Introduction
Typical characteristics of non-small cell lung
cancer (NSCLC) include the accumulation of multiple genetic
alterations, resulting from the inactivation of tumor-suppressor
genes, the activation of oncogenes and epigenetic changes. The
transmembrane epidermal growth factor receptor (EGFR) is detected
in 80–85% of patients with NSCLC, with the levels of expression
varying widely on a continuous scale. The expression and activity
levels of EGFR have been demonstrated to be closely associated with
tumor cell survival and proliferation (1,2).
EGFR mutations are associated with specific
characteristics, such as adenocarcinoma histological findings, a
non-smoking status, the female gender and East Asian ethnicity
(3,4). A previous study demonstrated that
activating mutations of the EGFR gene were associated with
sensitivity to EGFR tyrosine kinase inhibitors (TKIs), including
gefitinib and erlotinib (5).
Gefitinib and erlotinib are synthetic small molecules that are used
for the treatment of patients with unresectable or recurrent NSCLC
(3,5). The presence of EGFR mutations is a
useful predictor for the efficacy of such TKIs. Numerous EGFR
mutations types have been reported. For example, a deletion in exon
19 and point mutations of L858R in exon 21 are representative
mutations, since they comprise ~90% of the mutations (3,6). The
prevalence of Kirsten rat sarcoma (KRAS) mutation is known to be
associated with cigarette smoking (7). The mutated form of KRAS is
constitutively active, which results in the transformation of
immortalized cells and the promotion of cell proliferation. The
KRAS mutation status is a prognostic factor for survival; patients
with a KRAS mutation have been found to have a shorter survival
time compared with patients with the wild-type KRAS gene (8). In addition, the KRAS mutation status is
a predictor for poor therapeutic efficacy with EGFR-TKIs; however,
KRAS mutations have not been shown to affect chemotherapeutic
efficacy (9). EGFR and KRAS
mutations appear to be mutually exclusive (10). Currently, targeted therapy is not
available for patients with KRAS mutations, although
mitogen-activated protein kinase kinase inhibitors are being
investigated in clinical trials (11,12).
In recent years, the incidence of lung
adenocarcinoma, the most common histological subtype of lung cancer
in the majority of the world, has been increasing (13). Despite the improvement in numerous
treatment methods, the mortality rate from lung cancer has remained
high for several decades (14).
However, due to advances in computed tomography (CT) imaging and
the widening availability of lung cancer screening with the use of
spiral low-dose CT, the detection frequency of small and early lung
cancers, which are not visible on chest X-rays, is increasing.
Small nodules with ground-glass opacity (GGO) in the peripheral
lung can be more effectively detected, and small and early lung
cancer lesions may be observed in CT imaging as pure GGO (pGGO),
mixed GGO (mGGO) or solid patterned GGO (sGGO). Neoplastic small
nodules presenting GGO include the full spectrum of preinvasive to
invasive lesions, under the putative hypothesis that lung cancer
develops sequentially from atypical adenomatous hyperplasia to
bronchioloalveolar carcinoma (BAC) to adenocarcinoma with BAC
features. However, the mechanism underlying lesion progression over
time, in terms of radiological and molecular characteristics,
remains unclear. In the present study, associations between CT
features and the EGFR and KRAS mutation status were investigated in
patients with early lung adenocarcinomas. The aim of the study was
to determine whether evaluation of the CT findings may aid the
determination of the presence of EGFR and KRAS mutations,
particularly when a tumor specimen is unable to be obtained.
Patients and methods
Patients
This study was a retrospective cohort research and
was approved by the Institutional Review Board of the Chinese PLA
General Hospital (Beijing, China). All the patients provided
written informed consent. Between January 2011 and January 2014,
871 patients underwent surgical resection for lung adenocarcinoma
at the Chinese PLA General Hospital. Of these, 706 resected samples
were available for analysis of the EGFR and KRAS mutation status.
