Introduction
Myocardial hypertrophy (MH) is involved in the
pathogenesis of various cardiovascular diseases (1–3);
however, the molecular mechanism underlying MH is yet to be fully
understood. It has been shown that dysregulation of gene
transcription can promote MH and cardiac dysfunction (4). The acetylation and deacetylation of
core histones via histone acetyltransferases and histone
deacetylases (HDACs) are important regulatory mechanisms in the
pathogenesis of MH (5). A previous
study reported the presence of 18 different HDACs, divided in two
families: The first family includes HDACs belonging to Classes I
(HDAC1, HDAC2, HDAC3 and HDAC8), II (HDAC4, HDAC5, HDAC6, HDAC7,
HDAC9 and HDAC10) and IV (HDAC11); the second family of HDACs
comprises seven members belonging to the Class III HDACs or
sirtuins (SIRT1-7) (6).
Among the HDACs, HDAC2, HDAC5 and HDAC9 are the
major players actively involved in regulating the processes of MH
(7–9). It has been shown that the nonspecific
HDAC inhibitors trichostatin A and valproic acid, as well as the
HDAC-selective inhibitor SK-7041, can attenuate angiotensin II- and
aortic stenosis-induced MH (10).
Furthermore, experimental and clinical studies have demonstrated
the beneficial effects of certain angiotensin II receptor blockers
(ARBs) on inhibiting and attenuating MH (11–13).
Despite this, it has yet to be determined whether the
anti-hypertrophic effects of ARBs are partially mediated by
modulating the myocardial expression of HDAC2, HDAC5 and HDAC9. In
the present study, the association between myocardial HDAC2, HDAC5
and HDAC9 expression and MH was observed in rats with aortic
constriction (AC) and/or ARB blocker (valsartan) treatment.
Materials and methods
Ethics statement
All animal protocols in this study were approved by
the Animal Care and Use Committee of the Research Institute of
Medicine, Shanghai Jiao Tong University (Shanghai, China), in
accordance with National Institutes of Health guidelines and public
law. All surgery was performed under sodium pentobarbital
anesthesia, and all efforts were made to minimize suffering.
In vivo hypertrophy models and blood
pressure (BP) measurements
Five- to eight-week-old adult male Wistar rats were
purchased from the Shanghai SLAC Laboratory Animal Co., Ltd.
(Chinese Academy of Sciences, Shanghai, China) and housed
individually in plastic cages in a temperature-controlled room.
Rats were randomly divided into sham-operated control, MH and MH +
valsartan groups (n=6/group). MH was induced by abdominal aortic
banding as previously described (14). Briefly, the animals were anesthetized
with ketamine (16.65 mg/kg intramuscularly), a 22-gauge needle was
placed along the abdominal aorta above the renal arteries and both
the aorta and the needle were tied with a 7-0 silk thread. The
needle was removed, leaving an aortic lumen determined by the
diameter of the needle (60–65% stenosis). The sham-operated animals
were subjected to the same procedure without the aortic banding.
One day post-surgery, valsartan (20 mg/kg; Beijing Novartis Pharma
Co., Ltd., Beijing, China) was administered to rats in the MH +
valsartan group through gavage once daily for eight weeks. The
sham-operated animals and rats in the MH group received 1 ml
distilled water through gavage daily for eight weeks. The heart
weight to body weight (HW/BW) ratio was obtained and systolic BP
measurements were made using tail-cuff plethysmography (15) prior to surgery and every two weeks
after the surgery; mean values from three measurements at each
time-point were calculated.
Histology
Cross-sectional areas of cardiomyocytes and
myocardial morphological changes were observed under optical
microscope on hematoxylin and eosin-stained sections by an
investigator blinded to the study design.
Measurements of plasma atrial
natriuretic peptide (ANP) and brain natriuretic peptide (BNP)
levels and myocardial HDAC2, HDAC5 and HDAC9 mRNA expression
Plasma ANP and BNP levels were determined with
immunoradiometric assay as previously described (16). Myocardial HDAC2, HDAC5 and HDAC9 mRNA
expression was detected through a reverse transcription
semi-quantitative polymerase chain reaction (RT-qPCR) method. Total
RNA was extracted from the myocardial tissue with TRIzol®
(Invitrogen Life Technologies, Carlsbad, CA, USA). RT was carried
out with 1.0 g total RNA using the SuperScript® First-Strand
Synthesis System for RT-PCR (Invitrogen Life Technologies),
according to manufacturer's instructions. cDNA (10 ng) was
subjected to semi-quantitative PCR using TaqMan® gene expression
assays (Applied Biosystems, Foster City, CA, USA) to assess the
expression level of HDAC2 (cat. no. Mm01193631_m1), HDAC5 (cat. no.
