Introduction
The neuropeptide oxytocin (OT) is synthesized within
the hypothalamus in the paraventricular nucleus (PVN) and
supraoptic nucleus (SON) (1).
Central OT plays an important role in modulating a variety of
physiological functions in mammals, including parturition,
lactation, social behavior and memory (2).
Accumulating evidence shows that OT has analgesic
properties as a neurotransmitter or neuromodulator (3). Anatomically, OT neurons in the PVN
send direct descending fibers to several brain regions involved in
pain perception (4), including the
dorsal and ventral hippocampus, amygdala, periaqueductal gray (PAG)
and raphe nuclei, as well as the superficial dorsal horn of the
spinal cord (1,5). In addition, extensive clinical and
preclinical studies have demonstrated that OT in the PVN, rather
than that in the SON, is involved in anti-nociception or analgesia
(6,7). It has been reported that the
intrathecal injection of OT can significantly reduce withdrawal
responses to mechanical and cold stimulation in sciatic
nerve-ligated rats, and the effect can be blocked by intrathecal
injection of an OT antagonist (6,7). In
humans, Madrazo et al (8)
reported that the intractable thoracic pain of a patient with
diffuse mesothelioma could be reduced by 88% for 77 min by
intracerebroventricular injection of OT. Yang (9) reported that acute and chronic lower
back pain in humans resulted in a marked change of OT content in
the cerebral spinal fluid and plasma; OT could relieve this lower
back pain, and the effect of OT was blocked by an OT
antagonist.
While most previous studies have looked at the role
of OT in chronic pain, few investigations have been carried out
into the role of oxytocin in acute pain, particularly postoperative
pain. In the present study, the role of OT in incisional-induced
allodynia was explored for the first time, to the best of our
knowledge.
Materials and methods
Animals
The experiments were performed using male Sprague
Dawley rats (200–250 g) provided by the Animal Experimental Center
of Xiangya School of Medicine, Central South University (Changsha,
China). The rats were housed singly with food and water available
ad libitum in a temperature-controlled (25±2°C) room and
under a 12-h light/dark cycle. All experiments were conducted in
accordance with the guidelines of the International Association for
the Study of Pain (10). Every
effort was made to minimize any suffering of the animals and the
number of animals used.
Incisional pain model
The hindpaw incision model was performed as
described by Brennan et al (11). The model and control groups each
consisted of 30 mice. Briefly, rats were anesthetized with 2%
isoflurane (Sigma-Aldrich, St. Louis, MO, USA) delivered through a
nose cone. The plantar aspect of the right hindpaw was prepared in
a sterile manner with a 10% povidone-iodine solution
(Sigma-Aldrich), and the paw was positioned through a hole in a
sterile drape. A 1-cm longitudinal incision was made through the
skin and fascia of the plantar aspect of the foot, with a size 11
blade, starting at a point 0.5 cm away from the proximal edge of
the heel and extending toward the toes. The plantaris muscle was
raised and incised longitudinally, with its origin and insertion
remaining intact. The incision was closed with two mattress sutures
of 5-0 nylon. Following surgery, the animals were allowed to
recover in their home cages. Sham control groups, consisting of
rats that received anesthesia, antiseptic preparation and topical
antibiotic without an incision, were used in the study.
Intracerebroventricular injection of
OT
Animals were anesthetized by intraperitoneal
injection of chloral hydrate (300 mg/kg) and mounted on a
stereotaxic frame. A stainless steel guide cannula (0.8 mm outer
diameter) was directed into the lateral ventricle (anteroposterior,
−0.92 mm; lateral, 1.3 mm; dorsoventral, 4.0 mm) according to the
rat brain stereotaxic atlas of Paxinos and Watson (12). The cannula protruded 1 cm above the
skull and was fixed to the skull by dental acrylic. Finally, the
opening was sealed. If no abnormality was noted during the first
three days after implantation, a stainless steel needle (0.4 mm
diameter) was inserted directly into the lateral ventricle along
the guide cannula and 10 μl vehicle (0.9% saline) or OT
(100, 400 or 600 ng; Sigma-Aldrich) solution was injected over 10
min immediately after hindpaw incision.
Intrathecal injection of OT
Intrathecal catheter implantation was performed as
previously described (13), with
minor modifications. Following incision, OT (600 ng, total volume
10 μl) was injected intrathecally through the catheter,
followed by a 10-μl saline flush, and mechanical
hypersensitivity was assessed.