The records of the patients were reviewed to obtain further CT
findings, and clinical and pathological information. The tumor,
node and metastasis staging system was applied according to the 7th
edition of the tumor-node-metastasis classification of malignant
tumors for lung cancer outlined by the International Association
for the Study of Lung Cancer (IASLC) (15). In addition, the pathological
diagnoses were based on the 2004 World Health Organization
histological classification system (16) and the 2011 IASLC/American Thoracic
Society (ATS)/European Respiratory Society (ERS) lung
adenocarcinoma classification system (17). Patients who met the following
criteria were selected for the study: i) Solitary pulmonary nodule;
ii) adenocarcinoma; iii) lung tumor lesions completely resected
with radical lymph node dissection; and iv) EGFR and KRAS mutation
assays of tumor specimens had been performed. The excluding
criteria were as follows: i) Non-adenocarcinoma cell type; ii)
pathological stages IB, II, III and IV; iii) a tumor size of >3
cm; and iv) multiple lung cancers. A total of 212 patients were
included in the study.
CT findings
Patients underwent preoperative helical CT scanning
(LightSpeed™ Ultra; GE Medical Systems, Milwaukee, WI, USA) in the
month prior to surgery. The scanning parameters used for the chest
CT examination were as follows: Detector collimation, 1–5 mm; beam
pitch, 0.75–1.75; reconstruction thickness, 1–5 mm; reconstruction
interval, 1–5 mm; tube voltage, 120 kVp; tube current, 40 mA; and
reconstruction kernel, a high frequency algorithm. Two radiologists
who specialized in lung cancer independently reviewed the CT
images. Any differences in the interpretation of the nodule
categorization between the two readers were resolved by discussion
until a consensus was reached. The two radiologists had not been
informed of the pathological reports or the EGFR gene mutation
status. The image patterns of the tumors were categorized into
pGGO, mGGO or sGGO from the high-resolution CT scan. pGGO was
defined as a hazy increase in lung attenuation without obscuring
the underlying bronchial or vascular structures; mGGO was defined
as GGO with a solid section occupying <50% of the nodule; and
sGGO was defined as GGO with a section occupying >50% of the
nodules on high-resolution CT.
Histopathological analysis
In total, 212 lung tissue samples were fixed in
formalin, embedded in paraffin and stained with hematoxylin and
eosin for histological examination using an Olympus CX31 microscope
(Olympus Corporation, Tokyo, Japan). One board-certified
pathologist, with 20 years of experience performing pathological
diagnosis of lung cancer, who was blinded to the information
regarding the EGFR mutation status, reviewed the pathological
specimens and recorded the pathological subtype of each tumor
according to the IASLC/ATS/ERS classification of lung
adenocarcinoma (17). The categories
included were as follows: i) Adenocarcinoma in situ (AIS;
formerly known as BAC), which comprised a 3 cm nodule, lepidic
growth and mucinous, non-mucinous or mixed mucinous/non-mucinous
types; ii) minimally invasive adenocarcinoma (MIA), in which the
nodules were ≤3 cm with ≤5 mm of invasion, lepidic growth and
mucinous, non-mucinous or mixed mucinous/non-mucinous types; iii)
invasive adenocarcinoma with a predominant growth pattern of
lepidic growth, in which >5 mm of invasion was observed, with
acinar, papillary, micropapillary or solid types with mucin; and
iv) invasive adenocarcinoma variants, such as mucinous
adenocarcinoma, colloid, fetal and enteric morphologies. The latter
two categories were classified as invasive adenocarcinoma (IAC) as
its own class.