Mm00515917_m1), and HDAC9 (cat. no. Mm00458456_m1). The
semi-quantitative PCR cycling conditions were as follows: 94°C for
1 min, 55°C for 1 min and 72°C for 1 min for 35 cycles, followed by
72°C for 5 min. Quantity One V4.62 (Bio-Rad, Hercules, CA, USA)
software was used to analyse the results of the gray values from
the semi-quantitative PCR.
Statistical analysis
Results are expressed as the mean ± standard
deviation and analyzed using one-way analysis of variance with
Bonferroni post hoc comparison analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Myocardial hypertrophy
Eight weeks after surgery, the BW of the rats in the
MH + valsartan group was significantly reduced compared with that
in the MH rats. As expected, the HW and HW/BW were significantly
increased in the MH rats compared with those in the control rats,
and could be significantly reduced by valsartan treatment (Table I).
 | Table I.BW and HW of rats eight weeks after
aortic constriction surgery. |
Table I.
BW and HW of rats eight weeks after
aortic constriction surgery.
|
| Control group | MH group | Valsartan group |
|---|
| BW (g) |
262.4±8.0 |
257.3±10.1 |
241.7±10.1a |
| HW (mg) |
170.2±10.6 |
260.3±20.1a |
200.2±11.7a,b |
| HW/BW |
0.65±0.10 |
1.09±0.05a |
0.83±0.08b |
Blood pressure
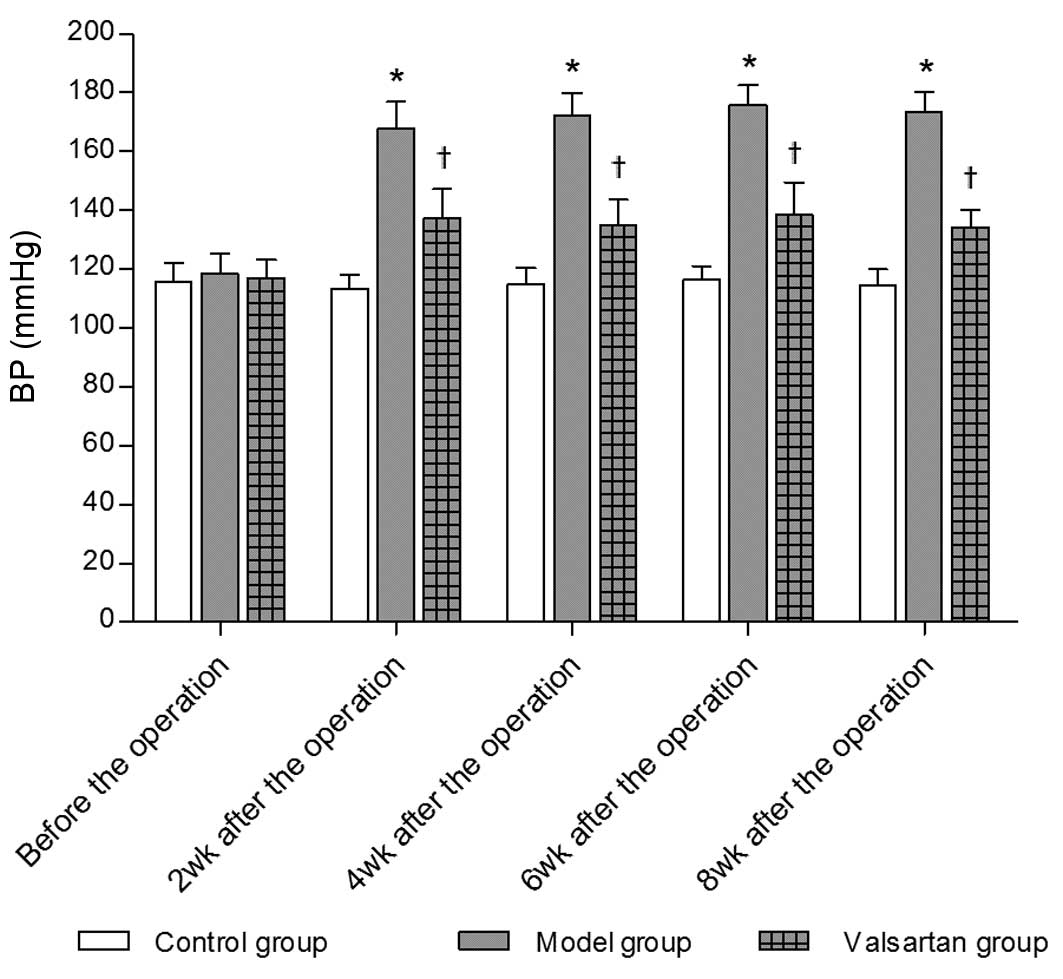
As shown in Fig. 1,
systolic BP was significantly increased in the MH rats compared
with that in the control rats, but was normalized with
valsartan.
