Introduction
For patients with acute myocardial infarction, early
reperfusion of the ischemic myocardium, either through thrombolytic
therapy or primary percutaneous coronary intervention, is the most
effective treatment to limit the infarct size (IS) and improve the
clinical outcome; however, the process of restoring blood flow to
the ischemic myocardium has the potential to induce additional
lethal injuries and reduce myocardial salvage. Inflammatory cells
and cytokines play an important role in the pathogenesis of
myocardial reperfusion injury, not only by triggering deleterious
responses but also by amplifying ongoing responses to build a
cascade of injury (1). Numerous
experimental studies have demonstrated the efficacy of
anti-inflammation therapies against myocardial ischemia/reperfusion
(I/R); however, the translation of these beneficial effects into
the clinical setting has been disappointing (1,2). The
efficacy that has been shown for most anti-inflammatory agents in
animal models has been difficult to confirm in clinical trials. The
trigger and the mechanism of inflammation propagation warrant
further studies.
It has previously been shown that high-mobility
group box-1 (HMGB1) is a potent innate ‘danger signal’. HMGB1 has
been demonstrated to initiate and amplify the inflammatory response
following I/R injury (3,4,5).
Recent studies have shown that HMGB1, acting via the receptor for
advanced glycation end products (RAGE) or members of the Toll-like
family of receptors (TLRs), is an early mediator of organ damage in
myocardial I/R injury (4,5). Blockage of HMGB1 with HMGB1 box A, a
specific antagonist of whole HMGB1 on the RAGE receptor, or
anti-HMGB1 monoclonal antibody has been proved to exert benefit
against I/R injury (6,7); however, these antagonists are
expensive and their source is limited. Ethyl pyruvate (EP), the
first described pharmacological inhibitor of HMGB1 secretion
(8), has been revealed to protect
various organs against I/R injury in vivo, including the
kidney and liver (9,10). The aims of the present study were
to investigate the role of HMGB1 in a myocardial I/R model, to
explore the association between HMGB1 and the inflammatory
mediators and to determine the benefit of EP.
Materials and methods
Animal care
Male Sprague Dawley rats (220–250 g) were purchased
from the National Rodent Laboratory Animal Resources, Shanghai
Branch (Shanghai, China). The animals were housed under standard
conditions, maintained under a diurnal 12-h light/dark cycle and
fed standard rat food and water ad libitum. The present
study was performed in accordance with the guidelines of the
National Institutes of Health (NIH) Guide for the Care and Use of
Laboratory Animals (NIH publication no. 85–23, revised 1996) and
was approved by the Animal Care and Use Committee of Fujian Medical
University (Fuzhou, China).
In vivo myocardial I/R model
The rats were anesthetized with pentobarbital sodium
(50 mg/kg, intraperitoneal). Additional doses were administered as
required to maintain anesthesia. The rats were intubated and
ventilated with 100% oxygen. The chest was opened via a left
thoracotomy through the fourth or fifth intercostal space and the
hearts were exposed. An 8-0 silk ligature was placed under the left
coronary artery (LCA) and tied using a shoestring knot. Myocardial
ischemia was confirmed by the presence of discoloration of the
ischemic area, left ventricular (LV) dyskinesia and ST-segment
elevation on an electrocardiogram. Following occlusion for 30 min,
reperfusion was initiated by releasing the knot. Reperfusion was
confirmed by the return of color to the ischemic area. The loosened
suture was left in place and then retied for the purpose of
evaluating the ischemic area. The chest wall was closed, the animal
extubated and body temperature maintained by use of a 37°C warm
plate. In the sham-operated animals, the same procedure was
performed with the exception of the coronary artery ligation. The
rats were sacrificed and the hearts were harvested 48 h after
reperfusion.
Treatment preparation
EP (Sigma-Aldrich, St. Louis, MO, USA) was dissolved
in a Ringer’s lactate solution (RLS; TianRui Pharmaceutical Group
Co., Ltd., Rui’an, China) containing sodium (130 mmol/l), potassium
(4 mmol/l), calcium (2.7 mmol/l) and chloride (139 mmol/l) ions (pH
7.0). EP was administered intravenously and the injection volume
was 0.5 ml per dose. Recombinant human HMGB1 (rhHMGB1; BD
Biosciences, Franklin Lakes, NJ, USA) was diluted with
phosphate-buffered saline (PBS) with a total volume of 0.5 ml.
