Introduction
The hyper-IgM syndromes (HIGMs) are a group of rare
primary immune deficiency diseases characterized by a normal or
elevated serum level of IgM and low or absent serum levels of IgG,
IgA and IgE with normal peripheral blood B lymphocyte counts
(1). The mechanism of HIGM is
immunoglobulin class-switch recombination (CSR) failure and somatic
hyper mutation (SHM), which is caused by molecular defects in the
CD40L/CD40 signaling pathway or defects involving enzymes required
for CSR and SHM (2). The X-linked
form of HIGM is caused by either the CD40L gene mutation or NF-κB
essential modulator defects (3).
To date, eight genetically defined forms of HIGM have been
documented, of which HIGM1 is the most common and the X-linked
form, caused by CD40L gene mutations (4).
Patients with HIGM are susceptible to recurrent
sino-pulmonary infections, neutropenia, autoimmune diseases and
malignancies, and opportunistic infections including pneumocystis
carinii pneumonia (PCP) and cryptosporidium (1,2). In
the treatment of patients with HIGM, immunoglobulin (Ig)
replacement can reduce the frequency and severity of infections,
but cannot prevent malignancies. Infections should be treated
aggressively with specific antimicrobial therapy and cases of PCP
require prophylaxis with oral trimethoprim-sulfamethoxazole. The
ideal length of prophylaxis remains unknown. Neutropenia can be
successfully treated with granulocyte-colony stimulating factor. At
present, stem cell transplantation remains the most effective
method of HIGM treatment. Genetic therapy for HIGM is currently in
the experimental stages (1). In
the present study, we report a case of X-linked HIGM with a new
CD40L gene mutation (HIGM1) presenting with eosinophilia in a young
Chinese boy.
Case report
Case presentation
A 1-year-old boy had been developing normally from
birth to 3 months old. However, he presented recurrent pulmonary
infection from the age of 4 months. The patient was admitted to the
Children’s Hospital of Zhejiang University School of Medicine
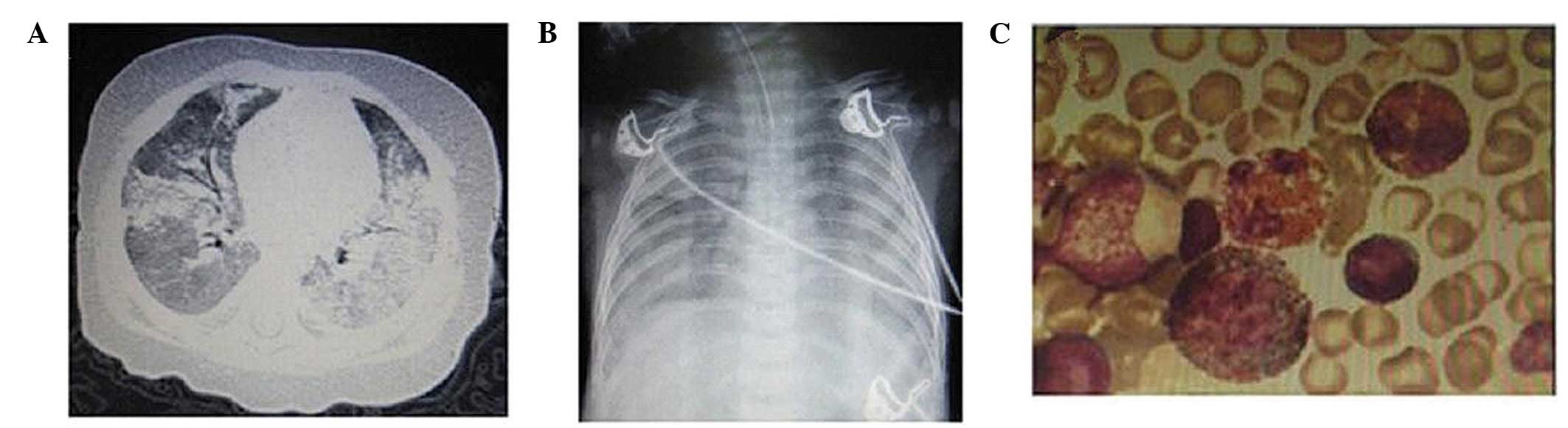
(Hangzhou, China) twice due to acute respiratory distress syndrome
(ARDS; Fig. 1A and B) at 4 and 7
months old of age, respectively. The present study was approved by
the Ethics Committee of the Children’s Hospital of the Zhejiang
University School of Medicine (Hangzhou, China). Written informed
consent was obtained from the patient’s family prior to
participation.