Histology
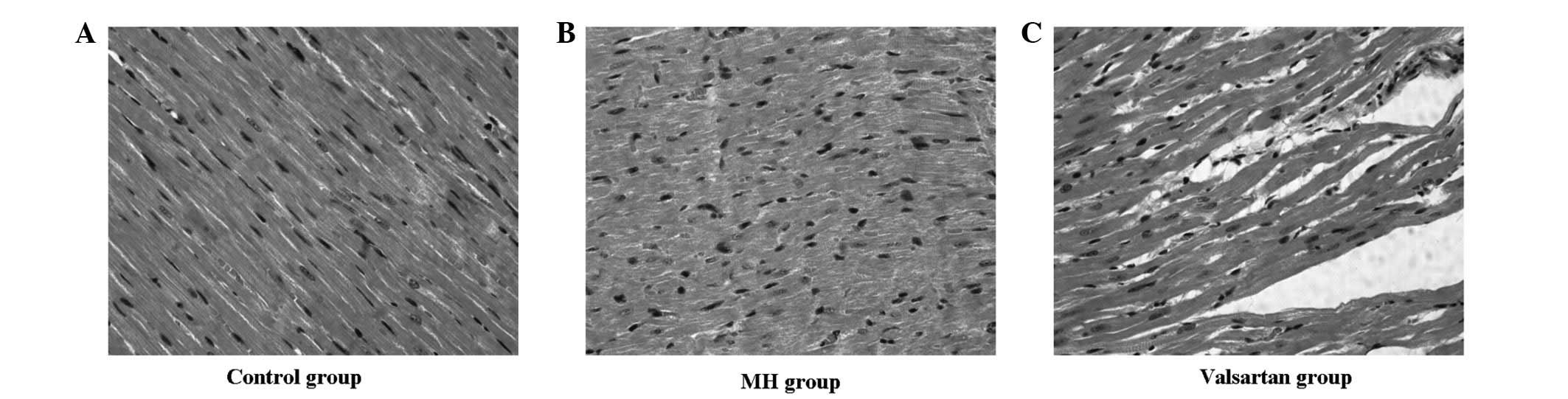
Histological observations were performed eight weeks
post-AC. Compared with the control group (Fig. 2A), moderate hypertrophy of the left
ventricular cardiomyocytes and enlarged nuclei were observed in MH
hearts (Fig. 2B). Only mild
hypertrophy of the cardiomyocytes could be observed following
valsartan treatment (Fig. 2C).
Plasma ANP and BNP levels
Plasma ANP and BNP levels were significantly
increased in the MH rats compared with those in the sham-operated
rats eight weeks after surgery. Valsartan treatment significantly
reduced the ANP and BNP levels (Table
II).
 | Table II.Plasma ANP and BNP levels. |
Table II.
Plasma ANP and BNP levels.
|
| Control group | MH group | Valsartan group |
|---|
| ANP (pg/ml) |
53.1±11.3 |
90.8±7.8a |
60.1±17.7b |
| BNP (pg/ml) |
136.6±11.2 |
174.4±43.8a |
121.5±20.8b |
Myocardial mRNA expression of HDAC2,
HDAC5 and HDAC9
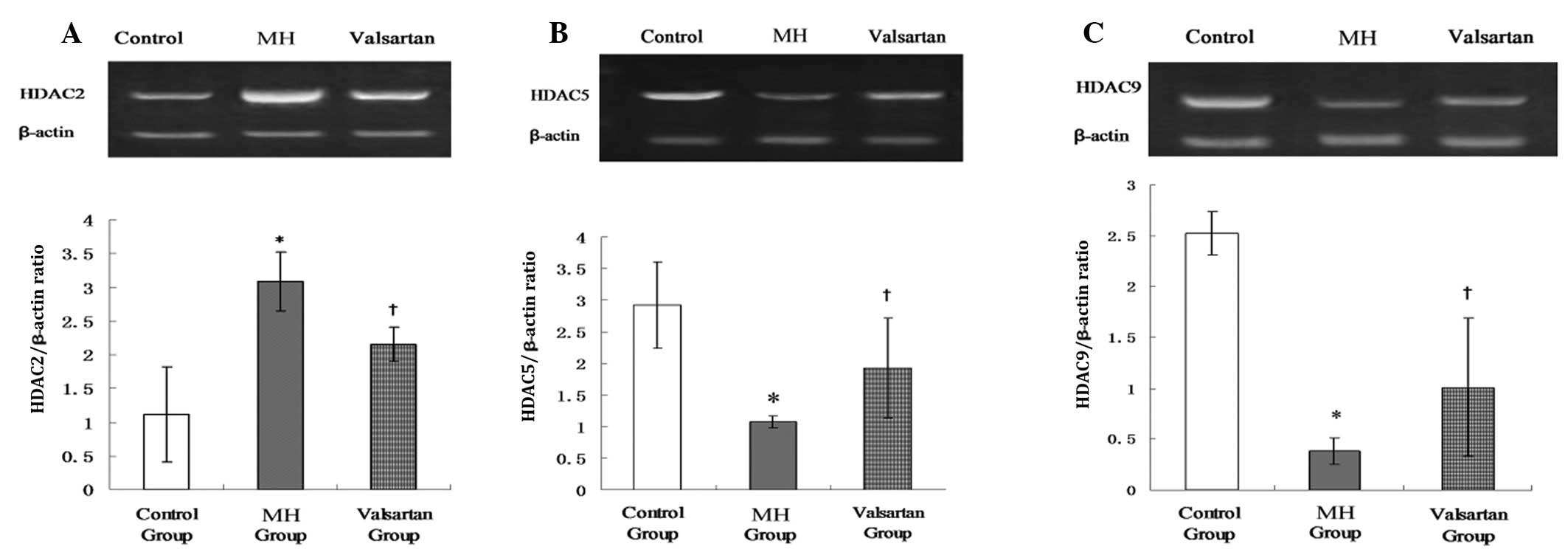
Eight weeks post-AC, the myocardial expression of
HDAC2 was significantly upregulated and the myocardial expression
of HDAC5 and HDAC9 was significantly downregulated in the MH hearts
compared with that in the sham-operated hearts. These changes could
be reversed by valsartan (Fig.
3A–C).
Discussion
In the present study, it was shown that AC-induced
MH was associated with myocardial HDAC expression changes: HDAC2
was upregulated while HDAC5 and HDAC9 were downregulated in MH
hearts. These changes could be reversed by valsartan, suggesting
that the anti-hypertrophic effects of valsartan could be partly
associated with the changes in myocardial HDAC expression. To the
best of our knowledge, that is the first report concerning the
effects of valsartan on myocardial HDAC expression in an AC-induced
model of MH.
It has previously been shown that the dysregulation
of gene transcription can promote cardiomyocyte hypertrophy and
embryonic gene expression and thus influence cardiac function
(17). Furthermore, it has been
shown that the enzymes controlling histone acetylation may serve as