Protocol assignment
The rats were divided into two groups, protocol 1
and protocol 2. The aim of protocol 1 was to determine the effect
of HMGB1 on myocardial reperfusion injury in vivo. The rats
in the protocol 1 group were further assigned to one of four groups
(n=16 per group): rhHMGB1 (three doses) or PBS. After 30 min
myocardial ischemia, rhHMGB1 (1, 10 or 100 μg/kg) was injected
intravenously 1 min prior to reperfusion; 0.5 ml PBS was used as a
vehicle in the vehicle group.
The aim of protocol 2 was to determine the effect of
EP on cardiac function. The rats assigned to protocol 2 were
further subdivided into four groups (n=16 per group): i) Sham
(surgical procedures were performed but ischemia was not applied);
ii) Control (0.5 ml RLS and 0.5 ml PBS were coadministered
intravenously 2 min prior to reperfusion); iii) EP (40 mg/kg EP was
administered intravenously 2 min prior to reperfusion); iv) EP +
rhHMGB1 (40 mg/kg EP and 100 μg/kg rhHMGB1 were coadministered
intravenously 2 min prior to reperfusion).
Determination of LV area at risk (AAR)
and IS
At the end of the 48-h reperfusion period, the LCA
was religated at the original site, and 1 ml 2% Evans blue dye
(Sigma-Aldrich) was injected via the external jugular vein. The
heart was quickly excised and the atria, right ventricle and fatty
tissues were removed. The left ventricle was sliced transversely
into 4–5 slices (~2-mm). The sections were then incubated in PBS
containing 2% triphenyltetrazolium chloride (Sigma-Aldrich) at 37°C
for 20 min. The AAR was shown by an absence of staining with Evans
blue. The AAR was separated from the nonischemic myocardium and
incubated in a 37°C 1% solution of buffered (pH 7.4)
triphenyltetrazolium chloride for 15 min. The AAR was then stored
in vials of 10% formaldehyde overnight, and the infarcted
myocardium was dissected from the AAR. The IS, AAR and LV weight
were determined gravimetrically. The AAR was expressed as a
percentage of the LV weight (AAR/LV weight), and the IS was
expressed as a percentage of the AAR (IS/AAR) (11).
LV function measurements. In vivo
cardiac hemodynamic function was measured with an
LMS-2B dual-trace physiological recorder (Chengdu Instrument
Factory, Chengdu, China) 48 h after reperfusion. The rats were
anesthetized with 2% isoflurane; a micromanometer was inserted into
the right carotid. Heart rate and the peak LV systolic pressure
(LVSP) and LV end-diastolic pressure were measured using this
catheter advanced into the LV cavity; the maximal slopes of
systolic pressure increase (+dp/dt max) and diastolic pressure
decrease (−dp/dt max) were analyzed.
Western blot analysis
The total protein was extracted from the AAR of the
heart. Nuclear and cytoplasmic proteins were isolated using a
Nuclear Extraction kit from Active Motif, Inc. (Carlsbad, CA, USA)
according to the manufacturer’s instructions. Extracts were frozen
in liquid nitrogen and stored at −80°C for subsequent western blot
analyses. The protein concentration was quantified using a protein
assay kit from Bio Rad Laboratories (Hercules, CA, USA).
Cytoplasmic or nuclear extracts (50 μg) were mixed with 2X sodium
dodecyl sulfate (SDS) sample buffer and separated via 15% [for
HMGB1, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)]
SDS-polyacrylamide gel electrophoresis. The separated proteins were
transferred onto Hybond™ enhanced chemiluminescence membranes (GE
Healthcare Life Sciences, Pittsburgh, PA, USA). The membranes were
then incubated at room temperature for 1 h with 5% skimmed milk in
Tris-buffered saline containing 0.1% Tween 20 (TBST) in order to
block nonspecific reactions. Following blocking, the membranes were
incubated overnight with primary antibody. The primary antibodies
used were as follows: Rabbit polyclonal anti-HMGB1 (1:1,000
dilution; #ab191583; Abcam, Cambridge, MA, USA), mouse monoclonal
anti-TNF-α (1:1,000 dilution; #ab1793; Abcam) and mouse monoclonal
anti-IL-6 (1:2,000 dilution; #MAB406; R&D Systems, Inc.,
Minneapolis, MN, USA). Subsequent to washing with TBST, the
membrane was incubated for 1 h in 5% skimmed milk and secondary
antibody (goat anti-rabbit or goat anti-mouse; 1:2,500; Abcam)
conjugated to horseradish peroxidase. The immunoreactions were
visualized using enhanced chemiluminescence according to the
manufacturer’s instructions (Merck Millipore, Darmstadt, Germany).
