Introduction
Breast cancer is the most common malignancy among
females throughout the world. Combined therapies of surgery,
chemotherapy, radiotherapy, endocrine therapy and targeted therapy
are important tools in the treatment of breast cancer; however,
current treatments can lead to a number of unsatisfactory outcomes,
predominantly due to the difficulty accessing certain tumor sites,
the dispersed nature of the disease and the toxicity of the
treatments. The development of a more effective therapy for breast
cancer has long been one of the most elusive goals of cancer gene
therapy (1).
Recently, there an been an increased focus on the
potential of mesenchymal stem cells (MSCs) for use in cell therapy
and tissue engineering due to their multipotency and ability to
secrete growth factors (2,3). Furthermore, as a result of their
characteristics of hypo-immunogenicity and immunomodulation, MSCs
have been shown to be effective as gene delivery vehicles for
therapeutic purposes (4–6). It has also been demonstrated that
MSCs can migrate towards tumors in response to chemokines produced
by tumor cells, when introduced into the organism by systemic
intravenous administration (7,8), and
contribute to the population of supportive stromal cells around
tumors (9,10). These observations support the
development of therapeutic strategies based on the local production
of tumoricidal biological agents by gene-manipulated MSCs.
Lentiviruses exhibit a number of desirable qualities
as vehicles for gene delivery for use in experimental studies and
gene therapy, as they can transfect dividing and non-dividing
cells, show stable transgene expression and have a low toxicity and
immunity and a large cloning capacity (9 kb) (11,12).
Interleukin-18 (IL-18), a cytokine that was formally known as
interferon-γ (IFN-γ)-inducing factor, exhibits structural and
functional similarities to IL-1. IL-18 is involved in numerous
biological activities, acting through its ability to stimulate
innate immunity and T-helper type 1 (Th1)- and Th2-mediated
responses (13,14). Furthermore, IL-18 can exert an
antitumor effect by enhancing the activity of natural killer (NK)
cells, reducing tumorigenesis, inducing tumor cell apoptosis and
inhibiting tumor angiogenesis (15). Contradictorily, recent studies have
shown that, in addition to the antitumor activity of IL-18 in the
immune system, IL-18 can exert pro-cancer effects when produced by
cancer cells, acting to promote cell proliferation and migration.
The autocrine action of IL-18 can, for example, induce cell
migration in gastric cancer and melanoma (16,17).
In addition, clinical studies have associated this marker in the
serum with the prognosis of bladder cancer, glioma and breast
cancer, particularly metastatic breast cancer (13,18,19).
Srabović et al (20) found
that patients with breast cancer who exhibited liver and bone
metastasis had significantly increased serum IL-18 levels relative
to healthy females. Taking into account the involvement of IL-18 in
breast cancer progression and metastasis, Yao et al
(21) suggested that IL-18 could
play dual functions in drug resistance and tumor metastasis.
Several studies have found that dendritic cells (DCs) loaded with
lysed tumor cells and IL-18 could stimulate Th1 responses against
glioma antigens and markedly enhance the cytotoxic efficacy of
cytotoxic T lymphocytes towards tumor cells (19,22);
however, few data exist regarding the effect of MSCs modified with
IL-18 gene in human tumors, and there is an urgent requirement for
their effect on different types of tumors to be studied. The
objective of the present study, therefore, was to transduce human
MSCs from umbilical cord (hUMSCs) with Lenti-IL-18 recombinant
virus and observe the antitumor effect, in order to determine
whether hUMSCs modified with IL-18 gene could suppress the
proliferation, invasion and migration of breast cancer cells in
vitro.
Materials and methods
MSC preparation
Umbilical cord was obtained from a healthy
25-year-old female who had given birth to a healthy, term fetus.
The female had no family history of genetic disorders or cancer and
had tested negative for hepatitis B, hepatitis C, human
immunodeficiency and Epstein-Barr viruses, as well as
cytomegalovirus and syphilis. The umbilical cord collection was
approved by the Institutional Medical Research Ethics Committee of
the Qingdao maternity hospital (Qingdao, China). Fully informed
consent was obtained from the pregnant female several weeks prior
to delivery.
The preparation of the hUMSCs was performed in the
laminar flow laboratory. Briefly, the umbilical cord was washed
twice with phosphate-buffered saline (PBS) and cut with scissors
into pieces measuring 1–2 mm3 in volume. These tissue
pieces were plated in a cell culture dish (Corning®
430597; Corning, Inc., Palo Alto, CA, USA) in low-glucose
Dulbecco’s modified Eagle’s medium (DMEM; HyClone, GE Healthcare
Life Sciences, South Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS; HyClone, GE Healthcare Life Sciences). The cell
cultures were maintained at 37°C in a humidified atmosphere with 5%
CO2. Following three days of culture, the medium was
replaced in order to remove the tissue and non-adherent cells;
medium replacement was performed twice weekly thereafter.
Subsequent to the cells reaching 80% confluence, 0.125% trypsin was
used to detach the adherent cells (passage 0), which were then
passaged in the cell culture dish. The MSC culture and expansion
was performed in a laminar flow laboratory for four passages to
prepare the final, sterile cell products. The cells were
subsequently stained with a double label and analyzed by flow
cytometry using a FACSCalibur™ flow cytometry system (Becton
Dickinson, Franklin Lakes, NJ, USA).
Transduction of hUMSCs
A lentivirus construct containing the green
fluorescent protein (GFP) and human IL-18 genes or blank lentivirus
vector was used (GenePharma, Beijing, China). The hUMSCs were
plated at a density of 104 cells per well in a 96-well
plate (Corning 3896; Corning, Inc.). After 24 h, the viruses were
added to the medium to infect the MSCs at 10, 30, 50, 70 and 100
pfu/cell (in order to explore the optimal dose), and the plates
were then centrifuged at 600 × g for 90 min at 37°C prior to
culture overnight. Following culture, the cells were washed with
PBS, the medium was replaced with a fresh culture medium and the
cells were further incubated for up to 72 h. hUMSCs transfected
with blank vector were used as controls. Transduction efficiency
was determined using an inverted fluorescence microscope based on
expression of the GFP gene. The percentage of cells positive for
GFP was determined using flow cytometry by setting a gate according
to the control; 10,000 cells were evaluated in each experiment.
Effective transduction was confirmed by IL-18 enzyme-linked
immunosorbent assay (ELISA) of the culture supernatant (KB1138;
Shanghai Kaibo Biochemical Reagent Co., Ltd., Shanghai, China) at
24, 48, 72 h and one week after transduction.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Extraction of total RNA from the cultured cells was
performed using TRIzol® reagent (Invitrogen™, Life
Technologies, Carlsbad, CA, USA) in accordance with the
manufacturer’s instructions. An ABI Prism® 7500 Sequence
Detection System (Applied Biosystems, Foster City, CA, USA) with
SYBR® Green I dye (Molecular Probes®, Life
Technologies) was used for cDNA amplification and quantification.