Diagnosis
Blood tests revealed a significantly increased white
blood cell count with eosinophilia (18–25%); however, neutrophil
counts were within the normal range. Bone marrow aspiration
(Fig. 1C) revealed an increased
proportion of eosinophils (23.5%) with no morphological evidence of
dysplasia. An investigation of pathogens, including tuberculosis,
parasites, atypical pathogens and viruses, revealed no
abnormalities. The ARDS condition was improved and the patient’s
eosinophil count was quickly reduced to the normal range with the
support of high-frequency oscillator ventilation and co-treatment
of antibiotics and glucocorticoids. However, the recurrent
pulmonary infections remained. Further examinations revealed that
serum immunoglobulin levels of IgG (0.16 g/l) and IgA (0.01 g/l)
were significantly reduced; however, the IgM (0.86 g/l) level was
within the normal range. No abnormal lymphocyte subsets were
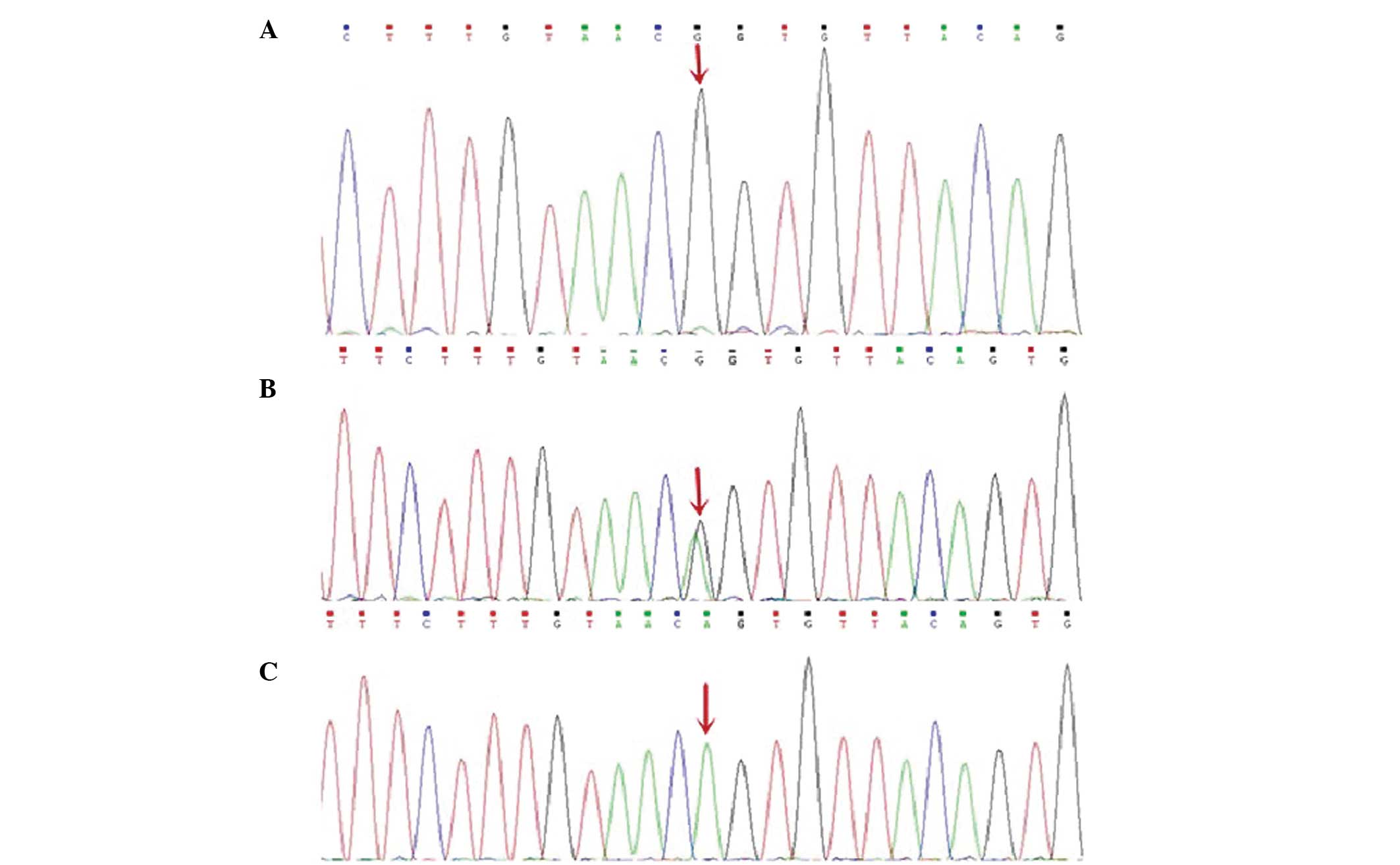
identified. Gene sequencing analysis of the CD40L gene revealed a
homozygous G to A substitution in exon 5 (c.410-2A>G; Fig. 2). Parental DNA analysis revealed
that the patient’s mother was a carrier. The diagnosis of X-linked
HIGM was confirmed.
Treatment
Following treatment of intravenous immunoglobulin
(IVIG), trimethoprim-sulfamethoxazole and glucocorticoid agents,
the pulmonary infection was significantly reduced. The oral
glucocorticoid agent was discontinued once the eosinophil count had
remained within normal range for 5 months. During the follow-up
period, the patient received regular IVIG replacement therapy every
3 to 4 weeks and intermittent infection prophylaxis with
trimethoprim-sulfamethoxazole. The patient is currently well
developed with normal immunoglobulin levels.