The protein signals were quantified using scanning densitometry,
and the results from each experimental group were expressed as a
relative integrated intensity compared with glyceraldehyde
3-phosphate dehydrogenase.
Statistical analysis
Data are presented as the mean ± standard error. IS
and the cardiac function parameters were analyzed using the
Student’s t-test or one-way analysis of variance followed by a
Least Significant Difference test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed using the SPSS statistical software version
13.0 (SPSS, Inc., Chicago, IL, USA).
Results
Mortality and exclusion of animals
Of the 128 rats used in the study, 13 died during
the surgical procedures due to bradycardia or ventricular
tachycardia and three were excluded as a result of severe
hypotension. Complete data sets were obtained from the remaining
112 rats.
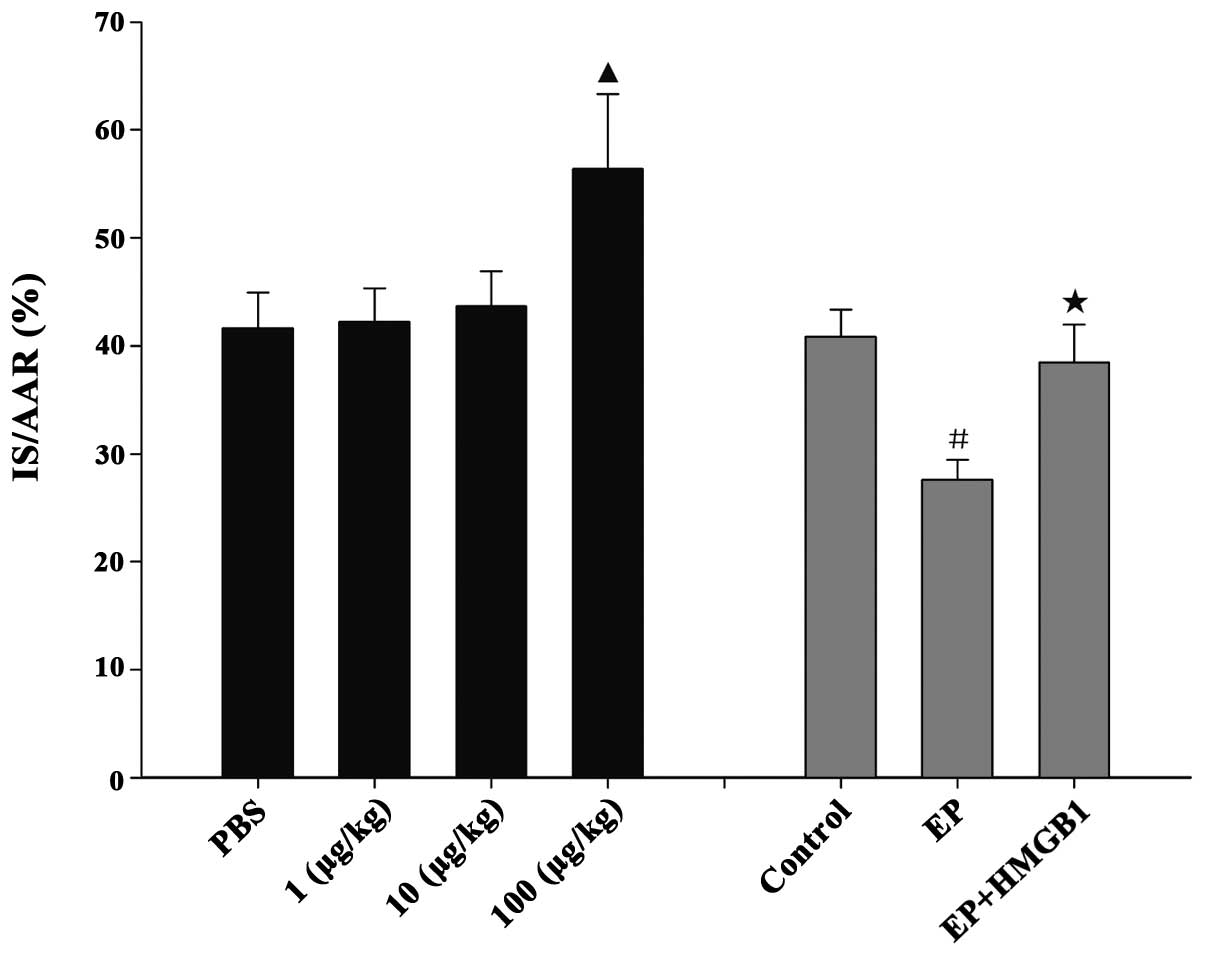
AAR and IS
The AAR/LV weight was comparable among the groups.