Mechanical hypersensitivity assay
Von Frey filaments (Stoelting Co., Wood Dale, IL,
USA) were used to assess the withdrawal threshold prior to incision
and at 0.5, 1.0, 3.0, 6.0, 24.0 and 72.0 h after incision,
according to the ‘up-down’ algorithm, as previously described
(14). The assay was performed by
an investigator blinded to the treatment. At the end of the
experiments, 20 μl methylthionine chloride was injected into
the lateral ventricle via the cannula, and a coronal section was
made across the lateral ventricle. Only data from rats whose
ventricle system was filled with methylthionine chloride were
included in the analysis.
Immunohistochemistry
Rats were deeply anesthetized with chloral hydrate
(300 mg/kg) and perfused transcardially with 4% paraformaldehyde.
The brain and lumbar enlargement from each rat were post-fixed with
4% paraformaldehyde for 4 h, and then immersed overnight in 0.01 M
phosphate buffer containing 30% sucrose. Cryostat-cut brain and
lumbar spinal cord sections (30-μm) were incubated overnight
at room temperature with polyclonal rabbit anti-human anti-OT
antibody (#O4389; 1:2,000 dilution; Sigma-Aldrich). Subsequent to
washing in 0.01 M phosphate-buffered saline, the sections were
incubated with biotinylated polyclonal goat anti-rabbit secondary
antibody (#A6154; 1:5,000 dilution; Sigma-Aldrich) followed by an
avidin-biotin-peroxidase complex (Sigma-Aldrich) for a further 2 h,
prior to being visualized with diaminobenzidine. Finally, the
sections were mounted on slides (in the dark for
