Introduction
Awake fiberoptic orotracheal intubation (AFOI) is
used in patients with expected difficult airways. Adequate topical
anesthesia and sedation techniques are important during the
procedure in order to maintain the patient’s airway and minimize
discomfort. Optimal conditions for modified AFOI should enable the
patients to be cooperative, comfortable and have blunted airway
reflexes, particularly when difficult laryngeal anatomy and/or
pathology are encountered. A number of agents have been used for
sedation during AFOI, including benzodiazepines, ketamine,
propofol, sevoflurane, remifentanil and dexmedetomidine (Dex).
Different protocols for sedation have been shown to improve the
success rate (1–5).
There are a number of advantages of using
remifentanil (Rem) for AFOI. Firstly, Rem is an ultra-short acting
drug with a constant half life. The drug exerts antitussive effects
that help to prevent coughing with tracheal manipulation. In
addition, Rem attenuates cardiovascular responses to airway
manipulation (6).
Dex is a selective α2-adrenergic receptor
agonist that exert anxiolysis and analgesia effects, without
respiratory depression. The drug has been used for intraoperative
sedation during surgery under regional anesthesia, for sedation to
mechanically ventilated patients in intensive care units, and for
sedation during procedures, including AFOI (7,8). A
previous study demonstrated that Dex and Rem were effective for
sedation in patients undergoing AFOI (9).
Good topical anesthesia is also key to successful
AFOI. Thyrocricocentesis is a useful technique; however, in cases
with huge tumors or wound infections in the neck area,
thyrocricocentesis is contraindicated. Thus, a modified method,
which involves using an epidural catheter threaded through the
suction channel of a fiberoptic bronchoscope to allow the patient
to be sprayed with lidocaine via an epidural catheter onto the
glottis and below the vocal cords, can be a useful alternative.
Furthermore, the modified AFOI technique produces less stimulation
compared with the application of a coarse bronchoscope below the
vocal cords. Previous studies used a fiberoptic bronchoscope to
spray lidocaine onto the glottis and below the vocal cords, which
was termed the spray-as-you-go technique (9,10,11).
Another study also used a fiberoptic bronchoscope to spray onto the
glottis and thyrocricocentesis below the vocal cords (12). In the present study, a modified
AFOI technique involving only a 1.1 mm three-orifice epidural
catheter to spray lidocaine onto the glottis and below the vocal
cords was used. This avoided cricothyroid membrane injection and
the use of a coarse bronchoscope below the vocal cords. Thus, the
present study investigated the efficacy of a modified AFOI method
in cases with anticipated difficult airways. In addition, the
efficacy of Rem and Dex as adjuvants were compared.
Materials and methods
Ethical approval
Following approval from the Institutional Review
Board of the First Affiliated Hospital of Guangzhou University of
Chinese Medicine (Guangzhou, China), written informed consent was
obtained from each patient during the year 2013. The study was also
registered as a clinical trial (http://www.clinicaltrials.gov, identifier:
ChiCTR-TRC-13003151).
Patients
A total of 90 adult patients with an American
Society of Anesthesiologists classification of grade I–II underwent
a modified AFOI procedure following airway evaluation. Patients
were excluded from the study if they were pregnant, under the age
of 18 years, had undergone emergency surgery, had an allergy to any
of the drugs used or were unable to communicate effectively. In
addition, patients were excluded if the surgeon requested nasal
intubation, if the patient refused and/or if the patients were
receiving long-term opioids or sedative medication.
Intubation procedure
An experienced consultant anesthetist, who was
certified in advanced airway life support, performed the airway
management for all the study subjects. While one resident performed
fiberoptic intubation, an additional resident controlled the drug
infusion. Anesthetic data and postoperative follow-ups were
documented by a study nurse. Intubation conditions were graded by
the consultant anesthetist who performed the fiberoptic intubation.
The intubating anesthetist, patients and the study nurse who
recorded the details of the procedures were all blinded to the
study.
The patients were randomized by pharmacy into one of
two groups, which included the Rem and Dex groups. The patients
were randomized at a ratio of 1:1 using a covariate adaptive
randomization algorithm. Study drugs [1 mg remifentanil,
intravenous (IV), Yichang Humanwell Pharmaceutical Co., Ltd.,
Yichang, China; and 200 mcg dexmedetomidine, IV, Jiangsu Hengrui
Medicine Co., Ltd., Lianyungang, China] were prepared in accordance
with the patient weight (kg), and were blinded to the anesthesia
care team (consultant and resident) and the patients. All the
residents had previously performed fiberoptic intubation at least
40 times.
Patients were informed and their consent was
obtained by one of investigators at the preoperative evaluation one
day prior to surgery. During the preoperative evaluation, an
extensive airway examination was performed and the difficulty of
the laryngoscopy or intubation procedure was assessed and assigned
a simplified airway risk index (SARI) score of ≥4. The SARI score,
as described by el-Ganzouri, comprised information regarding
previous airway difficulties, the Mallampati classification,
mobility of the neck, mouth opening, prognathism ability, the
thyromental distance and the body mass index (kg/m2)
(13).
