TNF-α, IL-6, ICAM-1,
E-selectin and VCAM-1, while downregulation of miR-23b promoted
inflammatory factor expression.
Numerous inflammatory factors interact with each
other to form a complex network, which is critical to sepsis
(52,53). Previously, NF-κB has been shown to
be activated in sepsis, regulating apoptosis, cell growth, the
stress reaction, the immune reaction and septic shock (54–56).
In addition, the levels of TNF-α and IL-6 have been shown to reach
a peak as early as 3 h after the onset of sepsis, and the degree of
increase may reflect the severity of sepsis (57,58).
ICAM-1, VCAM-1 and E-selectin are important cell adhesion factors
that regulate the activity of inflammatory and vascular endothelial
cells, pro- and anti-inflammatory factors, as well as inflammatory
cell migration to tissues and organs; thus, these factors play an
important role in sepsis (59–61).
In conclusion, the present study demonstrated that
miR-23b regulates sepsis through inhibiting the expression of
inflammatory factors in VECs. The affected inflammatory factors
include NF-κB, TNF-α, IL-6, ICAM-1, E-selectin and VCAM-1. These
results suggest that miR-23b may be a potential therapeutic target
for the treatment of sepsis. However, the present study only
observed the regulation of inflammatory factors by miR-23b, however
the underlying mechanisms require further study.
References
|
1
|
Dirkes S: Sepsis and inflammation: impact
on acute kidney injury. Nephrol Nurs J. 40:125–133. 2013.PubMed/NCBI
|
|
2
|
Gomez H, Ince C, De Backer D, et al: A
unified theory of sepsis-induced acute kidney injury: inflammation,
microcirculatory dysfunction, bioenergetics and the tubular cell
adaptation to injury. Shock. 41:3–11. 2014. View Article : Google Scholar :
|
|
3
|
Pinheiro da Silva F, Machado MC and
Velasco IT: Neuropeptides in sepsis: from brain pathology to
systemic inflammation. Peptides. 44:135–138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis DH, Chan DL, Pinheiro D,
Armitage-Chan E and Garden OA: The immunopathology of sepsis:
pathogen recognition, systemic inflammation, the compensatory
anti-inflammatory response and regulatory T cells. J Vet Intern
Med. 26:457–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hernandez MR, Palomo M, Fuste B, et al:
Effect of two different dialysis membranes on leukocyte adhesion
and aggregation. Nephron Clin Pract. 106:c1–c8. 2007.PubMed/NCBI
|
|
6
|
Rodella LF, Favero G, Foglio E, et al:
Vascular endothelial cells and dysfunctions: role of melatonin.
Front Biosci (Elite Ed). 5:119–129. 2013.
|
|
7
|
Zhang ZD and Ma XC: Injury of vascular
endothelial cell and microcirculation disturbance in sepsis.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 23:125–128. 2011.(In
Chinese). PubMed/NCBI
|
|
8
|
Li M and Yu YH: Vascular endothelial cell
dysfunction and its clinical strategy in severe sepsis. Zhonghua Er
Ke Za Zhi. 49:603–606. 2011.(In Chinese). PubMed/NCBI
|
|
9
|
Adamzik M, Schäfer S, Frey UH, et al: The
NFKB1 promoter polymorphism (−94ins/delATTG) alters nuclear
translocation of NF-κB1 in monocytes after lipopolysaccharide
stimulation and is associated with increased mortality in sepsis.
Anesthesiology. 118:123–133. 2013. View Article : Google Scholar
|
|
10
|
Raspé C, Höcherl K, Rath S, Sauvant C and
Bucher M: NF-κB-mediated inverse regulation of fractalkine and
CX3CR1 during CLP-induced sepsis. Cytokine. 61:97–103. 2013.
View Article : Google Scholar
|
|
11
|
Kaplan J, Nowell M, Chima R and Zingarelli
B: Pioglitazone reduces inflammation through inhibition of NF-κB in
polymicrobial sepsis. Innate Immun. 20:519–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Han W, Polosukhin V, et al: NF-κB
inhibition after cecal ligation and puncture reduces
sepsis-associated lung injury without altering bacterial host
defense. Mediators Inflamm. 2013:5032132013.
|
|
13
|
Lenkala D, LaCroix B, Gamazon ER, Geeleher
P, Im HK and Huang RS: The impact of microRNA expression on
cellular proliferation. Hum Genet. 133:931–938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dvinge H, Git A, Gräf S, et al: The
shaping and functional consequences of the microRNA landscape in
breast cancer. Nature. 497:378–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melton C, Judson RL and Blelloch R:
Opposing microRNA families regulate self-renewal in mouse embryonic
stem cells. Nature. 463:621–626. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Liu Z, Chen L, Zhou L and Yao Y:
MicroRNA-23b is an independent prognostic marker and suppresses
ovarian cancer progression by targeting runt-related transcription
factor-2. FEBS Lett. 588:1608–1615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pellegrino L, Krell J, Roca-Alonso L,
Stebbing J and Castellano L: MicroRNA-23b regulates cellular
architecture and impairs motogenic and invasive phenotypes during
cancer progression. Bioarchitecture. 3:119–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Donadelli M and Palmieri M: Roles for
microRNA 23b in regulating autophagy and development of pancreatic
adenocarcinoma. Gastroenterology. 145:936–938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishteiwy RA, Ward TM, Dykxhoorn DM and
Burnstein KL: The microRNA -23b/-27b cluster suppresses the
metastatic phenotype of castration-resistant prostate cancer cells.