immunofluorescence) and analyzed under a microscope (IX83; Olympus
Corporation, Beijing, China). Negative controls were set up by
performing the experiments without the primary antibodies.
Evaluation of immunostaining
Image-Pro Plus 6.0 software (Media Cybernetics Inc.,
Silver Spring, MD, USA) was used to quantify the optical density
value of OT in the PVN and SON and lamina I/II of the superficial
dorsal horn of the lumbar enlargement. According to the rat brain
stereotaxic atlas of Paxinos and Watson (12), the PVN and SON were located from
1.08 to 2.16 mm and from 0.92 to 1.44 mm posterior to the Bregma,
respectively (15). The mean
optical density of the OT staining was calculated by dividing the
optical density with the measurement area.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 11.0 (SPSS Inc., Chicago, IL, USA). Comparisons
of means between two groups were performed with Student’s t-tests
and among multiple groups with one-way analysis of variance
followed by post hoc pairwise comparisons using Dunnett’s tests.
Data are presented as the mean ± standard error of the mean. A
two-tailed P<0.05 was considered to indicate a statistically
significant difference in this study.
Results
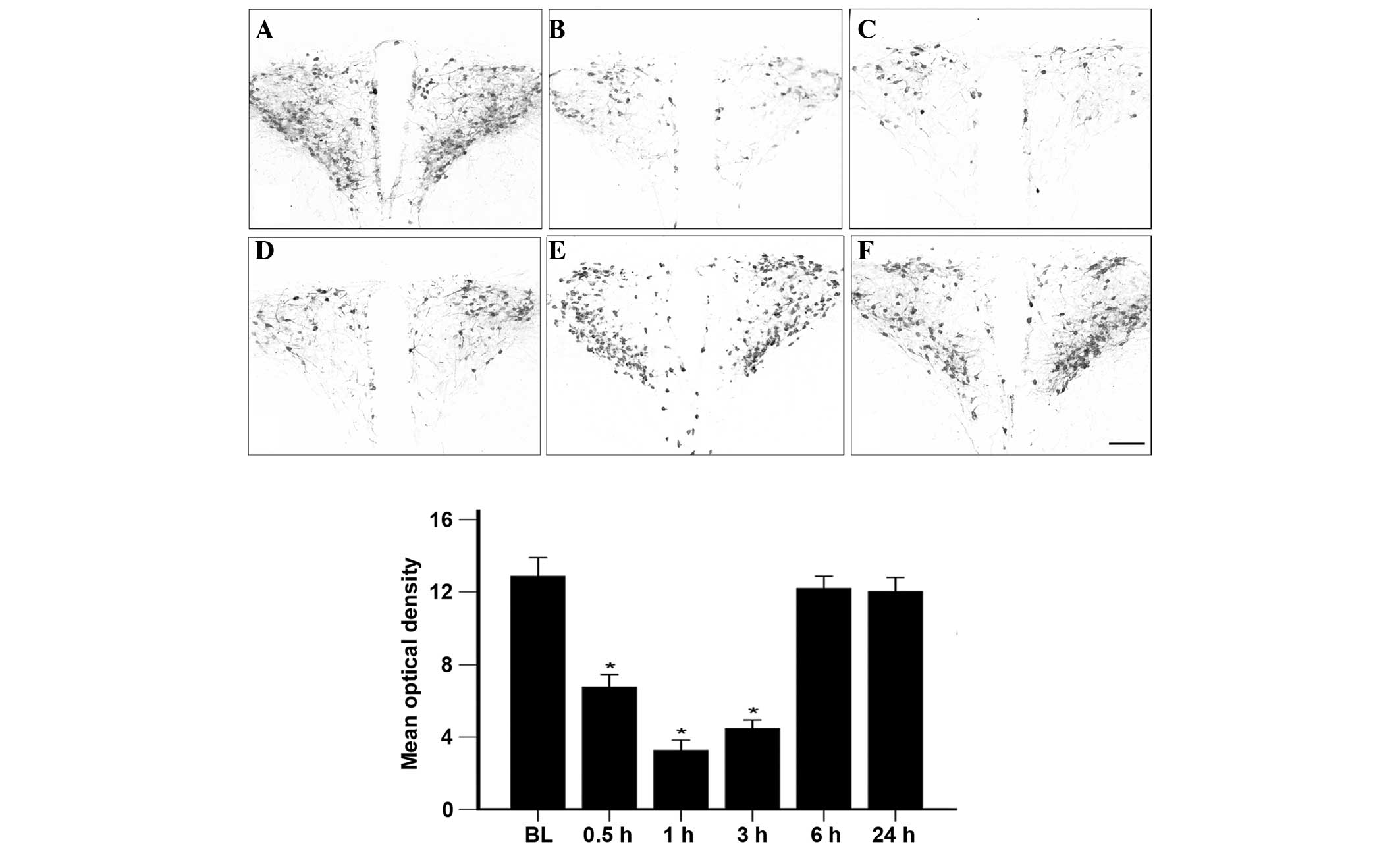
Hindpaw incision induces a reduction in
OT levels in the PVN
To determine whether surgical incision affected OT
in the PVN, OT in the PVN of animals with or without hindpaw
incision was immunostained and the OT content was quantified using