The primer sequences for IL-18, which were designed using Primer
Express v2.0 software (Applied Biosystems), were as follows:
forward: 5′-GAATAAAGATGGCTGCTGAACC-3′ and reverse:
5′-CCTGGGACACTTCTCTGAAA-3′. The reference gene GAPDH (146 bp) was
used as a control. The reaction conditions were as follows: Initial
incubation at 94°C for 5 min followed by 35 cycles of incubation at
98°C for 10 sec, 57°C for 30 sec and 72°C for 30 sec, with a final
extension step at 72°C for 10 min. The PCR products were
subsequently analyzed by electrophoresis and separated on the 2.0%
agarose gel. The expression of the IL-18 gene was normalized to the
geometric mean of the expression of the reference gene, GAPDH, in
order to control the variability in expression levels. Normalized
expression was calculated using the following formula: 2−[(Ct
of IL-18) − (Ct of GAPDH)], where Ct represents the threshold
cycle for each transcript. The results were expressed as the
average density of the positive bands obtained from three
independent experiments.
Western blot analysis
The cells were washed with ice-cold PBS and lysed
with M-PER® Mammalian Protein Extraction Reagent (78501;
Pierce Biotechnology, Thermo Scientific, Inc., South Logan, USA).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used
to separate equal quantities of proteins, which were subsequently
transferred to polyvinylidene fluoride membranes (IPVH00010
Immobilon-P; Merck-Millipore, Darmstadt, Germany). The membranes
were probed with antibody against IL-18 (rabbit anti human;
1:1,000; #ab68435; Abcam Trading (Shanghai) Company Ltd., Shanghai,
China). Horseradish peroxidase (HRP)-conjugated anti-rabbit
secondary antibody (Cell Signaling Technology, Inc., Beverly, MA,
USA) was used as a probe, and the immunoreactive bands were
visualized with the Immobilon Western Chemiluminescent HRP
Substrate (Merck-Millipore). The intensity of the bands was
measured using ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
ELISA
Culture supernatant was collected at different
time-points, and the level of IL-18 was quantified using an ELISA
kit (KB1138; Kangbo Co.) in accordance with the manufacturer’s
instructions. The absorbance was read at 450 nm. The minimum
sensitivity of detection was 1.0 pg/ml.
Direct coculture of MCF-7 and HCC1937
cells with hUMSCs/IL-18 in vitro
The MCF-7 and HCC1937 cells (donated by the Central
Laboratory of the Affiliated Hospital of Qingdao University,
Qingdao, China) were respectively seeded in the bottom chamber of a
24-well Transwell® culture system (Corning, Inc.)
containing 600 μl medium (low-glucose DMEM supplemented with 10%
FBS). The hUMSCs/IL-18 were dispersed onto the inserts of the
Transwell dishes with 0.4-μm pore size.
Four groups were included in the experiment: Tumor
cells (MCF-7 or HCC1937), hUMSCs + tumor cells, hUMSCs/vector +
tumor cells and hUMSCs/IL-18 + tumor cells. At least five wells
were randomly examined each time, and all experiments were repeated
twice.
Tumor cell proliferation assays
After one, three and five days of coculture,
triplicates of hUMSCs and tumor cells (1×104 cells per
well) in each group were respectively plated into 96-well plates
and incubated for 24 h at 37°C. A total of 10 μl cell counting kit
8 (CCK-8) solution was then added into the culture medium for
incubation for 3 h at 37°C. Following incubation, the plates were
subjected to the CellTiter 96® AQueous One Solution Cell
Proliferation Assay (G3582; Promega Corp., Madison, WI, USA) and
measured spectrophotometrically at 450 nm. The results were
presented as the percentage of proliferation, where the
proliferation of cells in culture medium without CCK-8 was set to
100%. Similar results were obtained in three independent
experiments.
Flow cytometry for cell cycle
analysis
Cell cycle analysis was performed using flow
cytometry following the harvesting and washing of the cells with
cold PBS. Briefly, the cells were fixed in 75% ethanol and stored
at −20°C for subsequent analysis. The fixed cells were subjected to
centrifugation at 200 × g at 4°C for 5 min and washed twice with
cold PBS. Ribonuclease A (final concentration, 20 μg/ml) and
propidium iodide staining solution (final concentration, 50 μg/ml;
Promega Corp.) were added to the cells, which were subsequently
incubated for 30 min at 37°C in the dark. Cell analysis (≥100,000
cells) was performed using a FACSCalibur flow cytometry system
(Becton Dickinson) equipped with CellQuest™ 3.3 software. ModFit™
LT 3.1 trial cell cycle analysis software (Verity Software House,
Topsham, MA, USA) was used to determine the proportion of the cells
in each of the different phases of the cell cycle.
Tumor cell migration assays
The MCF-7 and HCC1937 cell migration assays were
performed using a 24-well Transwell chamber. The MCF-7/HCC1937
cells from the four groups were separately resuspended in 100 μl
medium without FBS and placed into the upper chamber of the 24-well
Transwell culture system with 8.0-μm pore-size polycarbonate
membrane inserts. The lower wells were filled with 600 μl medium
supplemented with 20% FBS as a chemoattractant. Following 24 h of
culture, the cells in the upper surfaces of the filter were removed
with cotton swabs, and cells that had migrated to the lower
surfaces were fixed with 4% paraformaldehyde, stained with 0.05%
crystal violet and counted in five random fields on each filter.
The membranes were dried thoroughly prior to examination. The
number of cells that had migrated and adhered to the lower side of
the membrane was assessed under high-power light microscopy
(magnification, ×200). The average cell number (migrated cell
number) was taken from five random fields per well and used to
calculate the migration index: Migration index = migrated cell
number of the experimental group/migrated cell number of the
control group).