In protocol 1, no significant difference in IS was detected among
the PBS, 1 and 10 μg/kg rhHMGB1 groups (IS/AAR 41.6±3.3, 42.3±3.1
and 43.7±3.2% respectively; P>0.05, Fig. 1). Compared with the PBS group,
administration of rhHMGB1 at a dose of 100 μg/kg resulted in a
significant increase in IS/AAR (56.4±6.9% vs. PBS group, P<0.05,
Fig. 1). In protocol 2, the IS was
40.8±2.5% of the AAR in the control group after 30 min ischemia
followed by 48 h reperfusion. EP induced a significant reduction in
the IS/AAR to 27.7±1.8% (P<0.05, Fig. 1); however, the addition of rhHMGB1
blocked the IS-limiting effects of EP (38.5±3.5% vs. EP group,
P<0.05).
LV function parameters
In protocol 1, no significant difference was found
in the LVSP, +dp/dt max or -dp/dt max among the PBS, 1 and 10 μg/kg
rhHMGB1 groups (P>0.05, Table
I). Injection of 100 μg/kg rhHMGB1 caused a significant
deterioration in cardiac function (P<0.05, Table I).
 | Table IHemodynamic function of rats 48 h
after ischemia/reperfusion. |
Table I
Hemodynamic function of rats 48 h
after ischemia/reperfusion.
| Group | HR (bmp/min) | LVSP (mmHg) | +dp/dt max
(mmHg/sec) | −dp/dt max
(mmHg/sec) |
|---|
| Protocol 1 |
| PBS | 386±17 | 99±3 | 2890±217 | 1979±190 |
| 1 μg/kg HMGB1 | 394±11 | 97±3 | 2937±233 | 2007±219 |
| 10 μg/kg HMGB1 | 390±20 | 98±4 | 2744±261 | 2020±301 |
| 100 μg/kg HMGB1 | 389±20 | 85±3a | 2260±156a | 1588±119a |
| Protocol 2 |
| Sham | 382±16 | 131±7 | 4674±282 | 3531±243 |
| Control | 395±17 | 101±5 | 2768±219 | 2102±155 |
| EP | 386±16 | 116±4b | 3661±379b | 2821±144b |
| EP + HMGB1 | 382±20 | 104±4c | 3044±320c | 2347±226c |
In protocol 2, treatment with EP prior to
reperfusion preserved the cardiac function. The LVSP, +dp/dt max
and -dp/dt max were significantly improved in the EP group compared
with the control group (P<0.05; Table I). When rhHMGB1 was coadministered
with EP, the cardioprotective effect was blocked (Table I).