the optical density of the OT staining. As shown in Fig. 1, the OT content (optical density
value) in the PVN at baseline (prior to incision) was 13.5±1.2
(n=7). Following incision, the OT content in the PVN was 6.7±1.0,
3.5±0.8 and 4.8±0.9 at 0.5, 1.0 and 3.0 h, respectively (n=6 at
each time-point), indicating a significant decrease compared with
the baseline (P<0.05). Between 6 and 24 h after incision, the OT
content in the PVN returned to the baseline level (n=6 at each
time-point) (Fig. 1). By contrast,
the sham groups (n=5 at each time-point) showed no significant
differences in the OT content in the PVN prior to and subsequent to
incision (data not shown).
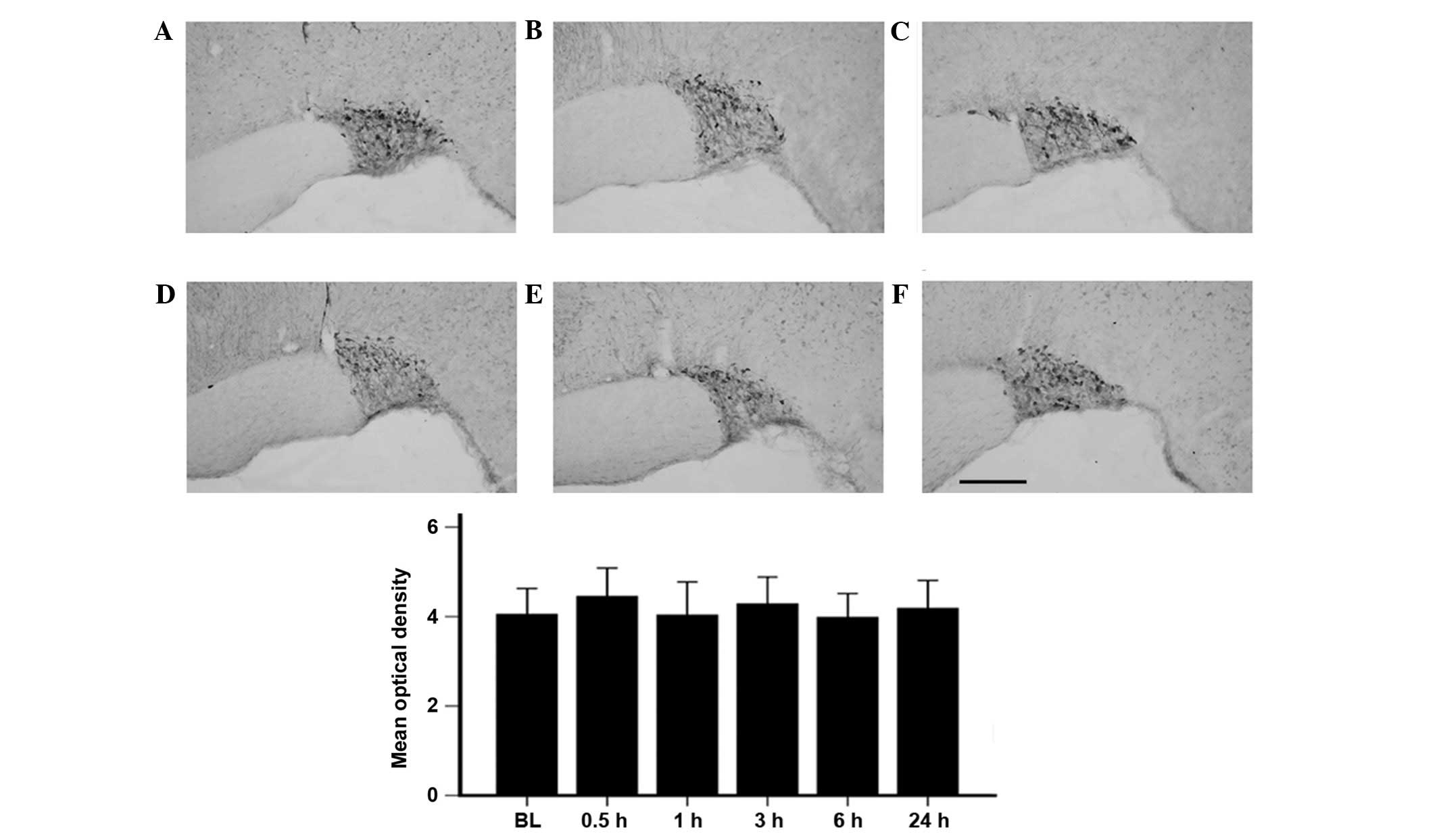
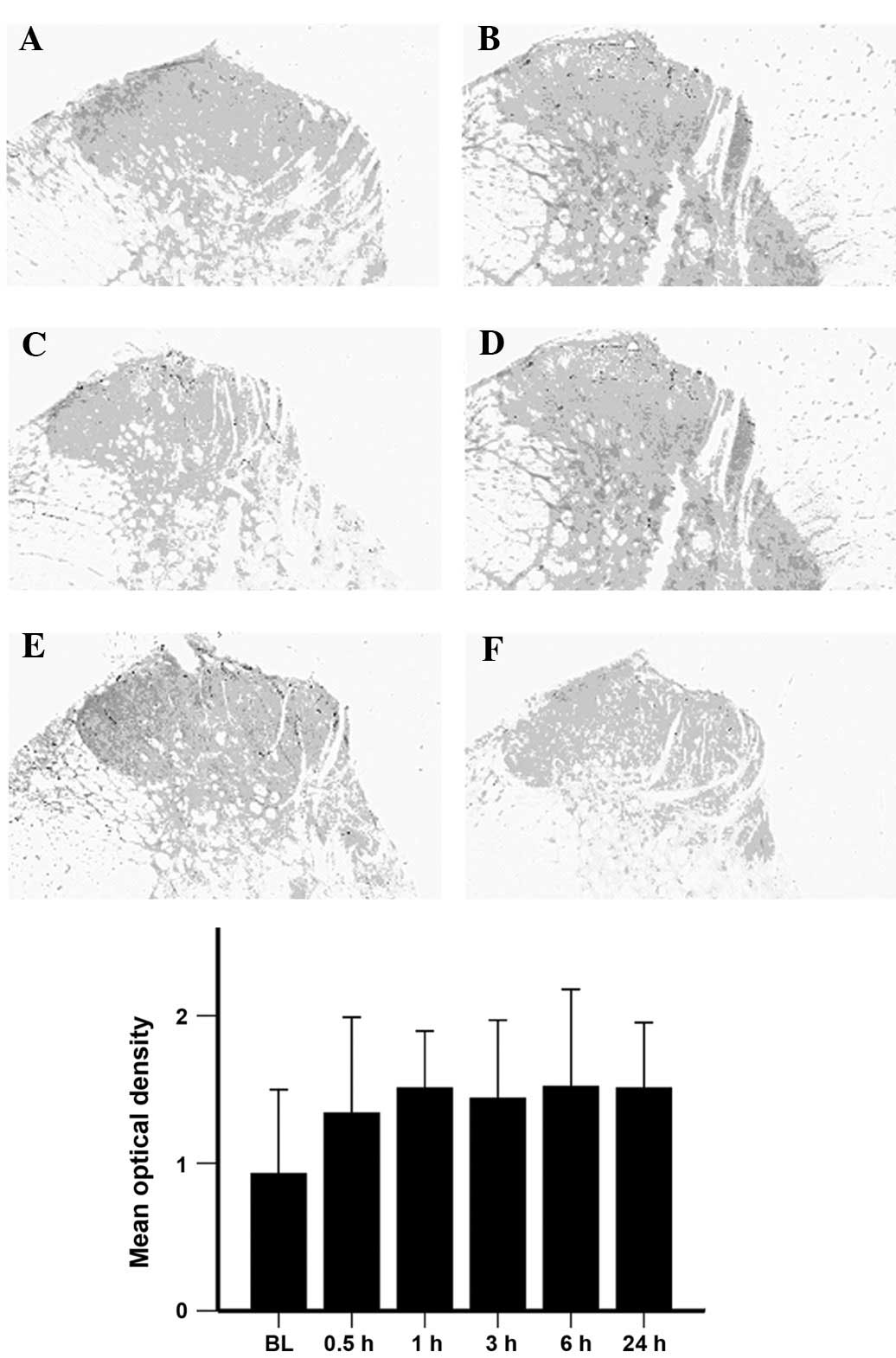
Expression of OT in the SON and spinal
cord remains unchanged following hindpaw incision
As shown in Fig. 2,
there was no significant difference in the OT content in the SON
prior to and subsequent to incision. Notably, the dorsal horn of
the spinal cord showed no significant difference in the OT content
prior to and following incision, despite being an important center
of pain modulation (Fig. 3).
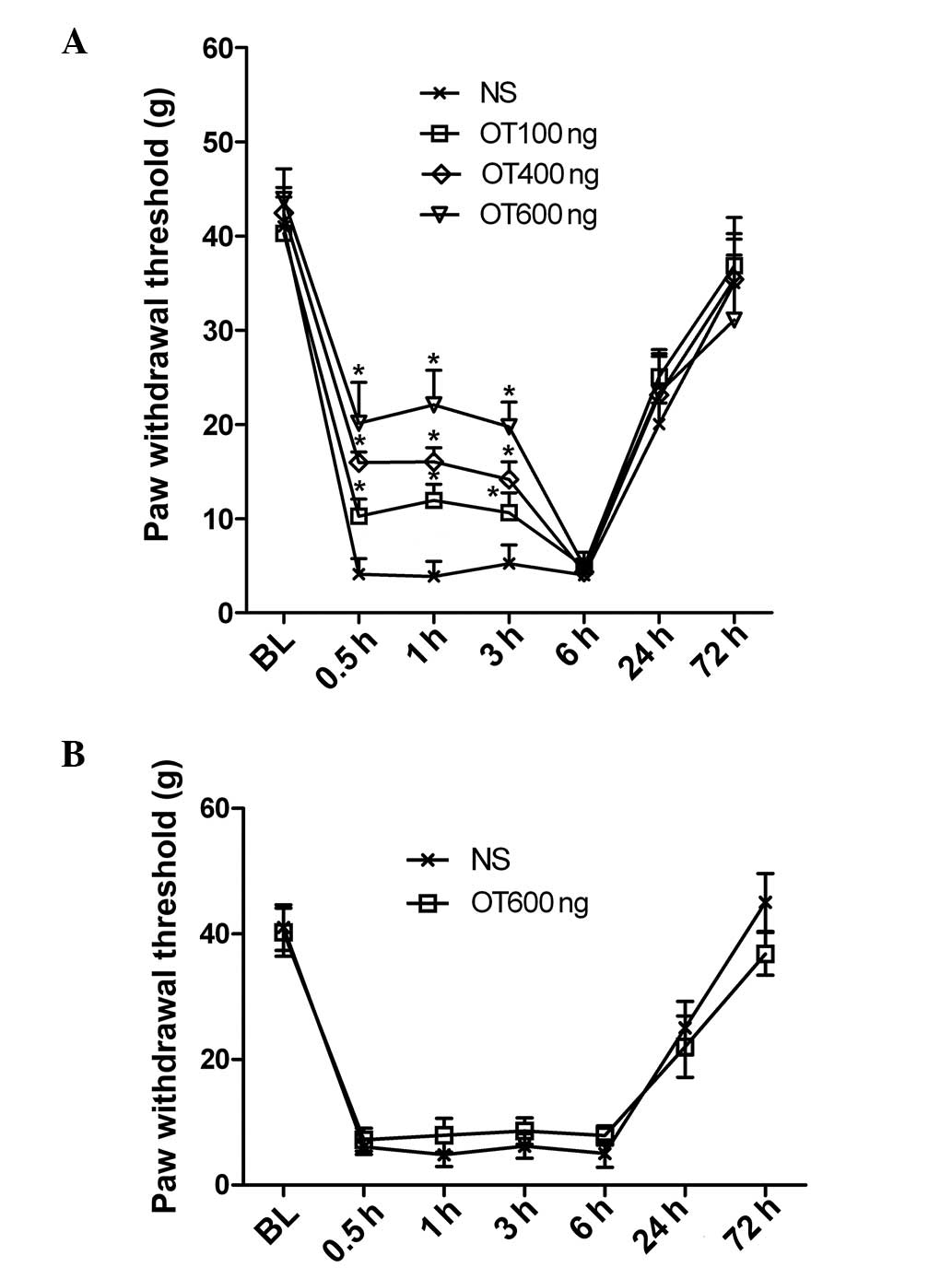
Intracerebroventricular but not
intrathecal injection of OT attenuates mechanical hypersensitivity
following hindpaw incision
To explore the role of OT in mechanical
hypersensitivity induced by incision, intrathecal or
intracerebroventricular injections of OT were performed immediately
subsequent to hindpaw incision. A dose-dependent inhibition of