The preparation of patients in each group was
standardized as much as possible. Following pretreatment with
intramuscular injections of 0.1 mg phenobarbital sodium and 0.5 mg
atropine, each patient was moved to the operating room where an
electrocardiogram, a non-invasive blood pressure cuff, a
respiratory rate and a pulse oximeter were placed with a Philips
monitor (Philips 865231; Philips Medizin Systeme Böblingen GmbH,
Böblingen, Germany). The conscious level of the patient was
evaluated using ‘state entropy monitoring’ (Datex-Ohmeda, Helsinki,
Finland).
Anesthesia application
Study drugs were administered in a 50-ml syringe as
200 μg Dex (2 ml) in 48 ml saline (0.9%), or in a 20-ml syringe as
1 mg Rem in 20 ml saline (0.9%). Patients in the Rem group received
a loading dose of 0.75 μg/kg infused at 0.15 μg/kg/min over 5 min,
followed by a continuous infusion of 0.1 μg/kg/min. Patients in the
Dex group received a loading dose of 1 μg/kg infused over 10 min,
followed by a continuous infusion of 0.3 μg/kg/h.
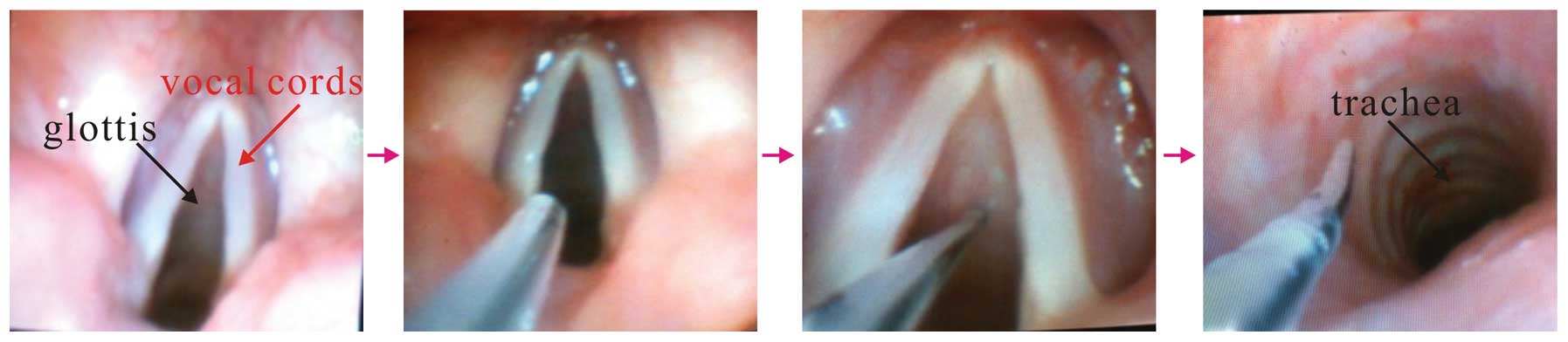
Simultaneously, topical anesthesia was applied.
Patients were asked to keep the lidocaine in their mouth for as
long as possible before swallowing. The patients were administered
4 ml lidocaine (2%) via a laryngeal anesthesia catheter through the
oral cavity and pharynx to reduce the gag reflex. While waiting for
the desired level of sedation to be achieved, an epidural catheter
was threaded through the suction channel of a fiberoptic
bronchoscope (FOB; PENTAX FB-15RBS; Pentax Medical, Tokyo, Japan),
which had an outer diameter of 4.8 mm. A longer (3–4 cm) flexible
fiberoptic was applied in order for the patients to be sprayed with
4 ml lidocaine (2%) via an epidural catheter onto the glottis and
below the vocal cords. This procedure was referred to as modified
topical anesthesia, and avoided cricothyroid membrane injection and
the application of a coarse bronchoscope below the vocal cords
(Fig. 1).
Anesthesia assessment
During the procedure, the anesthesiologists used the
Ramsay Sedation Scale (RSS) to assess the level of sedation of the
patients. If the RSS was <2, rescue doses of up to 20 mg
propofol were administered. In the two groups, drug infusion was
discontinued following successful intubation and general anesthesia
was induced with 1–2 mg/kg IV propofol (Precedex, 200 mg/20 ml;
Corden Pharma S.p.A. Caponago, Italy) and maintained with 4–5
mcg/kg IV fentanyl (Precedex, 100 mcg/2 ml; Yichang Humanwell
Pharmaceutical Co., Ltd.), 1–2% end-tidal isoflurane (Maruishi
Pharmaceutical Co., Ltd., Osaka, Japan) and 1 mg/kg IV vecuronium
(powder; 4 mg; Hainan Star Pharmaceutical Co., Ltd., Haikou, China)
for muscle relaxation. Fiberoptic intubation was initiated once the
RSS reached a score of two. The outer diameter (OD) 4.8-mm FOB was
loaded with an inner diameter (ID) 7.5-mm Parker Flex-Tip tube
(#215075H; Well Lead Medical Instrument Co., Ltd., Guangzhou,
China) for male patients, or an ID 7.0-mm Parker Flex-Tip
(#215070H; Well Lead Medical Instrument Co., Ltd.) for females. An
assistant performed a jaw thrust to expand the oropharyngeal space.