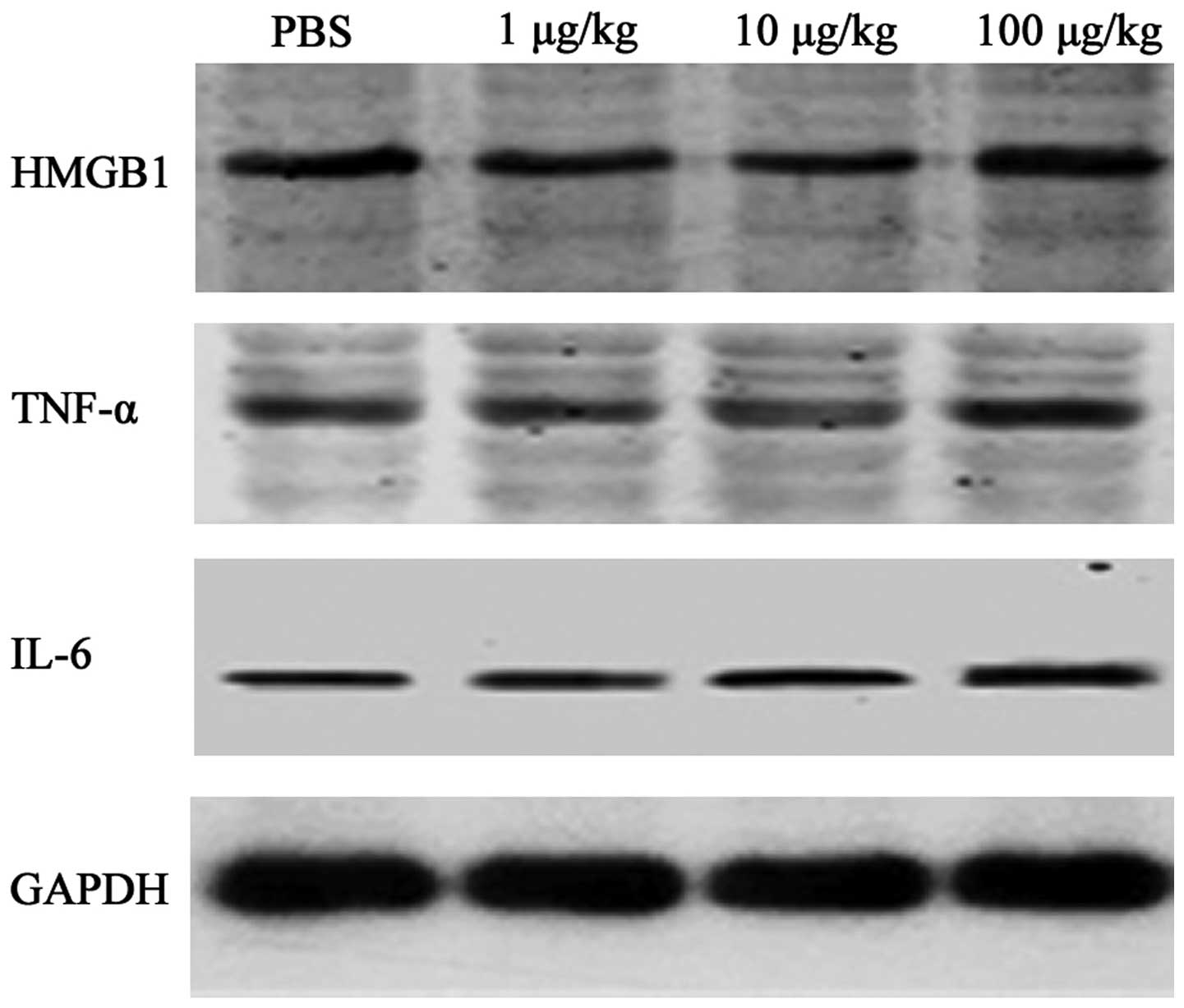
Inflammatory cytokine expression
following myocardial I/R
Western blotting was performed to assess the effect
of rhHMGB1 on TNF-α and IL-6 expression. I/R resulted in a marked
elevation in TNF-α and IL-6 levels. No significant increase in
TNF-α or IL-6 was detected when 1 or 10 μg/kg rhHMGB1 was injected.
When the dose of rhHMGB1 was increased to 100 μg/kg, the expression
of TNF-α (0.65±0.06 vs. 0.47±0.05, P<0.05) and IL-6 (0.60±0.08
vs. 0.46±0.06, P<0.05) was markedly elevated compared with that
in the PBS group (Fig. 2).
EP inhibits HMGB1 and inflammatory
cytokine expression following myocardial I/R
Compared with the sham group, HMGB1 expression was
significantly increased in the control group 48 h after reperfusion
(Fig. 3). Compared with the
control group, administration of 40 mg/kg EP significantly
inhibited the secretion of HMGB1 and decreased the inflammatory
cytokine expression, but this protective effect was abrogated by
the injection of rhHMGB1 (Fig.
3).