mechanical hypersensitivity was detected 30 min after
intracerebroventricular injection of OT (100, 400 or 600 ng) and
lasted for 3.0 h (n=5 per group) (Fig.
4A). By contrast, no significant difference was noted between
the intrathecal OT injection group (600 ng) and the control group
(n=5 per group) (Fig. 4B).
Discussion
In the present study, it was shown that OT is
involved in the response to incisional pain at the supraspinal
level, but not at the spinal cord as expected. Following incision,
a marked decrease in the OT content was observed in the PVN;
however, no significant change in the OT content was noted at the
spinal cord within 24.0 h of the incision. Administration of
exogenous OT at the supraspinal level by intracerebroventricular
injection attenuated mechanical hypersensitivity in a
dose-dependent manner, while injection at the spinal level did not
produce any analgesia. These results are noteworthy, given that the
spinal cord is an important center of pain modulation.
OT can exert a wide spectrum of central and
peripheral effects, including modulation of the neuroendocrine
reflex and analgesia (1,4). OT is synthesized in the PVN and SON
and then transported to the peripheral circulation system or
different regions of the central nervous system (1,4). In
the present study, it was found that the OT content in the PVN was
significantly reduced between 0.5 and 3.0 h after incision and
returned to the baseline level after 6.0 h. The OT content in the
SON, however, was not altered significantly following incision.