Gene mutation analysis
Formalin-fixed paraffin-embedded (FFPE) tissues were
analyzed to determine the EGFR and KRAS mutation status. Samples
considered suitable for downstream biomarker analysis were
progressed to biomarker analysis on the basis of quality, sample
source and tumor content (>100 tumor cells). The samples
underwent a central, histopathological review to ensure that they
were adequate for use and where appropriate, hematoxylin and
eosin-stained tissue was classified by suitably qualified
pathologists. DNA was extracted from the samples using a QIAamp DNA
FFPE tissue kit (Qiagen, Hilden, Germany), according to the
manufacturer's instructions. DNA was quantified to a final
concentration of 2 ng/µl using a NanoDrop2000 spectrophotometer
(NanoDrop; Thermo Fisher Scientific, Wilmington, DE, USA). EGFR
mutations at exons 18 to 21 and KRAS mutations at codons 12, 13 and
61 were analyzed with an amplification-refractory mutation system
using AmoyDx EGFR 29 and KRAS fluorescence polymerase chain
reaction (PCR) diagnostic kits (AmoyDx, Xiamen, China), according
to the manufacturer's instructions. Quantitative PCR amplification
was performed using an M×3000P™ quantitative PCR system
(Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA). PCR
cycling conditions were as follows: Initial denaturation at 95°C
for 5 min, followed by 15 cycles of denaturation at 95°C for 25
sec, annealing at 64°C for 20 sec and elongation at 70°C for 20
sec; and 31 cycles at 95°C for 25 sec, 60°C for 35 sec and 72°C for
20 sec. FAM and HEX fluorescence signals were collected in the
third step. The primers used are under patent; no. ZL2009101114992
for the EGFR kit and no. ZL2009101115016 for the KRAS kit. Data
were analyzed using MxPro software (version 4.10; Stratagene;
Agilent Technologies, Inc.). Relative expression levels were
determined using the cycle threshold (Ct), which was the cycle
number when the strength value of the fluorescence signal was
greater than the background signal. ΔCt values were equal to the Ct
value of the sample minus the control.
Statistical analysis
The χ2 test or Fisher's exact test were
used for comparisons of categorical variables. P<0.05 was
considered to indicate a statistically significant difference, and
all reported P-values were two-sided. Statistical analyses were
performed using SPSS for Windows (version 19.0; IBM SPSS, Armonk,
NY, USA).
Results
Clinical information and types of gene
mutation
Clinical features, pathological characteristics,
radiological findings and EGFR and KRAS gene mutation analyses are
summarized in Table I. The ages of
the 212 patients ranged between 36 and 76 years, and the median age
was 58 years. With regard to the smoking habits, 97 patients
(45.8%) had never smoked, 85 individuals (40.1%) were current
smokers and 30 patients (14.2%) were former smokers. Former smokers
were defined as patients who had quit smoking ≥1 year prior to
surgery (18). According to the
IASLC/ATS/ERS classification, 44 patients were diagnosed with AIS
(20.8%), 62 cases were classified as MIA (29.2%) and 106 patients
were diagnosed with IAC (50.0%). A pGGO pattern from the CT scan
was recorded in 39.2% of cases (n=83), while an mGGO pattern was
observed in 28.8% of cases (n=61) and an sGGO pattern was observed
in 32.0% of cases (n=68). The EGFR mutation rate was 36.8% (n=78),
in which 39 cases had an exon 19 deletion (18.4%), 34 cases
exhibited a L858R point mutation (16.0%) and five cases (2.4%) were
identified as other mutation types. The KRAS mutation rate was 8.5%
(n=18). Table II shows the types of
EGFR and KRAS mutations. The majority of EGFR mutations were due to
a point mutation of L858R in exon 21 (n=34, 43.6%) and a E746_A750
deletion in exon 19 (n=22, 28.2%). All types of KRAS mutation
occurred at codons 12 and 13. Two G12R mutations (11.1%), three
G12C mutations (16.7%), five G12V mutations (27.8%), six G12D
mutations (33.3%) and two G13D mutations (11.1%) were
identified.
 | Table I.Summary of clinical factors and gene
mutation status. |
Table I.