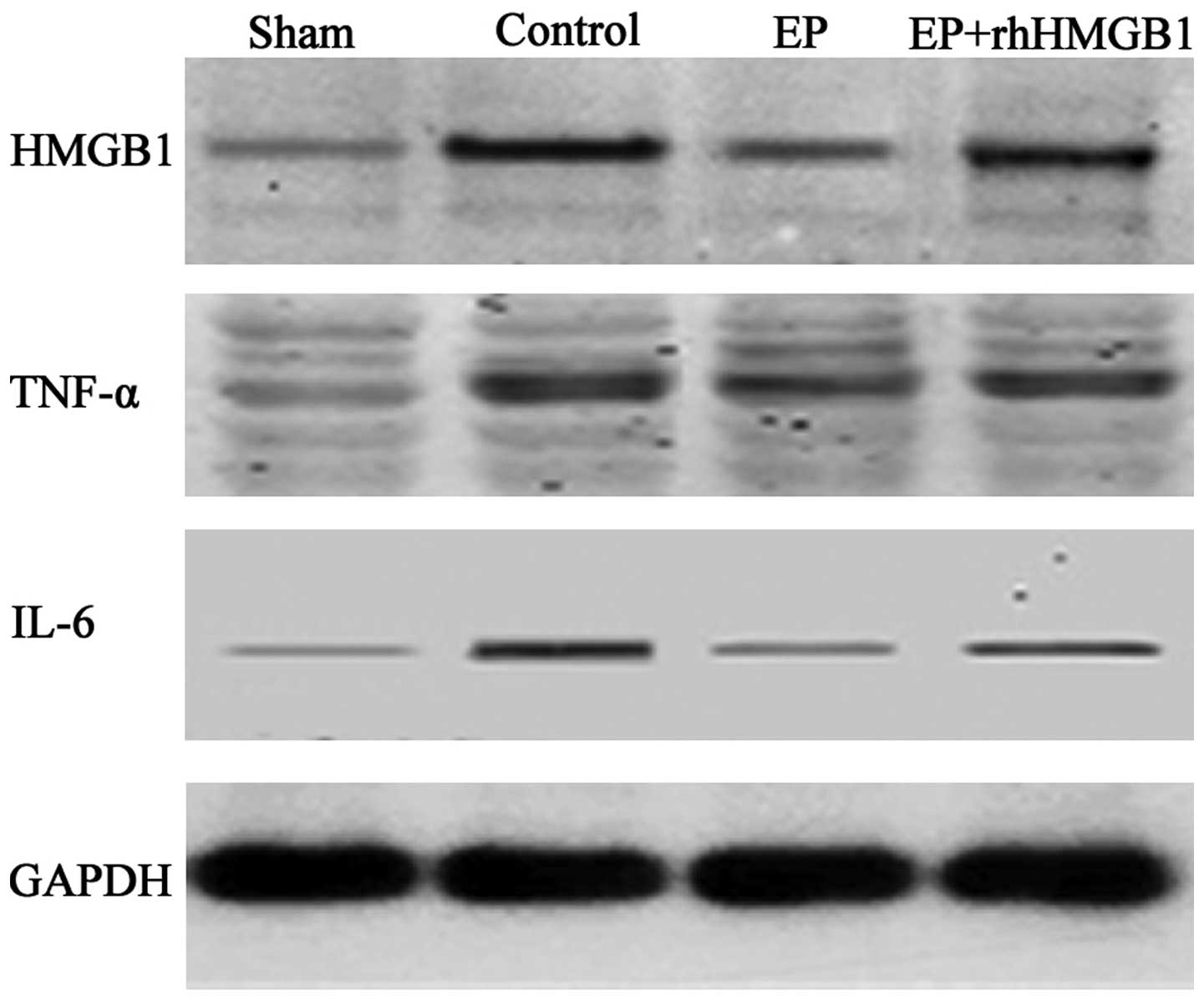 | Figure 3Effect of EP on the expression levels
of HMGB1, TNF-α and IL-6 in a rat myocardial ischemia/reperfusion
model. The expression levels of HMGB1, TNF-α, IL-6 and GAPDH were
analyzed in the sham, control, EP and EP + HMGB1 groups. The EP
group was administered EP at 40 mg/kg, while the EP + HMGB1 group
was administered EP at 40 mg/kg and rhHMGB1 at 100 μg/kg. Western
blot bands were quantified by densitometry. EP, ethyl pyruvate;
rhHMGB1, recombinant human high-mobility group box-1; TNF-α, tumor
necrosis factor-α; IL-6, interlukin-6; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Discussion
The pathogenesis of myocardial I/R injury is
multifactorial (1).
Proinflammatory cytokine release, endothelial cell activation and
inflammatory cell infiltration are among the known factors that
contribute to I/R injury. While the distal elements have been well
characterized, the early molecular events that initiate I/R injury
are poorly defined. HMGB1 can be passively released from damaged or
necrotic cells and secreted by activated innate immune cells.