Tumor cell invasion assays
The MCF-7 and HCC1937 cell invasion assays were
performed using a 24-well Transwell chamber. The MCF-7/HCC1937
cells from the four groups were separately resuspended in 100 μl
medium without FBS and placed into the upper chamber of a 24-well
Transwell culture system with 8.0-μm pore-size polycarbonate
membrane inserts coated with Matrigel™ for 1 h at 37°C. The lower
wells were filled with 600 μl medium supplemented with 20% FBS as a
chemoattractant. Following culture for 24 h, the cells on the upper
surfaces of the filter were removed with cotton swabs, and the
cells that had migrated to the lower surfaces were fixed with 4%
paraformaldehyde, stained with 0.05% crystal violet and counted in
five random fields on each filter. The membranes were dried
thoroughly prior to examination. The cells that had migrated and
adhered to the lower side of the membrane were counted under
high-power light microscopy (magnification, ×200). The average cell
number, taken from five random fields per well, was defined as the
invaded cell number.
hUMSCs/IL-18 migration assays
To assess the ability of the hUMSCs/IL-18 to migrate
to the tumor cells, 5×104 hUMSCs/IL-18 were dispersed
onto the inserts of Transwell dishes (8-μm pore-size; Falcon™;
Becton Dickinson Labware, Lincoln Park, MI, USA) and allowed to
adhere for 1 h at 37°C. The Transwell inserts were then transferred
to the bottom chamber, which contained 1×105 tumor cells
in 600 μl low-glucose DMEM supplemented with 10% FBS and cultured
for 24 h. The cells on the upper surfaces of the filter were
subsequently removed with cotton swabs, and any cells that had
migrated to the lower surfaces were fixed with 95% alcohol, stained
with 0.05% crystal violet and counted in five random fields on each
filter.
Statistical analysis
Statistical analyses were performed using a minimum
of three independently prepared cultures and are presented as the
mean ± standard deviation. Significant interactions were determined
by either one-way or two-way analysis of variance and Bonferroni
multiple comparison tests using Prism software (GraphPad Software,
Inc., San Diego, CA, USA). A two-sided P<0.05 was considered
statistically significant.
Results
Expression of IL-18 by hUMSCs
The hUMSCs that were expanded in vitro
appeared similar to fibroblasts, with a characteristic
spindle-shaped fusiform morphology (Fig. 1). Subsequent to the third passage,
the cells were of high purity, with cluster of differentiation
(CD)146+, CD105+, CD90+,
CD34− and CD45− expression. No changes in
cell shape were observed in the IL-18-transduced hUMSCs.
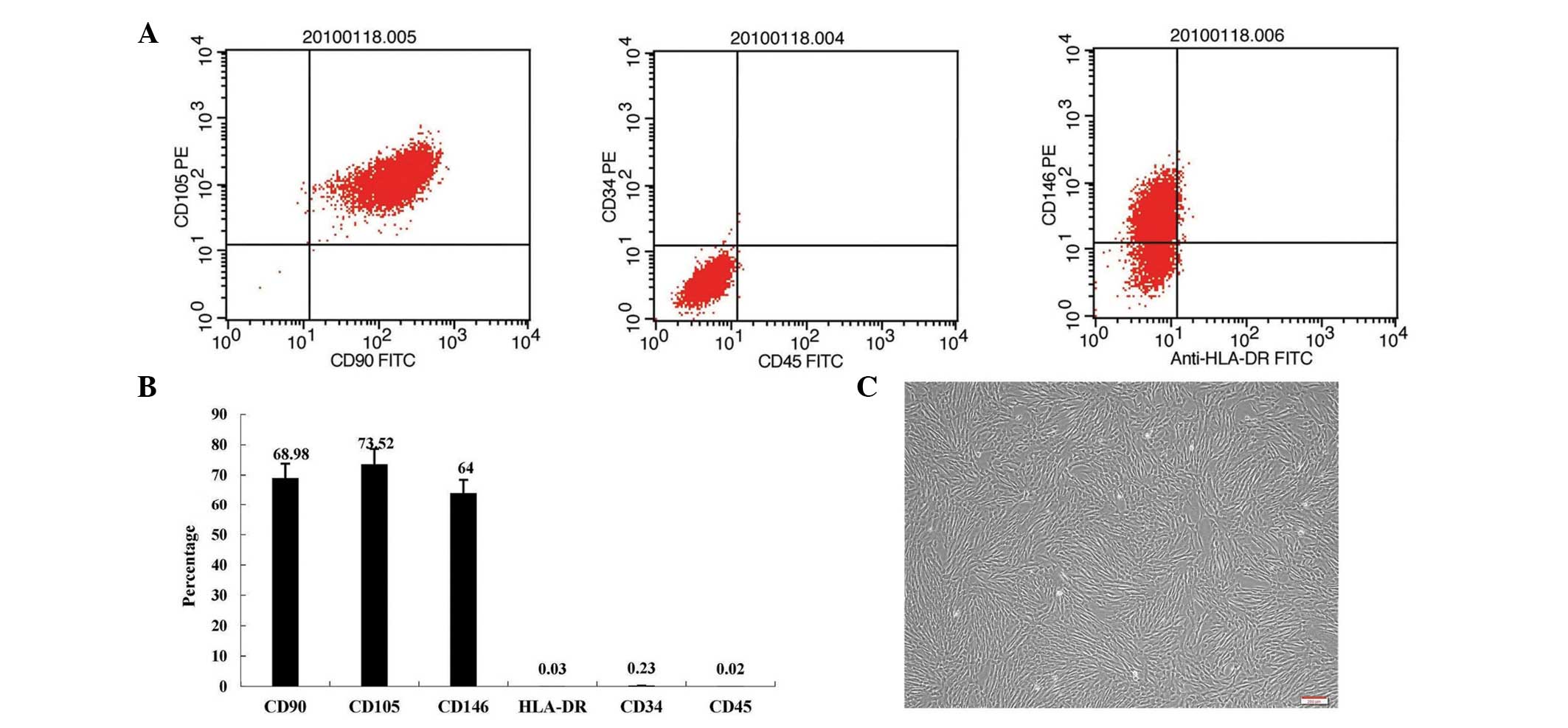 | Figure 1MSCs from human umbilical cord.
Following the third passage, MSCs (A and B) exhibited
CD146+, CD105+, CD90+,
CD34− and CD45− expression, as determined
using flow cytometry, and (C) were of high purity. MSC, mesenchymal
stem cell; CD, cluster of differentiation; HLA, human leukocyte
antigen; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Magnification, ×100. |
GFP-containing lentivirus was utilized to assess
transduction efficiency and the optimal viral infection conditions.
Fluorescence microscopy revealed that the majority of the cell
populations showed strongly positive GFP expression following
transduction (Fig. 2A). Flow
cytometric quantification of the GFP-positive cells showed a
transduction efficiency of 85–92% at a multiplicity of infection
(MOI) of 70; no significant benefit was obtained from increasing
the MOI to 100.