stress regulators in gene expression in the heart (18), and histone acetylation/deacetylation
may be a focal point for the control of cardiac growth and gene
expression in response to acute and chronic stress stimuli
(19).
The present results have shown that HDAC2 is
upregulated in MH rats. It is known that HDAC2 regulates the
expression of numerous fetal cardiac isoforms. HDAC2 deficiency or
chemical HDAC2 inhibition can prevent the re-expression of fetal
genes and attenuate cardiac hypertrophy in hearts exposed to
hypertrophic stimuli (20). The
present finding that upregulated HDAC2 expression in AC-induced MH
hearts can be partly reversed by valsartan is consistent with the
above results and suggests that one of the anti-hypertrophic
mechanisms of valsartan may be associated with the modulatory
effect of valsartan on myocardial HDAC2 expression.
In contrast to HDAC2, HDAC5 and HDAC9 are
hypertrophy suppressors, and mice lacking HDAC5 (20) or HDAC9 (21) have been shown to be prone to
hypertrophic stimuli. Consistent with the above results, the
present study found downregulated HDAC5 and HDAC9 myocardial
expression in MH hearts compared with the control hearts; however,
the myocardial HDAC5 and HDAC9 expression was significantly
upregulated following valsartan treatment.
Increases in BP and plasma ANP and BNP levels are
typical findings an AC rat model (22,23). As
expected, valsartan reduced the BP and plasma ANP and BNP levels,
indicating that BP reduction serves as an important mechanism in
the attenuation of AC-induced MH and that reduced plasma ANP and
BNP levels may be the consequence of reduced MH following valsartan
therapy.
In conclusion, the anti-hypertrophic effects of
valsartan may be partially mediated by changes in myocardial HDAC5,
HDAC9 and HDAC2 expression in this AC rat model. Further studies in
animals with silenced or overexpressed HDAC5, HDAC9 and HDAC2 gene
expression are required to establish the role of HDAC5, HDAC9 and
HDAC2 in the valsartan-induced effects on MH.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30670831 and
30871082).