Discussion
HIGM1 is the most common HIGM phenotype and accounts
for approximately 65–70% of all cases. HIGM1 patients usually have
an onset of symptoms before 2 years of age, presenting as recurrent
sino-pulmonary infections. The majority of patients are susceptible
to PCP and cryptosporidium associated with diarrhea, sclerosing
cholangitis and tumors of the liver, pancreas or biliary tract
(5,6). Autoimmune diseases and neutropenia
associated with stomatitis are relatively common (5,7).
HIGM1 patients require repeated IVIG replacement treatment at doses
of 400–600 mg/kg every 3 to 4 weeks and should receive prophylaxis
of trimethoprim-sulfamethoxazole for PCP. Stem cell transplantation
may cure X-linked HIGM as well as the associated neutropenia,
cholangiopathy and liver failure, in combination with a liver
transplant (8).
The CD40L gene is located in the long arm of the
X-chromosome (Xq26-27) and includes five exons. CD40L is a 39-kDa
type II membrane glycoprotein belonging to the superfamily of tumor
necrosis factors and is expressed on the surface of the activated
CD4+ T cells. CD40L is crucial for T-B cell interaction
by binding to CD40, which is expressed in B cells. CD40L gene
mutation results in the reduced expression of CD40L in
CD4+ T cells, which interferes with the interaction of
CD40L and CD40 or influences the formation of CD40 trimer molecules
(7,9). Therefore, the CD40L gene defect
destroys the T-B cell interaction and affects CSR. At present, 250
unique mutations of the CD40L gene have been identified (http://bioinf.uta.fi/CD40Lbase), mainly in exon 5
but also in exon 4 (7).
Our patient suffered recurrent pneumonia and ARDS.
Although no evidence of PCP was observed, his pulmonary infection
was significantly reduced following trimethoprim-sulfamethoxazole
treatment. Eosinophilia was a prominent clinical manifestation in
the patient. To date, there there has only been one report of
eosinophilia in patients with HIGM1 (10), and the pathogenesis of HIGM1
complicated with eosinophilia remains unclear. Neutropenia did not
exist in the patient. Immunological evaluation revealed a normal
level of serum IgM, significantly low levels of serum IgG and IgA,
and normal counts of peripheral blood B cells. Sequencing analysis
of the CD40L gene revealed a splice mutation within exon 5 at
nucleotide position 410 (c.410-2A>G), which has never been
reported previously in the literature. After the patient received
regular IVIG replacement therapy at 3 to 4 weeks intervals and
intermittent trimethoprim-sulfamethoxazole prophylaxis for PCP, his
immunoglobulin levels returned to normal with no further recurrent
pulmonary infection. The boy is currently developing normally.
Acknowledgements
This study was supported by grants from the Health
Bureau of Zhejiang Province, Zhejiang, China (no. 2011KYA096) and
the National Natural Science Foundation of China (no.
81270045).
References
|
1
|
Qamar N and Fuleihan RL: The hyper IgM
syndromes. Clin Rev Allergy Immunol. 46:120–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies EG and Thrasher AJ: Update on the
hyper immunoglobulin M syndromes. Br J Haematol. 149:167–180. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanson EP, Monaco-Shawver L, Solt LA, et
al: Hypomorphic nuclear factor-kappaB essential modulator mutation
database and reconstitution system identifies phenotypic and
immunologic diversity. J Allergy Clin Immunol. 122:1169–1177. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Herz W, Bousfiha A, Casanova JL, et al:
Primary immunodeficiency diseases: an update on the classification
from the international union of immunological societies expert
committee for primary immunodeficiency. Front Immunol.
5:1622014.PubMed/NCBI
|
|
5
|
Winkelstein JA, Marino MC, Ochs H, et al:
The X-linked hyper-IgM syndrome: clinical and immunologic features
of 79 patients. Medicine (Baltimore). 82:373–384. 2003. View Article : Google Scholar
|
|
6
|
Cabral-Marques O, Klaver S, Schimke LF, et
al: First report of the Hyper-IgM syndrome Registry of the Latin
American Society for Immunodeficiencies: novel mutations, unique
infections, and outcomes. J Clin Immunol. 34:146–156. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jesus AA, Duarte AJ and Oliveira JB:
Autoimmunity in hyper-IgM syndrome. J Clin Immunol. 28(Suppl 1):
S62–S66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobsohn DA, Emerick KM, Scholl P, et al:
Nonmyeloablative hematopoietic stem cell transplant for X-linked
hyper-immunoglobulin m syndrome with cholangiopathy. Pediatrics.
113:e122–e127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
An Y, Xiao J, Jiang L, Yang X, Yu J and
Zhao X: Clinical and molecular characterization of X-linked
hyper-IgM syndrome patients in China. Scand J Immunol. 72:50–56.
2010.PubMed/NCBI
|
|
10
|
Merchant RH, Ahmed J, Ahmed N and Picard
C: Type I hyper IgM syndrome with novel mutation from India. Indian
J Pediat. 81:620–622. 2014. View Article : Google Scholar
|