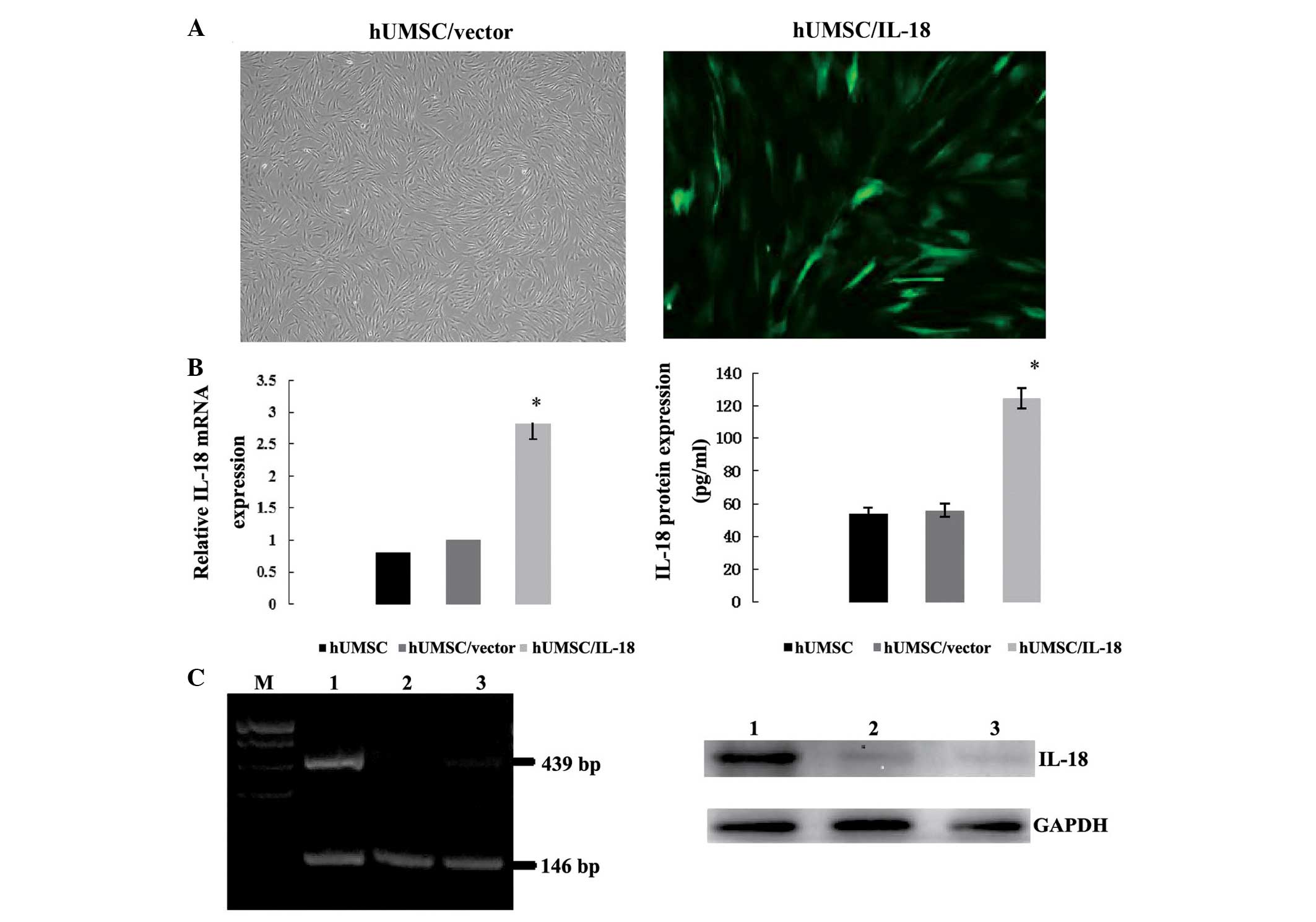 | Figure 2Expression of IL-18 protein by hUMSCs
following transduction. (A) hUMSCs transfected with vector control
and IL-18 gene by lentivirus (magnification, ×100). (B) Relative
mRNA and protein expression of IL-18 in hUMSC/IL-18 cells, as
determined by RT-PCR and western blotting, respectively. Relative
mRNA expression of IL-18 in the hUMSCs/IL-18 group was higher
compared with that in the hUMSCs/vector and hUMSCs groups, as
determined by RT-PCR (*P<0.001). Protein expression
of IL-18 in the hUMSCs/IL-18 group was higher compared with that in
the hUMSCs/vector and hUMSCs groups (*P=0.007 vs.
hUMSC/vector group, and P=0.008 vs. hUMSC group). (C) mRNA and
protein expression of IL-18 in hUMSCs, as determined by RT-PCR and
western blotting, respectively (lane 1, hUMSCs/IL-18; lane 2,
hUMSCs/vector; lane 3, hUMSCs; M, marker). IL-18, interleukin-18;
hUMSCs, human mesenchymal stem cells derived from umbilical cord;
RT-PCR, reverse transcription-polymerase chain reaction. |
To determine the expression of IL-18 in the hUMSCs,
the cells and medium were collected and assessed using RT-PCR one
week after transduction. RT-PCR showed that there was a
2.85±1.7-fold promotion of IL-18 expression in the hUMSCs/IL-18
group as compared with the hUMSCs/vector and hUMSCs groups
(P<0.001, Fig. 2B). Protein
expression was evaluated by western blotting and ELISA, which
showed that the IL-18 concentration in the hUMSCs/IL-18 group was
125±16.7 pg/ml, as compared with 54±6.1 and 56±5.9 pg/ml in the
hUMSCs/vector and hUMSCs groups, respectively (P=0.007 and 0.008,
Fig. 2B).