Extracellular HMGB1 can trigger inflammation in vitro
(12,13) and initiate and amplify inflammatory
responses in non-infectious inflammatory conditions (14). In contrast to its delayed release
following endotoxemia and microbial infection, HMGB1 is rapidly
released subsequent to tissue I/R injury. Tsung et al
(7) showed that HMGB1 levels were
increased during liver I/R as early as 1 h after reperfusion and
then increased in a time-dependent manner up to 24 h. In a mouse
myocardial I/R model, Andrassy et al (6) reported that the mRNA and protein
expression of HMGB1 increased after 30 min of ischemia and peaked
at 6 h after reperfusion. In the present study in a rat in
vivo model, the myocardial HMGB1 level increased significantly
after 30 min ischemia and 48 h reperfusion. Strong expression of
HMGB1 was observed on the infiltrating leukocytes in the ischemic
area of the myocardium. Furthermore, the elevated HMGB1 level was
positively correlated with the levels of myocardial TNF-α and IL-6.
These data supported the findings of Andrassy et al
(6) that HMGB1 is involved in the
inflammatory response following I/R.
To further investigate the role of exogenous HMGB1
in a rat I/R model, an escalating dose of rhHMGB1 was injected
intravenously prior to the onset of reperfusion. A previous study
demonstrated that the administration of rHMGB1 worsened liver I/R
injury (7). The deleterious effect
of HMGB1 in I/R injury was also observed in the cardiovascular
system. Furthermore, O’Connor et al (15) showed that intracerebroventricular
injections of HMGB1 induced an increased level of IL-1 and impaired
the central nervous system function in a dose-dependent manner
(15). A single dose of 10 μg per
mouse was selected in the study by Andrassy et al (6); however, the optimal dose for a rat
model was unknown. In the current study, rhHMGB1 at various doses
(1, 10 and 100 μg/kg) was injected to elucidate the effective dose
in the rat myocardial I/R model. The doses of 1 and 10 μg/kg failed
to induce further myocardial damage; however, administration of 100
μg/kg rhHMGB1 led to a significantly larger IS, elevated TNF-α and
IL-6 levels and exacerbated the cardiac dysfunction.
Since HMGB1 acts as a central mediator of I/R
injury, antagonism of HMGB1 may provide a novel therapeutic
intervention. Investigations have shown that anti-HMGB1 treatment
can ameliorate injury in multiple organ I/R models (6,16);
however, HMGB1 antagonists (neutralizing monoclonal antibodies or
HMGB1 box A) are expensive and require a complex technique.
EP, a stable and lipophilic derivative of pyruvate,
was the first described pharmacological inhibitor for HMGB1
secretion (8). EP is readily
available, and studies have demonstrated the protective effect of
EP on the brain and kidney following I/R injury (17,18).
Studies have also been conducted to investigate the
cardioprotective effect of EP. Woo et al (19) showed that, in a rat I/R model, EP
enhanced the myocardial adenosine triphosphate levels and
attenuated myocardial oxidative injury. The anti-apoptosis effect
was also found in an in vitro study (20). In the study by Woo et al
(19), EP was injected prior to
myocardial ischemia. It has previously been demonstrated that EP
can afford strong protection of the spinal cord (21), even when it is administered 6 h
after reperfusion. We speculated that EP could protect rats from
myocardial I/R injury when it was administered just prior to the
onset of reperfusion. This would be closer to the clinical
condition, as myocardial infarction is usually unpredictable. In
the current study, the intravenous injection of EP (40 mg/kg)
before reperfusion significantly suppressed the elevated HMGB1
level. TNF-α and IL-6 expression levels are known to increase in
the ischemic myocardium and play an important role in maintaining
the inflammation cascade following reperfusion (22). Treatment with EP blocked the
interaction between HMGB1 and the cytokines, reduced the TNF-α and
IL-6 levels and preserved cardiac function. When exogenous HMGB1
was injected, the cardioprotective effect was abrogated. This
indicated that the benefit of EP came from its inhibition of
HMGB1.
In this rat in vivo I/R experiment,
myocardial I/R resulted in a significant inflammatory response,
accompanied by a strong upregulation of cardiac HMGB1, TNF-α and
IL-6. Treatment with EP significantly attenuated cardiac I/R
injury, and its protective effect was associated with decreases in
the HMGB1 and local inflammatory cytokine levels. The beneficial
effect of EP could be abrogated by rhHMGB1 injected prior to
reperfusion. Thus, the current study revealed the potential role of
EP as a therapeutic option for myocardial I/R.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81170196).