PLoS One. 7:e521062012. View Article : Google Scholar
|
|
20
|
Au Yeung CL, Tsang TY, Yau PL and Kwok TT:
Human papillomavirus type 16 E6 induces cervical cancer cell
migration through the p53/microRNA-23b/urokinase-type plasminogen
activator pathway. Oncogene. 30:2401–2410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang KC, Garmire LX, Young A, et al: Role
of microRNA-23b in flow-regulation of Rb phosphorylation and
endothelial cell growth. Proc Natl Acad Sci USA. 107:3234–3239.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pellegrino L, Stebbing J, Braga VM, et al:
miR-23b regulates cytoskeletal remodeling, motility and metastasis
by directly targeting multiple transcripts. Nucleic Acids Res.
41:5400–5412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loftus JC, Ross JT, Paquette KM, et al:
miRNA expression profiling in migrating glioblastoma cells:
regulation of cell migration and invasion by miR-23b via targeting
of Pyk2. PLoS One. 7:e398182012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Han L, Zhang K, et al: VHL
regulates the effects of miR-23b on glioma survival and invasion
via suppression of HIF-1α/VEGF and β-catenin/Tcf-4 signaling. Neuro
Oncol. 14:1026–1036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu S, Pan W, Song X, et al: The microRNA
miR-23b suppresses IL-17-associated autoimmune inflammation by
targeting TAB2, TAB3 and IKK-α. Nat Med. 18:1077–1086. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu R and O’Connell RM: MiR-23b is a
safeguard against autoimmunity. Nat Med. 18:1009–1010. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duran-Bedolla J, Montes de Oca-Sandval MA,
Saldaña-Navor V, et al: Sepsis, mitochondrial failure and multiple
organ dysfunction. Clin Invest Med. 37:E58–E69. 2014.PubMed/NCBI
|
|
28
|
Stearns-Kurosawa DJ, Osuchowski MF,
Valentine C, Kurosawa S and Remick DG: The pathogenesis of sepsis.
Annu Rev Pathol. 6:19–48. 2011. View Article : Google Scholar
|
|
29
|
Brümmer A and Hausser J: MicroRNA binding
sites in the coding region of mRNAs: extending the repertoire of
post-transcriptional gene regulation. Bioessays. 36:617–626. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tarang S and Weston MD: Macros in microRNA
target identification: a comparative analysis of in silico, in
vitro and in vivo approaches to microRNA target identification. RNA
Biol. 11:324–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jernås M, Malmeström C, Axelsson M, et al:
MicroRNA regulate immune pathways in T-cells in multiple sclerosis
(MS). BMC Immunol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li YC, Chen Y, Liu W and Thadhani R:
MicroRNA-mediated mechanism of vitamin D regulation of innate
immune response. J Steroid Biochem Mol Biol. 144:81–86. 2014.
View Article : Google Scholar
|
|
34
|
Chen CZ, Schaffert S, Fragoso R and Loh C:
Regulation of immune responses and tolerance: the microRNA
perspective. Immunol Rev. 253:112–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lourenço AP, Guidugli-Lazzarini KR,
Freitas FC, Bitondi MM and Simões ZL: Bacterial infection activates
the immune system response and dysregulates microRNA expression in
honey bees. Insect Biochem Mol Biol. 43:474–482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jernås M, Nookaew I, Wadenvik H and Olsson
B: MicroRNA regulate immunological pathways in T-cells in immune
thrombocytopenia (ITP). Blood. 121:2095–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou R, O’Hara SP and Chen XM: MicroRNA
regulation of innate immune responses in epithelial cells. Cell Mol
Immunol. 8:371–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Du LL and Ma ZF: MicroRNA and immune
response, and sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
21:501–503. 2009.(In Chinese). PubMed/NCBI
|
|
39
|
Zhang ZD and Ma XC: Injury of vascular
endothelial cell and microcirculation disturbance in sepsis.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 23:125–128. 2011.(In
Chinese). PubMed/NCBI
|
|
40
|
Li M and Yu YH: Vascular endothelial cell
dysfunction and its clinical strategy in severe sepsis. Zhonghua Er
Ke Za Zhi. 49:603–606. 2011.(In Chinese). PubMed/NCBI
|
|
41
|
Herwig MC, Tsokos M, Hermanns MI,
Kirkpatrick CJ and Müller AM: Vascular endothelial cadherin
expression in lung specimens of patients with sepsis-induced acute
respiratory distress syndrome and endothelial cell cultures.