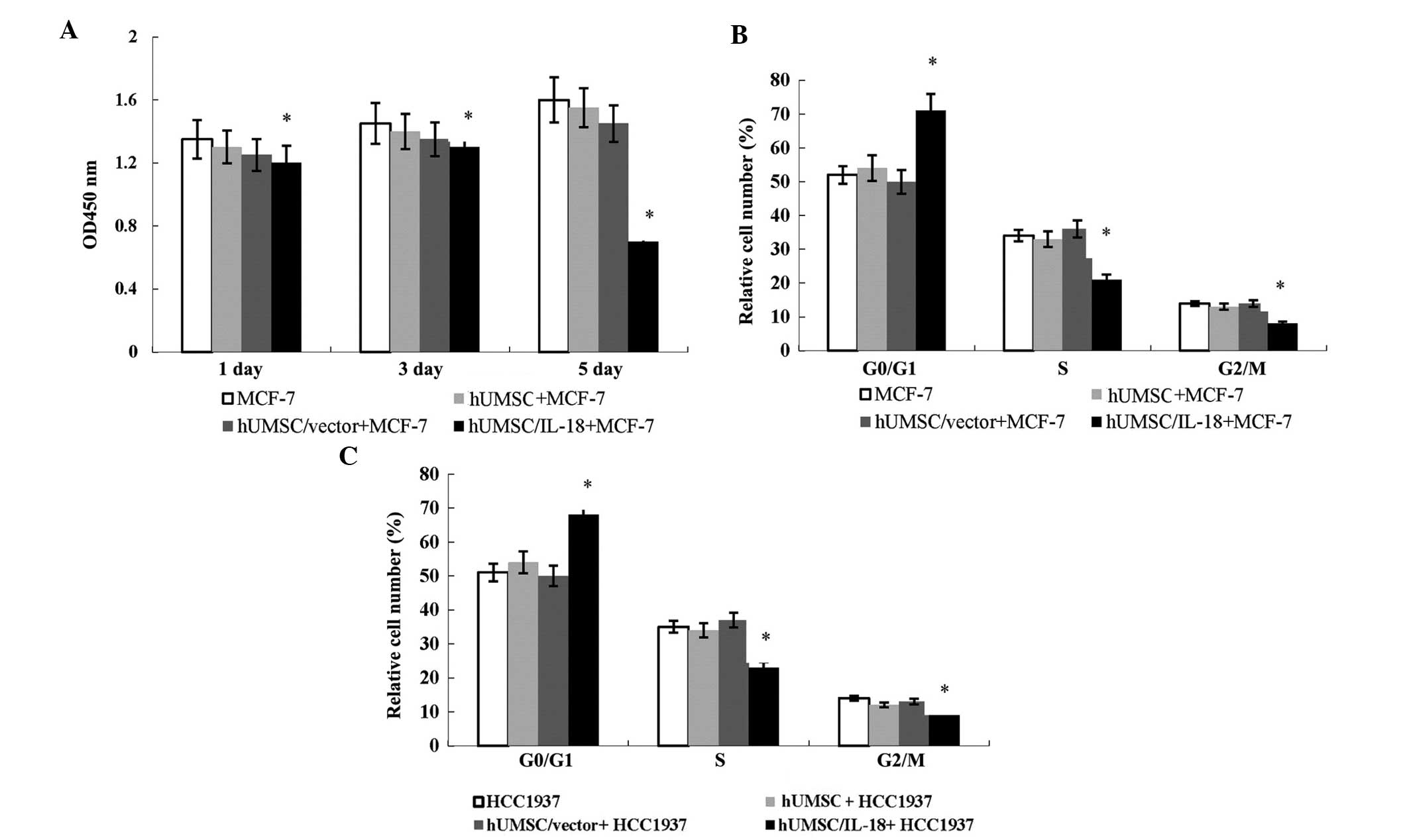
hUMSCs/IL-18 significantly suppress tumor
cell growth in vitro
To evaluate the bioactivity of hUMSCs/IL-18 on
cancer cell proliferation, the CCK-8 assay was performed in MCF-7
and HCC1937 cells. A marked reduction in cell proliferation was
observed in the MCF-7 and HCC1937 cells following coculture with
hUMSCs/IL-18, showing an evident decrease in cell number compared
with the vector-control group after a five-day culture period
(Fig. 3A).
To investigate the suppression mechanisms of
hUMSCs/IL-18 on breast cancer cells, cell cycle analysis was
performed. Flow cytometric analysis showed that hUMSCs/IL-18
significantly increased the percentage of cells in the
G0/G1 phase but decreased that in the S and
G2/M phase. As shown in Fig. 3B and C, the MCF-7 and HCC1937 cells
cocultured with hUMSCs/IL-18 exhibited a significant increase in
the percentages of cells in the G1 phase but a decreased
proportion in the S phase compared with the cells cocultured with
hUMSCs/vector and hUMSCs (P<0.05). This indicated that
hUMSCs/IL-18 suppressed cancer cell proliferation by inducing the
G1- to S-phase arrest of breast cancer cells.
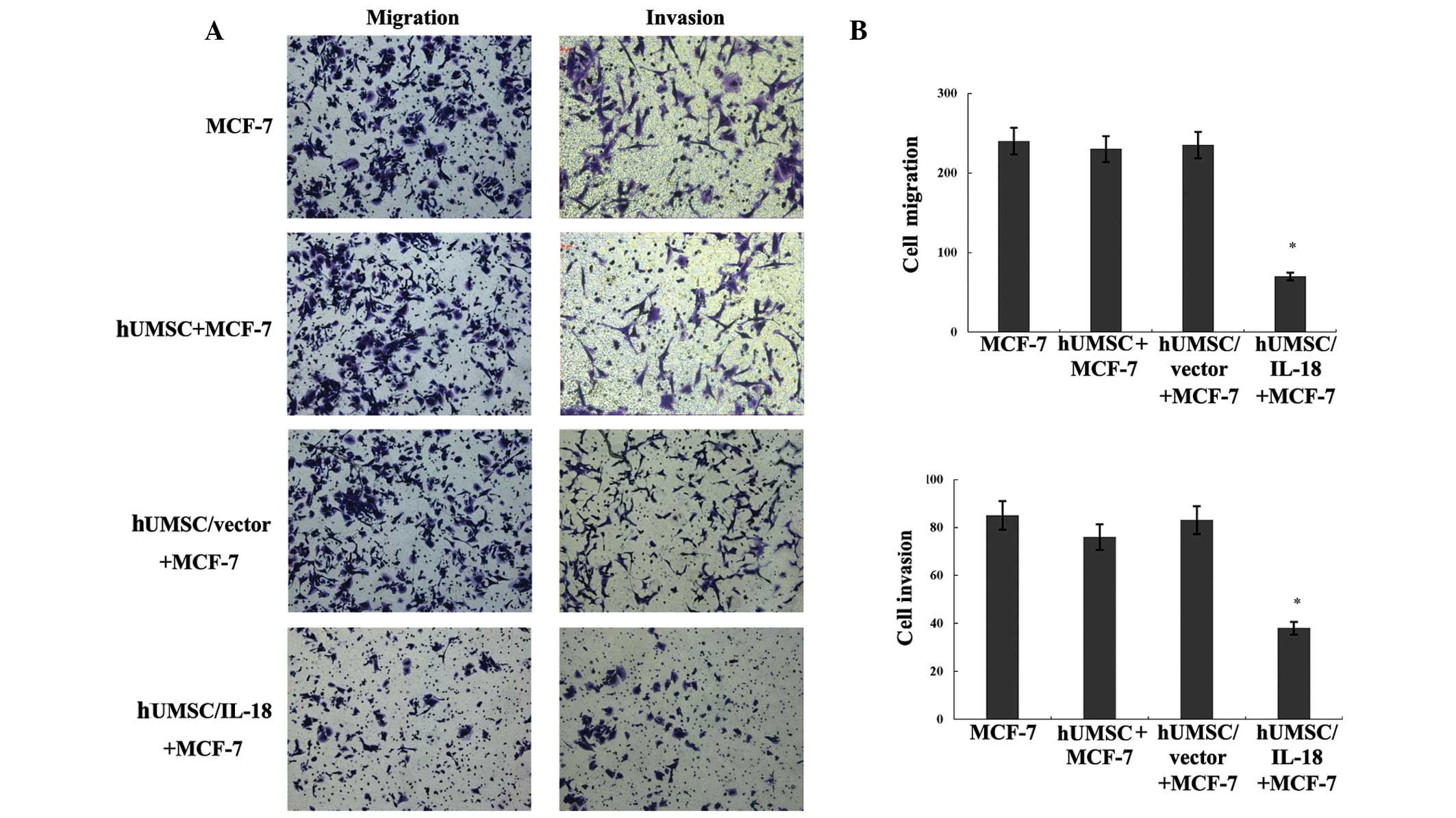
Effect of hUMSCs/IL-18 on the migration
and invasion of tumor cells
In order to investigate the effect of hUMSCs/IL-18
on the migration and invasion of MCF-7 and HCC1937 cells, cell
migration and invasion assays were performed in vitro and
the number of migrating and invading cells was counted. Compared
with the hUMSCs/vector and hUMSCs groups, hUMSCs/IL-18 markedly
suppressed the migration and invasion of the MCF-7 and HCC1937
cells (P<0.001), as shown in Fig.