Endotracheal tube placement was confirmed with capnography and
bilateral auscultation. The primary endpoint was the time to
tracheal intubation (TTI), as confirmed by capnography and measured
by the advancement of the flexible fiberscope behind the teeth
until the appearance of a capnography curve.
Clinical outcome assessment
Primary outcomes measured included the intubation
scores, as assessed by coughing (1, none; 2, slight; 3, moderate;
and 4, severe) and limb movement (1, none; 2, slight, 3, moderate;
and 4, severe). Secondly, patient tolerance was assessed by
intubation comfort scores (1, no reaction, no change or a single
change in the facial expression; 2, slight reaction, grimacing
facial expressions; 3, moderate reaction, severe facial grimace but
retained ability to follow verbal command and no reflex head
movements; 4 severe reaction, severe facial grimace associated with
head movements, but patient remains able to obey verbal commands;
5, very severe reaction, severe facial grimace associated with
protective head and limb movements hindering the procedure and an
inability to obey any verbal command; 6, uncooperative) (14). Furthermore, a three-point scale was
used to assess the clinical outcome immediately following the
tracheal intubation (1, cooperative; 2, restless with minimal
resistance; 3, severe resistance with immediate application of
general anesthesia). The lower the score, the better the patient
condition. Once tracheal intubation was complete and the tracheal
tube was secured, general anesthesia was administered.
Additional anesthetic parameters associated with the
modified AFOI method included the conscious level (state entropy
value and RSS level), airway obstruction score (1, patent airway;
2, airway obstruction relieved by neck extension; 3, airway
obstruction requiring jaw retraction) and the consumption of the
study drugs. In addition, the intubation time (time period between
FOB insertion and the confirmation of tracheal intubation), the
hypoxic episode (SpO2 of <90%) and the use of rescue
doses for conscious level support were recorded. Hemodynamic
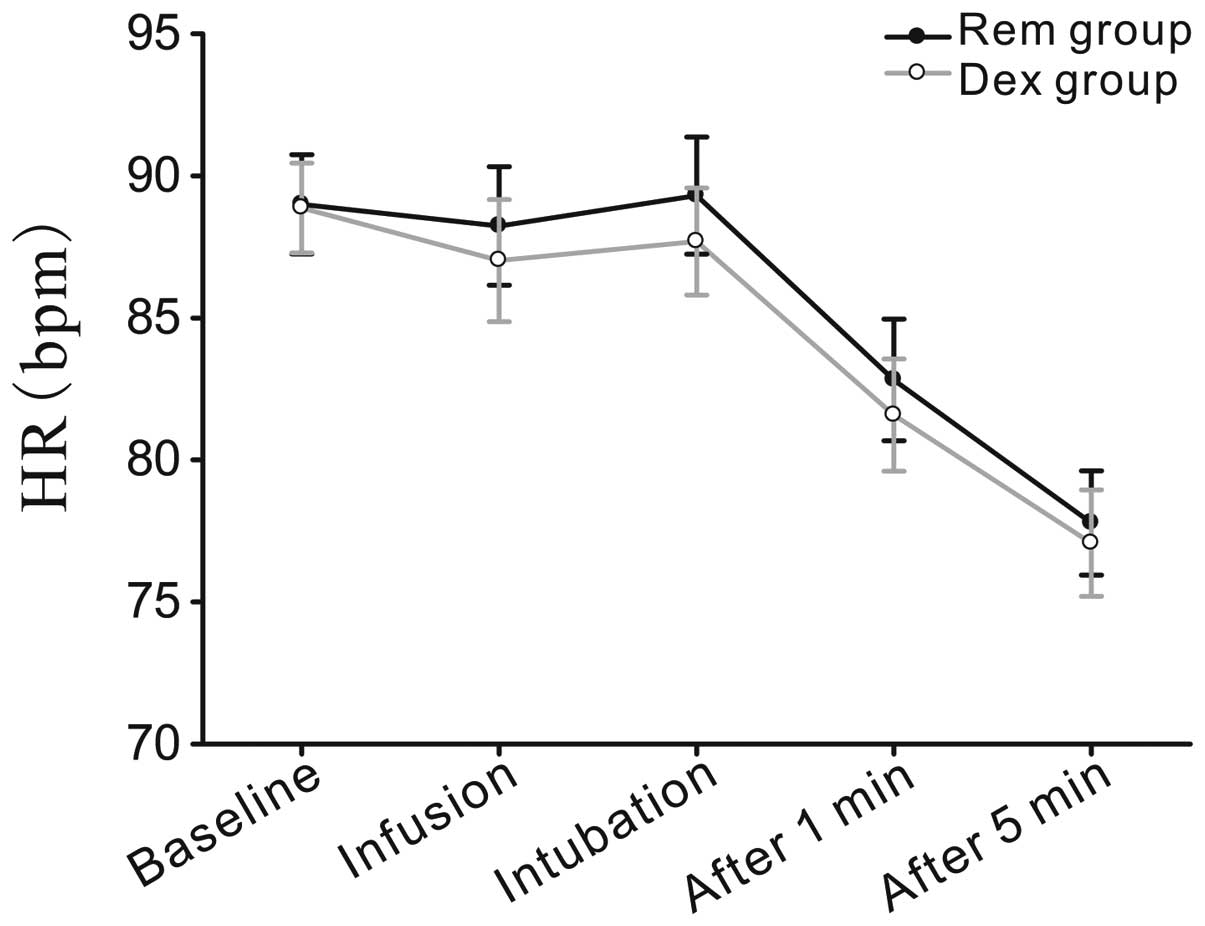
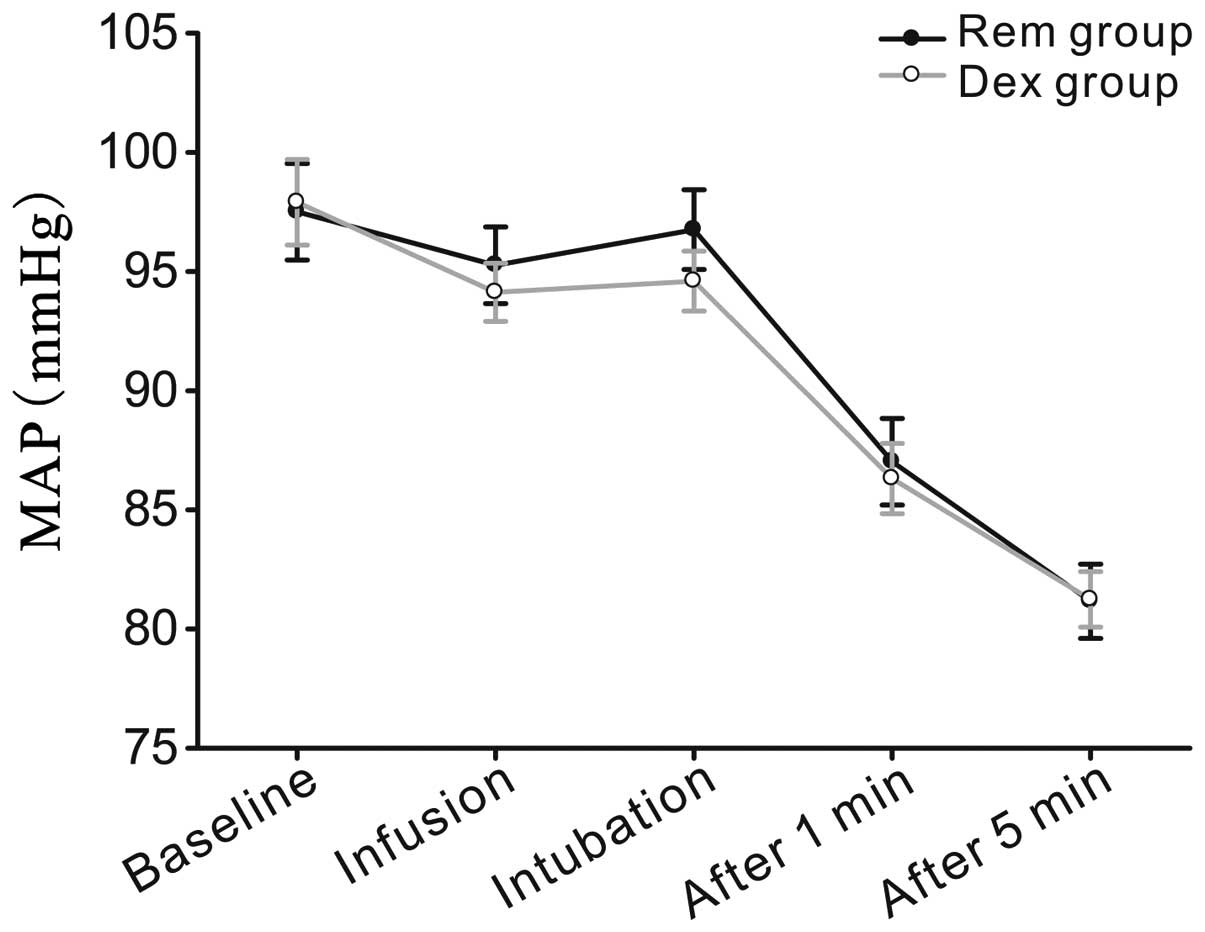
changes (heart rate and mean arterial blood pressure) were compared
between the two groups at five time points during the modified AFOI
procedure, including at the baseline (preanesthetic preparation),
at infusion (immediately prior to fiberoptic intubation), at
intubation and at 1 and 5 min after tracheal intubation.