This is in agreement with the observation by Rousselot et al
(16) that the OT that is involved
in an anti-nociceptive or analgesic response is likely to stem from
the PVN and not the SON.
OT fibers project to several regions involved in
pain modulation in the nervous system (17,18).
It has been reported that ≥25% of OT-positive neurons in the PVN
directly project to the superficial dorsal horn of the spinal cord
(19). Furthermore, the spinal
cord has overlap between OT fiber projections and the distribution
of OT binding sites (6).
Intrathecal injection of OT and electrical stimulation of the PVN
can reduce the hypersensitivity of neuropathic or nociceptive
responses in normal rats, and such effects can be inhibited by
pretreatment with intrathecal injection of an OT antagonist
(6). Condés-Lara et al
(20,21) suggested that intrathecal injection
of OT could reduce the activation of primary Aδ and C fibers evoked
by somatic stimulation or neuropathy. Yu et al (22) found that intrathecal administration
of OT dose-dependently attenuated carrageenan-induced inflammatory
pain. In the spinal cord, OT transported from the PVN activates
γ-aminobutyric acid (GABA)-ergic interneurons, which in turn
inhibit the activation of glutamatergic primary sensory neurons.
Such presynaptic inhibition prevents the transmission of
nociceptive signals to the brain (23–25).
In addition to the increase in GABA release, changes in the
reversal potential of GABAergic currents in nociceptive neurons
contribute to the analgesic effects of OT (26–29).