References
|
1
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dirksen MT, Laarman GJ, Simoons ML and
Duncker DJ: Reperfusion injury in humans: a review of clinical
trials on reperfusion injury inhibitory strategies. Cardiovasc Res.
74:343–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klune JR, Dhupar R, Cardinal J, Billiar TR
and Tsung A: HMGB1: endogenous danger signaling. Mol Med.
14:476–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu X, Xu W and Jiang H: HMGB1/IL-17A axis:
an important mechanism for myocardial ischemia-reperfusion injury.
Int J Cardiol. 174:447–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding HS, Yang J, Chen P, et al: The
HMGB1-TLR4 axis contributes to myocardial ischemia/reperfusion
injury via regulation of cardiomyocyte apoptosis. Gene.
527:389–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andrassy M, Volz HC, Igwe JC, et al:
High-mobility group box-1 in ischemia-reperfusion injury of the
heart. Circulation. 117:3216–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsung A, Kaizu T, Nakao A, et al: Ethyl
pyruvate ameliorates liver ischemia-reperfusion injury by
decreasing hepatic necrosis and apoptosis. Transplantation.
79:196–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ulloa L, Ochani M, Yang H, et al: Ethyl
pyruvate prevents lethality in mice with established lethal sepsis
and systemic inflammation. Proc Natl Acad Sci USA. 99:12351–12356.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rabadi MM, Ghaly T, Goligorksy MS and
Ratliff BB: HMGB1 in renal ischemic injury. Am J Physiol Renal
Physiol. 303:F873–F885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen M, Lu J, Dai W, et al: Ethyl pyruvate
ameliorates hepatic ischemia-reperfusion injury by inhibiting
intrinsic pathway of apoptosis and autophagy. Mediators Inflamm.
2013:4615362013.
|
|
11
|
Fryer RM, Hsu AK, Eells JT, Nagase H and
Gross GJ: Opioid-induced second window of cardioprotection:
potential role of mitochondrial KATP channels. Circ Res.
84:846–851. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rouhiainen A, Tumova S, Valmu L, Kalkkinen
N and Rauvala H: Pivotal advance: analysis of proinflammatory
activity of highly purified eukaryotic recombinant HMGB1
(amphoterin). J Leukoc Biol. 81:49–58. 2007. View Article : Google Scholar
|
|
13
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang R, Gallo DJ, Baust JJ, et al: Ethyl
pyruvate modulates inflammatory gene expression in mice subjected
to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol.
283:G212–G221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O’Connor KA, Hansen MK, Rachal Pugh C, et
al: Further characterization of high mobility group box 1 (HMGB1)
as a proinflammatory cytokine: central nervous system effects.
Cytokine. 24:254–265. 2003. View Article : Google Scholar
|
|
16
|
Tsung A, Sahai R, Tanaka H, et al: The
nuclear factor HMGB1 mediates hepatic injury after murine liver
ischemia-reperfusion. J Exp Med. 201:1135–1143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung KY, Park JJ and Kim YS: The role of
high-mobility group box-1 in renal ischemia and reperfusion injury
and the effect of ethyl pyruvate. Transplant Proc. 40:2136–2138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu YM, Kim JB, Lee KW, Kim SY, Han PL and
Lee JK: Inhibition of the cerebral ischemic injury by ethyl
pyruvate with a wide therapeutic window. Stroke. 36:2238–2243.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Woo YJ, Taylor MD, Cohen JE, et al: Ethyl
pyruvate preserves cardiac function and attenuates oxidative injury
after prolonged myocardial ischemia. J Thorac Cardiovasc Surg.
127:1262–1269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, Zhang K, Ji Y, Jiang X and Zuo S:
Effects of ethyl pyruvate on myocardial apoptosis and expression of
Bcl-2 and Bax proteins after ischemia-reperfusion in rats. J
Huazhong Univ Sci Technolog Med Sci. 28:281–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Q, Ding Q, Zhou Y, et al: Ethyl
pyruvate attenuates spinal cord ischemic injury with a wide
therapeutic window through inhibiting high-mobility group box 1
release in rabbits. Anesthesiology. 110:1279–1286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M and Chen L: Status of cytokines in
ischemia reperfusion induced heart injury. Cardiovasc Hematol
Disord Drug Targets. 8:161–172. 2008. View Article : Google Scholar : PubMed/NCBI
|