A postoperative follow-up was conducted the day
following surgery, and amnesia (memory of preanesthetic
preparations, topical anesthesia, endoscopy and intubation),
incidence of adverse events (hoarseness and sore throat) and
satisfaction score (1, excellent, 2, good, 3, fair, 4, poor) were
assessed.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Normally distributed and
continuous variables are presented as the mean ± standard deviation
or mean ± standard error of the mean. Continuous data were compared
using an unpaired t-test, while the Mann-Whitney U test was used to
compare non-continuous data and non-normally distributed data.
Intragroup comparisons of hemodynamic data at the various time
points were performed using repeated measures analysis of variance.
Where statistical significance was determined, Fisher’s protected
least significant difference post hoc test was applied. The
χ2 test or Fisher’s exact test were used to compare the
categorical data between the two groups. Sample size calculation
was based on a pilot study. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient clinical data
In total, 90 patients completed the study. No
statistically significant differences were observed between the
baseline data of the two groups (Table
I). The modified SARI score was 4.5±2.7 in the Rem group and
4.3±2.6 in the Dex group (Table
I). SARI scores represented the sum of the individual risk
factor weightings. All of the patients had no history of anesthesia
usage; however, the preoperative interview indicated a suspicion of
a difficult laryngoscopy intubation.
 | Table IDemographic data of the patients. |
Table I
Demographic data of the patients.
| Characteristics | Rem group | Dex group |
|---|
| Age, years | 45.9±11.7 | 41.5±12.8 |
| Weight, kg | 61.9±11.1 | 63.6±11.0 |
| Height, cm | 162.72±6.5 | 166.5±7.1 |
| BMI,
kg/m2 | 23.5±3.3 | 22.9±3.4 |
| ASA status | 1.7±0.5 | 1.6±0.5 |
| Modified SARI | 4.5±2.7 | 4.3±2.6 |
Assessment of intubating conditions
All the patients underwent a successful fiberoptic
intubation. As shown in Table II,
three patients in the Rem group and two patients in the Dex group
required a rescue infusion of propofol to achieve adequate
sedation. The mean time to achieve sedation with Rem was 531.2 sec,
while for Dex, the mean time was 673.1 sec. Thus, the TTI was lower
in the Rem group compared with the Dex group. During endoscopy
insertion, the Rem group exhibited more favorable intubation scores
with regard to coughing. However, no statistically significant
differences were observed in the sedation scale, intubation times
and patient reactions when comparing the two groups.
 | Table IIAnesthetic data during the modified
AFOI procedure. |
Table II
Anesthetic data during the modified
AFOI procedure.
| Intubation
scores | Rem group | Dex group |
|---|
| Cough, 1/2/3/4,
n | 23/17/4/1a | 19/16/7/3 |
| Movement, 1/2/3/4,
n | 23/13/7/2 | 21/14/7/3 |
| Intubation time,
sec | 52.0±20.2 | 50.1±28.3 |
| Drug requirements,
μg | 137.4±47.6a | 61.4±15.2 |
| RSS at
intubation | 2.2±0.7 | 2.3±0.6 |
| State entropy at
intubation | 88.1±0.7 | 89.2±1.1 |
| Rescue requirement
for consciousness, n (%) | 3 (10.0) | 2 (6.7) |
| Time to tracheal
intubation, sec | 531.2±7.2a | 673.1±8.3 |
Adverse events in patients during
procedure
In total, four patients from the Rem group and three
patients in the Dex group were associated with severe airway
obstruction, with five patients from the Rem group and four
individuals from the Dex group developing transient hypoxia
(Table III). An additional
patient from each group exhibited transient hypoxia due to a
sensitivity to the drugs. In the Rem group, the SpO2
values of the patients decreased to 88–90%, while their respiratory
rates decreased to 8 or 9 bpm. Four patients in the Dex group had
SpO2 values that decreased to 88%, with the respiratory
rate decreasing to 10 bpm. Loud auditory stimuli and high-flow mask
oxygen (8 l/min) were required to resolve the transient hypoxia.
The mean respiratory rate with Rem was 12 bpm, and 11 bpm with Dex.