In combination, the previous studies indicate that OT contributes
to pain relief at the spinal level; however, in the present study,
it was found that the expression of OT in the ipsilateral lumbar
enlargement of the spinal cord remained unchanged until 24 h after
incision, and intrathecal injection of OT did not improve
mechanical hypersensitivity. By contrast, intracerebroventricular
injection of OT dose-dependently elevated the mechanical
hypersensitivity threshold. As intrathecal injection acts at the
spinal cord, the results from the present study suggest that OT
attenuates acute incisional pain at the supraspinal level rather
than at the spinal level (30).
The PVN sends projections to numerous brain regions implicated in
pain modulation, such as the PAG, raphe magnus and dorsal raphe
nuclei (31–33). Injection of OT into the cerebral
ventricle can elevate pain thresholds in rats and humans (8,34).
In addition, a previous study reported that OT could exert
analgesic effects in neonatal rats during delivery, even when the
descending OT fibers were cut by decerebration at the upper pons
level (35). This suggests that OT
in the brain can exert analgesic effects independently of the
spinal cord and corroborates the findings from the present
study.
The reduction of the OT content in the PVN following
incision could be due to an alteration of OT transportation in the
brain, as the OT content in the PVN was restored to the baseline
level within 6 h of the incision. We aim to explore the underlying
mechanisms in future studies.
In conclusion, the present study provides the first
in vivo evidence, to the best of our knowledge, that OT in
the PVN attenuates incision-induced mechanical allodynia at the
supraspinal, but not the spinal, level. This suggests that OT is
involved in supraspinal analgesia for postoperative pain.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81070897). The
authors would like to thank Ms. Dan Liu and Dr Jian-Wei Zhang for
their technical support.
References
|
1
|
Gimpl G and Fahrenholz F: The oxytocin
receptor system: structure, function, and regulation. Physiol Rev.
81:629–683. 2001.PubMed/NCBI
|
|
2
|
Russell JA and Leng G: Sex, parturition
and motherhood without oxytocin? J Endocrinol. 157:343–359. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee HJ, Macbeth AH, Pagani JH and Young WS
III: Oxytocin: The great facilitator of life. Prog Neurobiol.
88:127–151. 2009.(Review.). PubMed/NCBI
|
|
4
|
Lundeberg T, Uvnäs-Moberg K, Agren G and
Bruzelius G: Anti-nociceptive effects of oxytocin in rats and mice.
Neurosci Lett. 170:153–157. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martínez-Lorenzana G, Espinosa-López L,
Carranza M, Aramburo C, Paz-Tres C, Rojas-Piloni G and Condés-Lara
M: PVN electrical stimulation prolongs withdrawal latencies and
releases oxytocin in cerebrospinal fluid, plasma, and spinal cord
tissue in intact and neuropathic rats. Pain. 140:265–273. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miranda-Cardenas Y, Rojas-Piloni G,
Martínez-Lorenzana G, Rodríguez-Jiménez J, López-Hidalgo M,
Freund-Mercier MJ and Condés-Lara M: Oxytocin and electrical
stimulation of the paraventricular hypothalamic nucleus produce
antinociceptive effects that are reversed by an oxytocin
antagonist. Pain. 122:182–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Padhy BM and Kumar VL: Inhibition of
Calotropis procera latex-induced inflammatory hyperalgesia by
oxytocin and melatonin. Mediators Inflamm. 6:360–365. 2005.
View Article : Google Scholar
|
|
8
|
Madrazo I, Franco-Bourland RE, León-Meza
VM and Mena I: Intraventricular somatostatin-14, arginine
vasopressin, and oxytocin: analgesic effect in a patient with
intractable cancer pain. Appl Neurophysiol. 50:427–431.
1987.PubMed/NCBI
|
|
9
|
Yang J: Intrathecal administration of
oxytocin induces analgesia in low back pain involving the
endogenous opiate peptide system. Spine (Phila Pa 1976).
19:867–871. 1994. View Article : Google Scholar
|
|
10
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brennan TJ, Vandermeulen EP and Gebhart
GF: Characterization of a rat model of incisional pain. Pain.
64:493–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. 4th edition. Academic Press; Sydney:
1998
|
|
13
|
Wang Y, Liu C, Guo QL, Yan JQ, Zhu XY,
Huang CS and Zou WY: Intrathecal 5-azacytidine inhibits global DNA
methylation and methyl-CpG-binding protein 2 expression and
alleviates neuropathic pain in rats following chronic constriction
injury. Brain Res. 1418:64–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao DQ and Ai HB: Oxytocin and
vasopressin involved in restraint water-immersion stress mediated
by oxytocin receptor and vasopressin 1b receptor in rat brain. PLoS
One. 6:e233622011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rousselot P, Papadopoulos G, Merighi A,
Poulain DA and Theodosis DT: Oxytocinergic innervation of the rat
spinal cord. An electron microscopic study. Brain Res. 529:178–184.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hashimoto H, Fukui K, Noto T, Nakajima T
and Kato N: Distribution of vasopressin and oxytocin in rat brain.