4.
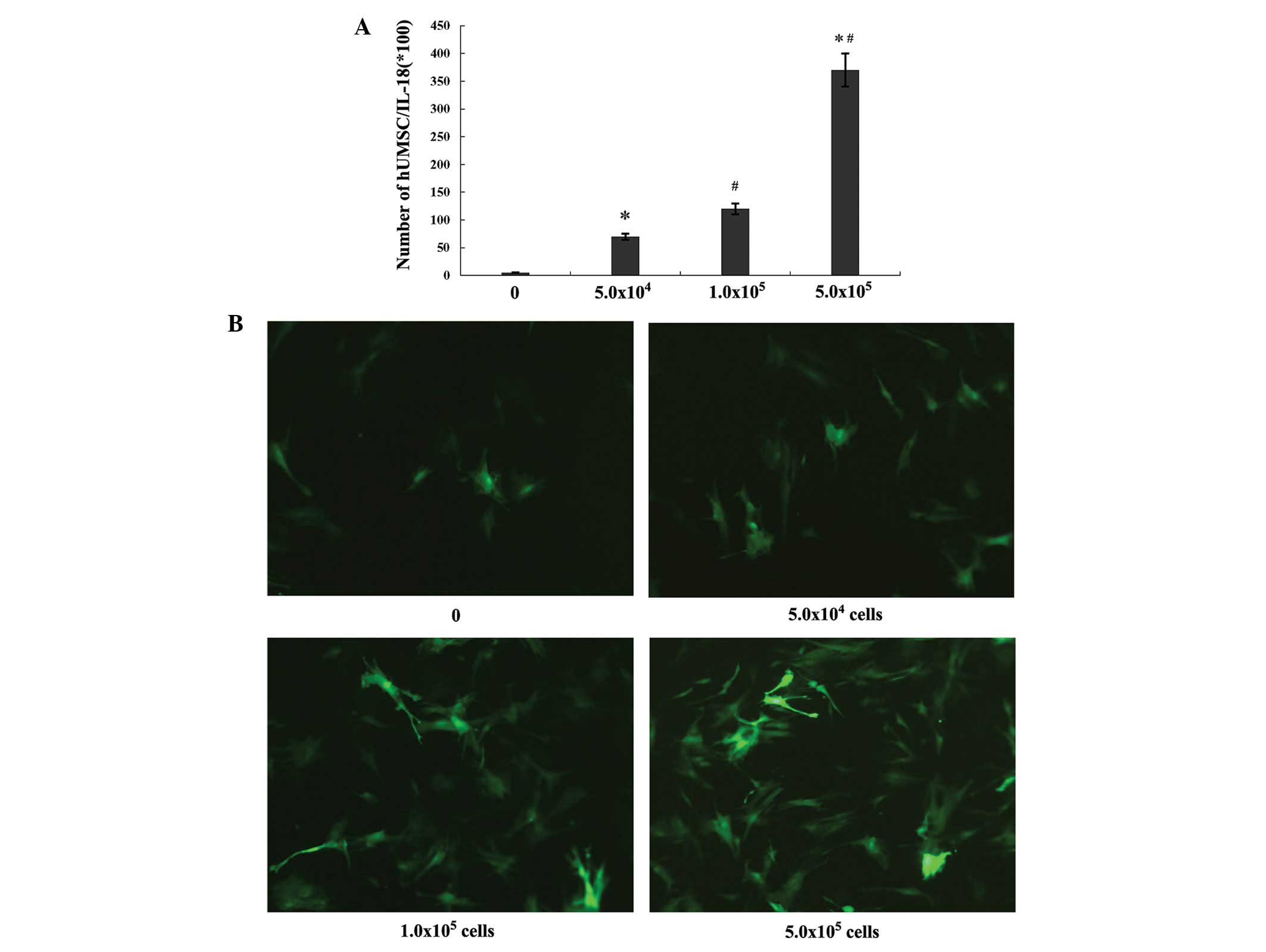
hUMSCs/IL-18 migration towards tumor
cells in vitro
The hUMSCs/IL-18 did not migrate to the lower
chamber when the tumor cells were not present, but they were
stimulated to migrate by the addition of tumor cells into the lower
chamber. Of the hUMSCs/IL-18 (5.0×104 cells) placed in
the upper chamber, 14.0% (7×103 cells) moved to the
lower side in the presence of 5×104 HCC1937 cells, 24%
(1.2×104 cells) moved to the lower side in the presence
of 1.0×105 HCC1937 cells and 74% (3.7×104
cells) moved to the lower side in the presence of
5.0×105 HCC1937 cells. The migration of the hUMSCs
increased in a dose-dependent manner with increasing numbers of
tumor cells (P<0.001, Fig.
5).
Discussion
In the present study, hUMSCs genetically modified
with IL-18 gene were used to study the effect of hUMSCs/IL-18 on
the growth, migration and invasion of two breast cancer cell lines
in vitro. Through the use of a lentivirus, IL-18 protein was
successfully secreted from the hUMSCs/IL-18 at an MOI of 70.
Furthermore, the results showed that hUMSCs/IL-18, but not hUMSCs,
significantly inhibited the growth, migration and invasion of MCF-7
and HCC1937 cells in vitro.
The present study showed that hUMSCs could be
efficiently modified by lentiviral systems and could stably express
the transgene. In a previous study by Brennen et al
(23), it was shown that MSCs
engrafted in tumors could act as stromal precursor cells and
successfully function as cellular vehicles for gene delivery and
contribute to the local production of biological agents. Compared
with other types of cells, MSCs are simpler to obtain and can be
more easily propagated in vitro; furthermore, their use is
associated with fewer ethical issues and immune response problems.
hUMSCs possess specific chemoresistance and migration properties
and should therefore be considered as a valuable type of adult stem
cell for use in cancer therapy (1,24).