No serious complications occurred in the two groups throughout the
AFOI procedures.
 | Table IIIAdverse events in patients receiving
Rem or Dex during modified AFOI. |
Table III
Adverse events in patients receiving
Rem or Dex during modified AFOI.
| Adverse event | Rem group | Dex group |
|---|
| Airway obstruction
score, 1/2/3, n | 34/7/4 | 35/7/3 |
| Hypoxia, n (%) | 5 (11.1) | 4 (8.9) |
| Respiratory rate,
bpm | 12±3.4 | 11±3.9 |
Hemodynamic level
Heart rate and mean arterial pressure were evaluated
at five time points, as shown in Figs.
2 and 3. No statistically
significant differences were observed between the predicted mean
values for the Rem and Dex groups.
Postoperative follow-up
A postoperative follow-up examination was conducted
the day following surgery, and the interview parameters are shown
in Table IV. With regard to
patient tolerance, the lowest median (interquartile range) comfort
score during the procedure was 2 (1–2) for
the Dex and Rem groups (P>0.05). The levels of memory for
preanesthetic events, topical anesthesia, endoscopy and intubation
were 82.2, 57.8 and 26.7%, respectively, in the Dex group compared
with 88.9, 62.2 and 31.1%, respectively, in the Rem group
(P>0.05). The occurrence of postoperative adverse events did not
differ significantly between the two groups (Table III).
 | Table IVPostoperative follow-up data. |
Table IV
Postoperative follow-up data.
| Follow-up
parameters | Rem group | Dex group |
|---|
| Sore throat, n
(%) | 10 (22.2) | 11 (24.4) |
| Hoarseness, n
(%) | 3 (6.7) | 2 (4.4) |
| Satisfaction score
(1–4) | 2 (1–2) | 2 (1–2) |
| Recall of topical
anesthesia, n (%) | 40 (88.9) | 37 (82.2) |
| Recall of
endoscopy, n (%) | 28 (62.2) | 26 (57.8) |
| Recall of
intubation, n (%) | 14 (31.1) | 12 (26.7) |
Discussion
The primary aims of the present study were to
determine whether there were differences in the safety and efficacy
of using remifentanil (Rem) and dexmedetomidine (Dex) regimens for
sedation during modified AFOI. The modified AFOI method uses an
improved topical anesthesia technique, where the drug is sprayed
into the airway through an epidural catheter that has been passed
through the suction channel of a FOB (15). The modified procedure avoids
cricothyroid membrane injections and coarse bronchoscope
application below the vocal cords (Fig. 1), which is particularly important
for patients with large neck tumors, infection or who are unable to
bear the stimulation of the coarse bronchoscope.
The current study demonstrated relatively similar
efficacy of Rem and Dex as adjuvants to modified AFOI. During
endoscopy insertion, the Rem group exhibited more favorable
intubation scores with regard to coughing. However, there were no
statistically significant differences in the sedation scale,
intubation times and the patient reaction in the two groups.
Recently, Hu et al (9)
compared Rem and Dex treatment and demonstrated that Dex therapy
results in improved endoscopy scores. The two differing results may
due to the topical anesthesia applied. Hu et al advanced the
tip of the FOB into the site below the glottis to prevent the FOB
from slipping out of the trachea when spraying the endotracheal
region, as a result of coughing or movement. However, in the
present study, an epidural catheter was used for translaryngeal
spraying of lidocaine, since a previous study had demonstrated that
this method can produce a more effective airway topical anesthesia
for fiberoscopy due to the more proximal site in the airway
(12). The technical spreading of
lidocaine through cricothyroid membrane injections is achieved
primarily by the coughing of the patient immediately after the
injection. Xue et al hypothesized that it was impossible to
ensure that lidocaine was well-distributed along the infraglottic
area and tracheal wall using this method (11).
During AFOI, it is crucial that the patient is
relaxed and cooperative. Thus, conscious sedation is important. A
number of studies have reported success with various agents,
including Rem, Dex, midazola, propofol and ketamine. However Rem
and Dex have been demonstrated to be more efficient compared with
others (1–9).
Rem provides profound analgesia, suppresses airway
reflexes and has minimal effect on cognitive function (6). These characteristics make the drug
useful as an adjunct and also as a primary agent to provide
sedation during AFOI (2,5,7,9,13,12).
Vennila et al (16)
revealed the mean effective site concentration for Rem as 6.3±3.87
ng/ml during nasal endoscopy and 8.06±3.52 ng/ml during tracheal
intubation, as a single agent without the use of other
sedatives/premedication and/or spray-as-you-go local
anesthesia.
Dex activates the postsynaptic
α2-adrenergic receptors in the locus coeruleus, and
induced conscious sedation involves activation of the endogenous
sleep-promoting pathway. In addition, Dex exhibits analgesic,
anxiolytic and antisialagogue properties (17,18).
Chu et al (10)
demonstrated that combining Dex loading with topical anesthesia
provides a significant benefit for AFOI with regard to intubation
conditions, patient tolerance and hemodynamic parameters.