References
|
1
|
Zhang CL, McKinsey TA, Chang S, et al:
Class II histone deacetylases act as signal-responsive repressors
of cardiac hypertrophy. Cell. 110:479–488. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berridge MJ: Remodelling Ca2+
signalling systems and cardiac hypertrophy. Biochem Soc Trans.
34:228–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bisping E, Ikeda S, et al: Transcription
factor GATA4 is activated but not required for insulin-like growth
factor 1 (IGF1)-induced cardiac hypertrophy. J Biol Chem.
287:9827–9834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toko H, Minamino T and Komuro I: Role of
heat shock transcriptional factor 1 and heat shock proteins in
cardiac hypertrophy. Trends Cardiovasc Med. 18:88–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie M and Hill JA: HDAC-dependent
ventricular remodeling. Trends Cardiovasc Med. 23:229–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Marcotullio L, Canettieri G, Infante P,
et al: Protected from the inside: endogenous histone deacetylase
inhibitors and the road to cancer. Biochim Biophys Acta.
1815:241–252. 2011.PubMed/NCBI
|
|
7
|
Agalioti T, Chen G and Thanos D:
Deciphering the transcriptional histone acetylation code for a
human gene. Cell. 111:381–392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hang CT, Yang J, Han P, et al: Chromatin
regulation by Brg1 underlies heart muscle development and disease.
Nature. 466:62–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pedram A, Razandi M, Narayanan R, et al:
Estrogen regulates histone deacetylases to prevent cardiac
hypertrophy. Mol Biol Cell. 24:3805–3818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kee HJ, Sohn IS, Nam KI, et al: Inhibition
of histone deacetylation blocks cardiac hypertrophy induced by
angiotensin II infusion and aortic banding. Circulation. 113:51–59.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prisant LM: Management of hypertension in
patients with cardiac disease: use of renin-angiotensin blocking
agents. Am J Med. 121:(8 Suppl). S8–S15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimada YJ, Passeri JJ, Baggish AL, et al:
Effects of losartan on left ventricular hypertrophy and fibrosis in
patients with nonobstructive hypertrophic cardiomyopathy. JACC
Heart Fail. 1:480–487. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Zhou N, Gong H, et al: Comparison of
angiotensin II type 1-receptor blockers to regress pressure
overload-induced cardiac hypertrophy in mice. Hypertens Res.
33:1289–1297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao S, Long CL, Wang RH, et al: K(ATP)
activation prevents progression of cardiac hypertrophy to failure
induced by pressure overload via protecting endothelial function.
Cardiovasc Res. 83:444–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lovenberg W: Animal models for
hypertension research. Prog Clin Biol Res. 229:225–240.
1987.PubMed/NCBI
|
|
16
|
Del Ry S, Clerico A, Giannessi D, et al:
Measurement of brain natriuretic peptide in plasma samples and
cardiac tissue extracts by means of an immunoradiometric assay
method. Scand J Clin Lab Invest. 60:81–90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang N, Frank GD, Ding R, et al:
Promyelocytic leukemia zinc finger protein activates GATA4
transcription and mediates cardiac hypertrophic signaling from
angiotensin II receptor 2. PLoS One. 7:e356322012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colussi C, Illi B, Rosati J, et al:
Histone deacetylase inhibitors: keeping momentum for neuromuscular
and cardiovascular diseases treatment. Pharmacol Res. 62:3–10.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu CH, Lo JF, Hu WS, et al: Histone
acetylation is essential for ANG-II-induced IGF-IIR gene expression
in H9c2 cardiomyoblast cells and pathologically hypertensive rat
heart. J Cell Physiol. 227:259–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eom GH, Nam YS, Oh JG, et al: Regulation
of acetylation of histone deacetylase 2 by p300/CBP-associated
factor/histone deacetylase 5 in the development of cardiac
hypertrophy. Circ Res. 114:1133–1143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greco TM, Yu F, Guise AJ and Cristea IM:
Nuclear import of histone deacetylase 5 by requisite nuclear
localization signal phosphorylation. Mol Cell Proteomics.
10:M110.0043172011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nader L, Lahoud L, Chouery E, et al:
B-type natriuretic peptide receptors in hypertrophied adult rat
cardiomyocytes. Ann Cardiol Angeiol (Paris). 59:20–24. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito H, Hiroe M, Hirata Y, et al:
Endothelin ETA receptor antagonist blocks cardiac hypertrophy
provoked by hemodynamic overload. 89:2198–2203. 1994.PubMed/NCBI
|