Pathobiology. 80:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Anand AR, Bradley R and Ganju RK:
LPS-induced MCP-1 expression in human microvascular endothelial
cells is mediated by the tyrosine kinase, Pyk2 via the p38
MAPK/NF-kappaB-dependent pathway. Mol Immunol. 46:962–968. 2009.
View Article : Google Scholar :
|
|
43
|
Chen L, Zhang K, Shi Z, et al: A
lentivirus-mediated miR-23b sponge diminishes the malignant
phenotype of glioma cells in vitro and in vivo. Oncol Rep.
31:1573–1580. 2014.PubMed/NCBI
|
|
44
|
Zaman MS, Thamminana S, Shahryari V, et
al: Inhibition of PTEN gene expression by oncogenic miR-23b-3p in
renal cancer. PLoS One. 7:e502032012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Geng J, Luo H, Pu Y, et al: Methylation
mediated silencing of miR-23b expression and its role in glioma
stem cells. Neurosci Lett. 528:185–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leone V, Langella C, D’Angelo D, et al:
MiR-23b and miR-130b expression is downregulated in pituitary
adenomas. Mol Cell Endocrinol. 390:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Majid S, Dar AA, Saini S, et al: mir-23b
represses proto-oncogene Src kinase and functions as
methylation-silenced tumor suppressor with diagnostic and
prognostic significance in prostate cancer. Cancer Res.
72:6435–6446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin L, Wessely O, Marcusson EG, et al:
Prooncogenic factors miR-23b and miR-27b are regulated by Her2/Neu,
EGF and TNF-α in breast cancer. Cancer Res. 73:2884–2896. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pellegrino L, Stebbing J, Braga VM, et al:
miR-23b regulates cytoskeletal remodeling, motility and metastasis
by directly targeting multiple transcripts. Nucleic Acids Res.
41:5400–5412. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu R and O’Connell RM: MiR-23b is a
safeguard against autoimmunity. Nat Med. 18:1009–1010. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ouda R, Onomoto K, Takahasi K, et al:
Retinoic acid-inducible gene I-inducible miR-23b inhibits
infections by minor group rhinoviruses through down-regulation of
the very low density lipoprotein receptor. J Biol Chem.
286:26210–26219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu Y, Li C, He Y, et al: Relationship
between expression of microRNA and inflammatory cytokines plasma
level in pediatric patients with sepsis. Zhonghua Er Ke Za Zhi.
52:28–33. 2014.(In Chinese). PubMed/NCBI
|
|
53
|
Chen W, Zhao L, Niu S, et al: The
diagnostic value of different pro-inflammatory factor in early
diagnosis of sepsis in patients with bloodstream infection.
Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 26:165–170. 2014.(In
Chinese). PubMed/NCBI
|
|
54
|
Brown MA and Jones WK: NF-kappaB action in
sepsis: the innate immune system and the heart. Front Biosci.
9:1201–1217. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thair S and Russell JA: Noncanonical
nuclear factor kappaB (NF-κB) signaling and potential for
therapeutics in sepsis. Curr Infect Dis Rep. 15:364–371. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liang Y, Li X, Zhang X, et al: Elevated
levels of plasma TNF-α are associated with microvascular
endothelial dysfunction in patients with sepsis through activating
the NF-kB and p38 mitogen-activated protein kinase in endothelial
cells. Shock. 41:275–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Song R, Kim J, Yu D, Park C and Park J:
Kinetics of IL-6 and TNF-α changes in a canine model of sepsis
induced by endotoxin. Vet Immunol Immunopathol. 146:143–149. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Shahkar L, Keshtkar A, Mirfazeli A, Ahani
A and Roshandel G: The role of IL-6 for predicting neonatal sepsis:
a systematic review and meta-analysis. Iran J Pediatr. 21:411–417.
2011.
|
|
59
|
Hildebrand F, Pape HC, Harwood P, et al:
Role of adhesion molecule ICAM in the pathogenesis of polymicrobial
sepsis. Exp Toxicol Pathol. 56:281–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Figueras-Aloy J, Gómez-López L,
Rodríguez-Miguélez JM, et al: Serum soluble ICAM-1, VCAM-1,
L-selectin, and P-selectin levels as markers of infection and their
relation to clinical severity in neonatal sepsis. Am J Perinatol.
24:331–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zaki Mel-S and el-Sayed H: Evaluation of
microbiologic and hematologic parameters and E-selectin as early
predictors for outcome of neonatal sepsis. Arch Pathol Lab Med.
133:1291–1296. 2009.
|