In the present study, the GFP and IL-18 genes did not show any
cytotoxic effects on the transduced hUMSCs, and a high active
expression of IL-18 was observed. A possible risk associated with
lentiviral transduction is insertional oncogenesis following
multiple vector integrations into the host genome. Cell-based
therapy with systemic delivery of MSCs has been used in animal
experiments and clinical trials, and no adverse effects have been
reported to date (11,25).
MCF-7 and HCC1937 cells are human breast cancer cell
lines and respectively represent luminal and triple-negative breast
cancer cells. The migration of hUMSCs towards the two cancer cell
lines was demonstrated in vitro in the present study. This
observation was consistent with previous reports (26,27)
and represents an important characteristic when enforced in
vivo; however, the mechanisms underlying the homing and
engrafting of the hUMSCs to the tumors have yet to be fully
elucidated. It is likely that hUMSCs express a wide range of
cytokine and chemokine receptors, and that differential gene
regulation may occur following the exposure of the hUMSCs to
different microenvironments. Since tumor cells and their
microenvironments secrete chemokines or cytokines, these may be
responsible for upregulating the expression of the chemokine and
cytokine receptors on the hUMSCs (28,29),
such as C-X-C chemokine receptor type 4 (which is upregulated in
hUMSCs) and stromal cell-derived factor 1 (secreted by hUMSCs),
leading to an effect on MSC migration (30,31).
The antitumor activities of hUMSCs/IL-18, i.e. the
inhibition of tumor cell growth, as well as migration and invasion,
were confirmed by coculture in Transwell chambers in the present
study. Compared with hUMSCs and hUMSCs/vector, hUMSCs/IL-18 could
significantly suppress the proliferation, migration and invasion of
the MCF-7 and HCC1937 cells. The mechanisms by which hUMSCs/IL-18
caused the growth attenuation of cancer cells were determined by
cell cycle analysis with flow cytometry. The results indicated that
the hUMSC/IL-18-dependent tumor cell growth attenuation occurred
predominantly due to cell cycle arrest at the G1/S check
point.
IL-18 has been demonstrated to promote the
production of Th1-type cytokines, which are involved in the
antitumor cytotoxic T-cell response (22). Consistent with the theory that Th1
cells are associated with immunity to cancer, the administration of
IL-18 can result in notable antitumor effects. IL-18 has been found
to induce IFN-γ and granulocyte-macrophage colony stimulaing factor
secretion by T and NK cells, enhance the cytolytic activity of NK
cells, increase the proliferation of T cells and activate
CD8+ cytotoxic T lymphocytes (32–34).
IL-18 can also induce IL-2 secretion by Th1 clones, stimulated by
immobilized anti-CD3, and enhance NK cell expression of the
DC-attracting chemokines C-C motif chemokine 3 (CCL3), CCL4,
Chemokine (C-X-C motif) ligand 8 and XC chemokine ligand 1,
resulting in the attraction of immature DCs. Previous studies have
demonstrated that DCs loaded with lysed tumor cells and IL-18 may
induce Th1 responses against glioma antigens (19,22).
Such IL-18-driven enhancement in the expression of DC-attracting
chemokines corresponds closely to the previously reported
regulation of IFN-γ and tumor necrosis factor-α (TNF-α), factors
essential for the NK cell-mediated activation of DCs, in
IL-18-primed human NK cells (34).
These results indicate that IL-18 can be used in cancer
immunotherapy as a promoter of a strong cellular response.
Although hUMSCs/IL-18 significantly suppressed the
growth and invasion of cancer cells, IL-18 has also been found to
promote tumor progression. Increased levels of IL-18 have been
detected in certain types of cancer, and elevated IL-18 expression
has been associated with tumor growth and metastasis in breast
cancer (20). In the present
study, a novel possibility that IL-18 could have a function in the
treatment of breast cancer was revealed; however, further
experiments are necessary to verify the effect of hUMSCs/IL-18 on
breast cancer in vivo. Furthermore, combinations with other
cytokines, such as IL-12 and TNF-α, or with a DNA vaccine, could
enhance the antitumor efficacy in the future.
In conclusion, the present study has demonstrated
the therapeutic potential of hUMSCs/IL-18 in breast cancer
treatment. It was demonstrated that genetically engineering hUMSCs
to produce IL-18 had synergistic therapeutic benefits and that such
cells could contribute to an adoptive immunotherapy for breast
cancer and thereby provide a promising new treatment option. Thus,
further investigation into the use of hUMSCs/IL-18 as vehicles of
tumor therapy in vivo, including pilot clinical trials, and
in combination with other therapies, such as surgery, chemotherapy,
endocrine therapy and targeted therapy, is warranted.
Acknowledgements
This study was supported by the Shandong Provincial
Natural Science Foundation (grant no. 2013ZRB01426) and by the
Department of Central Laboratory, the Affiliated Hospital of
Qingdao University (Qingdao, China).
References
|
1
|
Mohr A, Lyons M, Deedigan L, et al:
Mesenchymal stem cells expressing TRAIL lead to tumour growth
inhibition in an experimental lung cancer model. J Cell Mol Med.
12:2628–2643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Donega V, van Velthoven CT, Nijboer CH,
Kavelaars A and Heijnen CJ: The endogenous regenerative capacity of
the damaged newborn brain: boosting neurogenesis with mesenchymal
stem cell treatment. J Cereb Blood Flow Metab. 33:625–634. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsuda K, Falkenberg KJ, Woods AA, Choi
YS, Morrison WA and Dilley RJ: Adipose-derived stem cells promote
angiogenesis and tissue formation for in vivo tissue engineering.
Tissue Eng Part A. 19:1327–1335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang R, Liu Y, Yan K, et al:
Anti-inflammatory and immunomodulatory mechanisms of mesenchymal
stem cell transplantation in experimental traumatic brain injury. J
Neuroinflammation. 10:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang YS, Wang Y, Wang L, et al: Labeling
human mesenchymal stem cells with gold nanocages for in vitro and
in vivo tracking by two-photon microscopy and photoacoustic
microscopy. Theranostics. 3:532–543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu WT, Lin CH, Chiang BL, Jui HY, Wu KK
and Lee CM: Prostaglandin E2 potentiates mesenchymal stem
cell-induced IL-10+IFN-γ+CD4+
regulatory T cells to control transplant arteriosclerosis. J
Immunol. 190:2372–2380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Panciani PP, Fontanella M, Tamagno I, et
al: Stem cells based therapy in high grade glioma: why the
intraventricular route should be preferred? J Neurosurg Sci.
56:221–229. 2012.PubMed/NCBI
|
|
8
|
Zhu X, Su D, Xuan S, et al: Gene therapy
of gastric cancer using LIGHT-secreting human umbilical cord
blood-derived mesenchymal stem cells. Gastric Cancer. 16:155–166.