In the present study, the patients in the Dex group
had a delayed intubation start time, possibly due to the different
mechanisms of sedation between the two agents. The optimum sedation
dose of Dex for AFOI has not been established, although a loading
dose between 0.4 μg/kg and 1 mg/kg over a minimum of 10 min has
been used to attain sedation. Cattano et al selected lower
loading doses, which resulted in insufficient sedation and
analgesia for a successful first attempt at AFOI (19). In the present study, a relatively
high loading dose of 1 μg/kg over 10 min was applied, followed by a
lower infusion rate of 0.3 μg/kg/h. The optimum drug dose for a
sedative to achieve a careful balance of airway relaxation versus
collapse is difficult to ascertain. Patients in the Rem group
received a loading dose of 0.75 μg/kg infused at 0.15 μg/kg/min
over 5 min, followed by a continuous infusion of 0.1 μg/kg/min. An
RSS score of two was achieved almost immediately following the
loading dose of the two groups. Dex administration achieved an RSS
score of two at a slower rate compared with Rem. Four patients in
the Rem and three patients in the Dex groups experienced severe
airway obstruction, with five patients from the Rem and four
patients from the Dex groups developing transient hypoxia (Table III). The further patient from
each group exhibited transient hypoxia due to a sensitivity to the
drugs. An additional patient in the Rem group exhibited transient
hypoxia due to sensitivity to the drug. Loud auditory stimuli and
high-flow mask oxygen (8 l/min) were required to resolve transient
hypoxia. In the study by Scher and Gitlin, 1 μg/kg Dex was applied;
however, a 15-mg ketamine bolus was also administered, followed by
an infusion at 20 mg/h to achieve excellent intubating conditions
for AFOI, including satisfactory sedation, patient cooperation and
a dry airway (20). By contrast,
Belda et al stated that addition of ketamine to Rem
target-controlled infusion did not offer any advantages, and
ketamine administration alone was not adequate sedation for AFOI
(5).
With regard to hemodynamic stability, no
statistically significant differences were observed in the mean
arterial pressure and heart rate during intubation for the Dex or
Rem groups. Hu et al reported statistically significant
differences in the heart rate at the end of endoscopy and
intubation, but no significant differences in the mean arterial
pressure between the Rem and Dex groups at any time point (9). However, Hu et al used larger
doses of Dex compared with the present study. The difference in
results between the study by Hu et al and the present study
may be due to the method of local anesthesia used as the modified
AFOI technique in the current study used only a 1.1 mm
three-orifice epidural catheter onto the glottis and below the
vocal cords. It avoided using cricothyroid membrane injection and a
coarse bronchoscope below the vocal cords, which may have reduced
the release of noradrenaline (21). Dex infusion may cause adverse
effects, including hypotension, hypertension, nausea, bradycardia,
atrial fibrillation and hypoxia (22). In the present study, a
SpO2 of <90% was observed in two patients from the
Dex group and three patients in the Rem group. The symptom was
easily managed by asking the patients to force inspiration, and the
condition of all the patients improved. The intubation time and
postoperative sore throat and hoarseness did not differ
significantly between the two groups.
In the results of the present study, state entropy
values of the two groups revealed consciousness levels comparable
with clinical observation at tracheal intubation (state entropy,
~89; Table II). Patients
receiving the Rem infusion revealed a comparative incidence of
amnesia to topical anesthesia (88.9 vs. 86.7%), endoscopy (62.2 vs.
57.8%) and intubation (31.1 vs. 26.7%) when compared with the Dex
group. In the present study, Dex loading did not decrease the state
entropy values to a greater extent compared with Rem.
In conclusion, the Rem and Dex regimes utilized in
the present study provided satisfactory intubating conditions and
patient satisfaction in the majority of patients undergoing the
modified AFOI procedure. Comparable upper airway patency to awake
patients was observed, and only temporary hemodynamic adverse
effects occurred in the patients. These properties indicate that
Dex and Rem are useful drugs for providing conscious sedation;
however, one limitation is that their administration may be
associated with a greater incidence of recall.
The present study demonstrated that modified AFOI is
a feasible and effective method for dealing with difficult airways,
while Rem and Dex administration for sedation exert a similar
efficacy.
Abbreviations:
|
AFOI
|
awake fiberoptic tracheal
intubation
|
|
Rem
|
remifentanil
|
|
Dex
|
dexmedetomidine
|
|
TTI
|
time to tracheal intubation
|
|
SARI
|
simple airway risk index
|
|
FOB
|
fiberoptic bronchoscope
|
|
RSS
|
Ramsay Sedation Scale
|
References
|
1
|
Cafiero T, Esposito F, Fraioli G, et al:
Remifentanil-TCI and propofol-TCI for conscious sedation during
fibreoptic intubation in the acromegalic patient. Eur J
Anaesthesiol. 25:670–674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Péan D, Floch H, Beliard C, et al:
Propofol versus sevoflurane for fiberoptic intubation under
spontaneous breathing anesthesia in patients difficult to intubate.