Endocrinol Jpn. 32:89–97. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sofroniew MV, Weindl A, Schrell U and
Wetzstein R: Immunohistochemistry of vasopressin, oxytocin and
neurophysin in the hypothalamus and extrahypothalamic regions of
the human and primate brain. Acta Histochem Supp. 24:79–95.
1981.
|
|
19
|
Sawchenko PE and Swanson LW:
Immunohistochemical identification of neurons in the
paraventricular nucleus of the hypothalamus that project to the
medulla or to the spinal cord in the rat. J Comp Neurol.
205:260–272. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Condés-Lara M, González NM,
Martínez-Lorenzana G, Delgado OL and Freund-Mercier MJ: Actions of
oxytocin and interactions with glutamate on spontaneous and evoked
dorsal spinal cord neuronal activities. Brain Res. 976:75–81. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Condés-Lara M, Maie IA and Dickenson AH:
Oxytocin actions on afferent evoked spinal cord neuronal activities
in neuropathic but not in normal rats. Brain Res. 1045:124–133.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu SQ, Lundeberg T and Yu LC: Involvement
of oxytocin in spinal antinociception in rats with inflammation.
Brain Res. 983:13–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson DA, Wei F, Wang GD, Li P, Kim SJ,
Vogt SK, Muglia LJ and Zhuo M: Oxytocin mediates stress-induced
analgesia in adult mice. J Physiol. 540:593–606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Condés-Lara M, Rojas-Piloni G,
Martínez-Lorenzana G, Rodríguez-Jiménez J, López Hidalgo M and
Freund-Mercier MJ: Paraventricular hypothalamic influences on
spinal nociceptive processing. Brain Res. 1081:126–137. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Condés-Lara M, Rojas-Piloni G,
Martínez-Lorenzana G, López-Hidalgo M and Rodríguez-Jiménez J:
Hypothalamospinal oxytocinergic antinociception is mediated by
GABAergic and opiate neurons that reduce A-delta and C fiber
primary afferent excitation of spinal cord cells. Brain Res.
1247:38–49. 2009. View Article : Google Scholar
|
|
26
|
De Koninck Y: Altered chloride homeostasis
in neurological disorders: a new target. Curr Opin Pharmacol.
7:93–99. 2007. View Article : Google Scholar
|
|
27
|
Price TJ, Cervero F, Gold MS, Hammond DL
and Prescott SA: Chloride regulation in the pain pathway. Brain Res
Rev. 60:149–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Granados-Soto V, Arguelles CF and
Alvarez-Leefmans FJ: Peripheral and central antinociceptive action
of Na+-K+-2Cl− cotransporter
blockers on formalin-induced nociception in rats. Pain.
114:231–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valencia-de Ita S, Lawand NB, Lin Q,
Castañeda-Hernandez G and Willis WD: Role of the
Na+-K+-2Cl− cotransporter in the
development of capsaicin-induced neurogenic inflammation. J
Neurophysiol. 95:3553–3561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yaksh TL and Rudy TA: Chronic
catheterization of the spinal subarachnoid space. Physiol Behav.
17:1031–1036. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Antunes JL and Zimmerman EA: The
hypothalamic magnocellular system of the rhesus monkey: an
immunocytochemical study. J Comp Neurol. 181:539–565. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buijs RM, De Vries GJ, Van Leeuwen FW and
Swaab DF: Vasopressin and oxytocin: distribution and putative
functions in the brain. Prog Brain Res. 60:115–122. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jenkins JS, Ang VT, Hawthorn J, Rossor MN
and Iversen LL: Vasopressin, oxytocin and neurophysins in the human
brain and spinal cord. Brain Res. 291:111–117. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Yang Y, Chen JM, Liu WY, Wang CH
and Lin BC: Central oxytocin enhances antinociception in the rat.
Peptides. 28:1113–1119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mazzuca M, Minlebaev M, Shakirzyanova A,
Tyzio R, Taccola G, Janackova S, Gataullina S, Ben-Ari Y,
Giniatullin R and Khazipov R: Newborn analgesia mediated by
oxytocin during delivery. Front Cell Neurosci. 5:32011. View Article : Google Scholar : PubMed/NCBI
|