2013. View Article : Google Scholar
|
|
9
|
Chen Q, Cheng P, Song N, et al: Antitumor
activity of placenta-derived mesenchymal stem cells producing
pigment epithelium-derived factor in a mouse melanoma model. Oncol
Lett. 4:413–418. 2012.
|
|
10
|
Belmar-Lopez C, Mendoza G, Oberg D, et al:
Tissue-derived mesenchymal stromal cells used as vehicles for
anti-tumor therapy exert different in vivo effects on migration
capacity and tumor growth. BMC Med. 11:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moriyama H, Moriyama M, Sawaragi K, Okura
H, Ichinose A, Matsuyama A and Hayakawa T: Tightly regulated and
homogeneous transgene expression in human adipose-derived
mesenchymal stem cells by lentivirus with tet-off system. PLoS One.
8:e662742013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luetzkendorf J, Mueller LP, Mueller T,
Caysa H, Nerger K and Schmoll HJ: Growth inhibition of colorectal
carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem
cells requires their substantial intratumoral presence. J Cell Mol
Med. 14:2292–2304. 2010. View Article : Google Scholar
|
|
13
|
Jaiswal PK, Singh V, Srivastava P and
Mittal RD: Association of IL-12, IL-18 variants and serum IL-18
with bladder cancer susceptibility in North Indian population.
Gene. 519:128–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuppala MB, Syed SB, Bandaru S, Varre S,
Akka J and Mundulru HP: Immunotherapeutic approach for better
management of cancer - role of IL-18. Asian Pac J Cancer Prev.
13:5353–5361. 2012. View Article : Google Scholar
|
|
15
|
Ni J, Miller M, Stojanovic A, Garbi N and
Cerwenka A: Sustained effector function of IL-12/15/18-preactivated
NK cells against established tumors. J Exp Med. 209:2351–2365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen Z, Seppänen H, Vainionpää S, Ye Y,
Wang S, Mustonen H and Puolakkainen P: IL10, IL11, IL18 are
differently expressed in CD14+ TAMS and play different
role in regulating the invasion of gastric cancer cells under
hypoxia. Cytokine. 59:352–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crende O, Sabatino M, Valcárcel M, et al:
Metastatic lesions with and without interleukin-18-dependent genes
in advanced-stage melanoma patients. Am J Pathol. 183:69–82. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taheri M, Hashemi M, Eskandari-Nasab E,
Fazaeli A, Arababi F, Bahrani-Zeidabadi M and Bahari G: Association
of -607 C/A polymorphism of IL-18 gene (rs1946518) with breast
cancer risk in Zahedan, Southeast Iran. Prague Med Rep.
113:217–222. 2012.PubMed/NCBI
|
|
19
|
Xu G, Jiang XD, Xu Y, et al:
Adenoviral-mediated interleukin-18 expression in mesenchymal stem
cells effectively suppresses the growth of glioma in rats. Cell
Biol Int. 33:466–474. 2009. View Article : Google Scholar
|
|
20
|
Srabović N, Mujagić Z,
Mujanović-Mustedanagić J, Muminović Z and Cickusić E: Interleukin
18 expression in the primary breast cancer tumour tissue. Med Glas
(Zenica). 8:109–115. 2011.
|
|
21
|
Yao L, Zhang Y, Chen K, Hu X and Xu LX:
Discovery of IL-18 as a novel secreted protein contributing to
doxorubicin resistance by comparative secretome analysis of MCF-7
and MCF-7/Dox. PLoS One. 6:e246842011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan X, Ye M, Xue B, Ke Y, Wong CK and Xie
Y: Human dendritic cells engineered to secrete interleukin-18
activate MAGE-A3-specific cytotoxic T lymphocytes in vitro. Immunol
Invest. 41:469–483. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brennen WN, Denmeade SR and Isaacs JT:
Mesenchymal stem cells as a vector for the inflammatory prostate
microenvironment. Endocr Relat Cancer. 20:R269–R290. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dwyer RM, Ryan J, Havelin RJ, et al:
Mesenchymal stem cell-mediated delivery of the sodium iodide
symporter supports radionuclide imaging and treatment of breast
cancer. Stem Cells. 29:1149–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matuskova M, Hlubinova K, Pastorakova A,
Hunakova L, Altanerova V, Altaner C and Kucerova L: HSV-tk
expressing mesenchymal stem cells exert bystander effect on human
glioblastoma cells. Cancer Lett. 290:58–67. 2010. View Article : Google Scholar
|
|
26
|
Ciavarella S, Grisendi G, Dominici M,
Tucci M, Brunetti O, Dammacco F and Silvestris F: In vitro
anti-myeloma activity of TRAIL-expressing adipose-derived
mesenchymal stem cells. Br J Haematol. 157:586–598. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Birnbaum T, Hildebrandt J, Nuebling G,
Sostak P and Straube A: Glioblastoma-dependent differentiation and
angiogenic potential of human mesenchymal stem cells in vitro. J
Neurooncol. 105:57–65. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang J, Huang W, Yu X, et al: Suicide
gene reveals the myocardial neovascularization role of mesenchymal
stem cells overexpressing CXCR4 (MSC(CXCR4)). PLoS One.
7:e461582012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu LN, Wang G, Hendricks K, Lee K,
Bohnlein E, Junker U and Mosca JD: Comparison of drug and
cell-based delivery: engineered adult mesenchymal stem cells
expressing soluble tumor necrosis factor receptor II prevent
arthritis in mouse and rat animal models. Stem Cells Transl Med.
2:362–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jezierska-Drutel A, Rosenzweig SA and
Neumann CA: Role of oxidative stress and the microenvironment in
breast cancer development and progression. Adv Cancer Res.
119:107–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Creighton CJ, Gibbons DL and Kurie JM: The
role of epithelial-mesenchymal transition programming in invasion
and metastasis: a clinical perspective. Cancer Manag Res.
5:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Srivastava S, Pelloso D, Feng H, et al:
Effects of interleukin-18 on natural killer cells: costimulation of
activation through Fc receptors for immunoglobulin. Cancer Immunol
Immunother. 62:1073–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seo SH, Kim KS, Park SH, Suh YS, Kim SJ,
Jeun SS and Sung YC: The effects of mesenchymal stem cells injected
via different routes on modified IL-12-mediated antitumor activity.
Gene Ther. 18:488–495. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong JL, Berk E, Edwards RP and Kalinski
P: IL-18-primed helper NK cells collaborate with dendritic cells to
promote recruitment of effector CD8+ T cells to the
tumor microenvironment. Cancer Res. 73:4653–4662. 2013. View Article : Google Scholar : PubMed/NCBI
|