Minerva Anestesiol. 76:780–786. 2010.PubMed/NCBI
|
|
3
|
Lallo A, Billard V and Bourgain JL: A
comparison of propofol and remifentanil target-controlled infusions
to facilitate fiberoptic nasotracheal intubation. Anesth Analg.
108:852–857. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boehm CA, Carney EL, Tallarida RJ, et al:
Midazolam enhances the analgesic properties of dexmedetomidine in
the rat. Vet Anaesth Analg. 37:550–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belda I, Cubas MG, Rivas E, et al:
Remifentanil target controlled infusion (TCI) vs ketamine or
ketamine in combination with remifentanil TCI for conscious
sedation in awake fiberoptic intubation: A randomized controlled
trial: 19AP1-5. Eur J Anaesthesiol. 28:2262011. View Article : Google Scholar
|
|
6
|
Bürkle H, Dunbar S and Van Aken H:
Remifentanil: a novel, short acting, mu-opoid. Anesth Analg.
83:646–651. 1996.
|
|
7
|
Bergese SD, Khabiri B, Roberts WD, et al:
Dexmedetomidine for conscious sedation in difficult awake
fiberoptic intubation cases. J Clin Anesth. 19:141–144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Madhere M, Vangura D and Saidov A:
Dexmedetomidine as sole agent for awake fiberoptic intubation in a
patient with local anesthetic allergy. J Anesth. 25:592–594. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu R, Liu JX and Jiang H: Dexmedetomidine
versus remifentanil sedation during awake fiberoptic nasotracheal
intubation: a double-blinded randomized controlled trial. J Anesth.
27:211–217. 2013. View Article : Google Scholar
|
|
10
|
Chu KS, Wang FY, Hsu HT, et al: The
effectiveness of dexmedetomidine infusion for sedating oral cancer
patients undergoing awake fibreoptic nasal intubation. Eur J
Anaesthesiol. 27:36–40. 2010. View Article : Google Scholar
|
|
11
|
Xue FS, Liu HP, He N, et al:
Spray-as-you-go airway topical anesthesia in patients with a
difficult airway: a randomized, double-blind comparison of 2% and
4% lidocaine. Anesth Analg. 108:536–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Webb AR, Fernando SS, Dalton HR, et al:
Local anaesthesia for fibreoptic bronchoscopy: transcricoid
injection or the ‘spray as you go’ technique? Thorax. 45:474–477.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
el-Ganzouri AR, McCarthy RJ, Tuman KJ, et
al: Preoperative airway assessment: predictive value of a
multivariate risk index. Anesth Analg. 82:1197–1204.
1996.PubMed/NCBI
|
|
14
|
Kundra P, Kutralam S and Ravishankar M:
Local anaesthesia for awake fibreoptic nasotracheal intubation.
Acta Anaesthesiol Scand. 44:511–516. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez A, Iyer RR and Morrison DE:
Preparation of the patient for awake intubation. Benumof’s Airway
Management; Principles and Practice. Hagberg CA: 2nd edition.
Mosby-Year Book, Inc; St. Louis, MO: pp. 263–277. 2007
|
|
16
|
Vennila R, Hall A, Ali M, et al:
Remifentanil as single agent to facilitate awake fibreoptic
intubation in the absence of premedication. Anesthesia. 66:368–372.
2011. View Article : Google Scholar
|
|
17
|
Jaakola ML, Ali-Melkkilä T, Kanto J, et
al: Dexmedetomidine reduces intraocular pressure, intubation
responses and anaesthetic requirements in patients undergoing
ophthalmic surgery. Br J Anaesth. 68:570–575. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Venn RM, Bradshaw CJ, Spencer R, et al:
Preliminary UK experience of dexmedetomidine, a novelagent for
postoperative sedation in the intensive care unit. Anaesthesia.
54:1136–1142. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cattano D, Lam NC, Ferrario L, et al:
Dexmedetomidine versus remifentanil for sedation during awake
fiberoptic intubation. Anesthesiol Res Pract.
2012:7531072012.PubMed/NCBI
|
|
20
|
Scher CS and Gitlin MC: Dexmedetomidine
and low-dose ketamine provide adequate sedation for awake
fibreoptic intubation. Can J Anaesth. 50:607–610. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bloor BC, Ward DS, Belleville JP and Maze
M: Effects of intravenous dexmedetomidine in humans. II Hemodynamic
changes. Anesthesiology. 77:1134–1142. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ebert TJ, Hall JE, Barney JA, et al: The
effects of increasing plasma concentrations of dexmedetomidine in
humans. Anesthesiology. 93:382–394. 2000. View Article : Google Scholar : PubMed/NCBI
|