Summary of clinical factors and gene
mutation status.
| Variables | Cases, n (%) |
|---|
| Median age, years
(range) | 58 (36–76) |
| Gender |
|
|
Male | 130 (61.3) |
|
Female | 82 (38.7) |
| Smoking
history |
|
|
Never | 97 (45.8) |
|
Formera/current | 115 (54.3) |
| Pathological
diagnosis |
|
|
AIS | 44 (20.8) |
|
MIA | 62 (29.2) |
|
IAC | 106 (50.0) |
| Image patterns |
|
|
pGGO | 83 (39.2) |
|
mGGO | 61 (28.8) |
|
sGGO | 68 (32.0) |
| EGFR mutation
status |
|
| Wild
type | 134 (63.2) |
|
L858R | 34 (16.0) |
| Exon 19
deletion | 39 (18.4) |
|
Others | 5 (2.4) |
| KRAS mutation
status |
|
| Wild
type | 194 (91.5) |
|
Mutation | 18 (8.5) |
 | Table II.Information on the gene mutation
status. |
Table II.
Information on the gene mutation
status.
| A, EGFR |
|---|
|
|---|
| Exon | Amino acid
change | Nucleotide
change | Cases, n (%) |
|---|
| 18 | G719A | 2156G>C | 1 (1.28) |
|
| G719S | 2155G>A | 1 (1.28) |
| 19 | E746_A750del | 2235–2249del
15 | 22 (28.2) |
|
| E746_A750del | 2236–2250del
15 | 2 (2.56) |
|
| L747_P753>S | 2240–2257del
18 | 5 (6.41) |
|
| E746_T751>A | 2237–2251del
15 | 1 (1.28) |
|
| E746_T751>I | 2235–2252>AATdel
18 | 1 (1.28) |
|
| L747_A750>P | 2238–2248>GCdel
11 | 2 (2.56) |
|
| L747_A750>P | 2239–2248>Cdel
10 | 1 (1.28) |
|
| L747_T751del | 2239–2253del
15 | 1 (1.28) |
|
| L747_T751del | 2240–2254del
15 | 1 (1.28) |
|
| L747_S752del | 2239–2256del
18 | 1 (1.28) |
|
| L747_T751>P | 2239–2251>Cdel
13 | 1 (1.28) |
|
| E746_S752>V | 2237–2255>Tdel
19 | 1 (1.28) |
| 20 | S768I | 2303G>T | 1 (1.28) |
|
|
V769_D770insASV |
2307–2308insGACAACGTG | 1 (1.28) |
|
| T790M | 2369C>T | 1 (1.28) |
| 21 | L858R | 2573T>G | 34 (43.6) |
|
| B, KRAS |
|
| Exon | Amino acid
change | Nucleotide
change | Cases, n (%) |
|
| 2 | G12R | 34G>C | 2 (11.1) |
|
| G12C | 34G>T | 3 (16.7) |
|
| G12V | 35G>T | 5 (27.8) |
|
| G12D | 35G>A | 6 (33.3) |
|
| G13D | 38G>A | 2 (11.1) |
Image patterns and pathological
diagnosis
Associations between image patterns and pathological
diagnosis are shown in Table III.
AIS was found in 25.3% (21/83) of the pGGO group, 21.3% (13/61) of
the mGGO group and 14.7% (10/68) of the sGGO group. MIA was
observed in 36.1% (30/83) of the pGGO group, 29.5% (18/61) of the
mGGO group and 20.6% (14/68) of the sGGO group. Furthermore, IAC
was found in 38.6% (32/83) of the pGGO group, 49.2% (30/61) of the
mGGO group and 64.7% (44/68) of the sGGO group. Therefore, AIS and
MIA were shown to exhibit a decreasing trend with regard to the
solid section of the tumors, whereas IAC exhibited an increasing
trend. The χ2 test was performed (χ2=10.254)
and the differences were determined to be statistically significant
(P=0.036). Thus, the solid portion of the CT image patterns and the
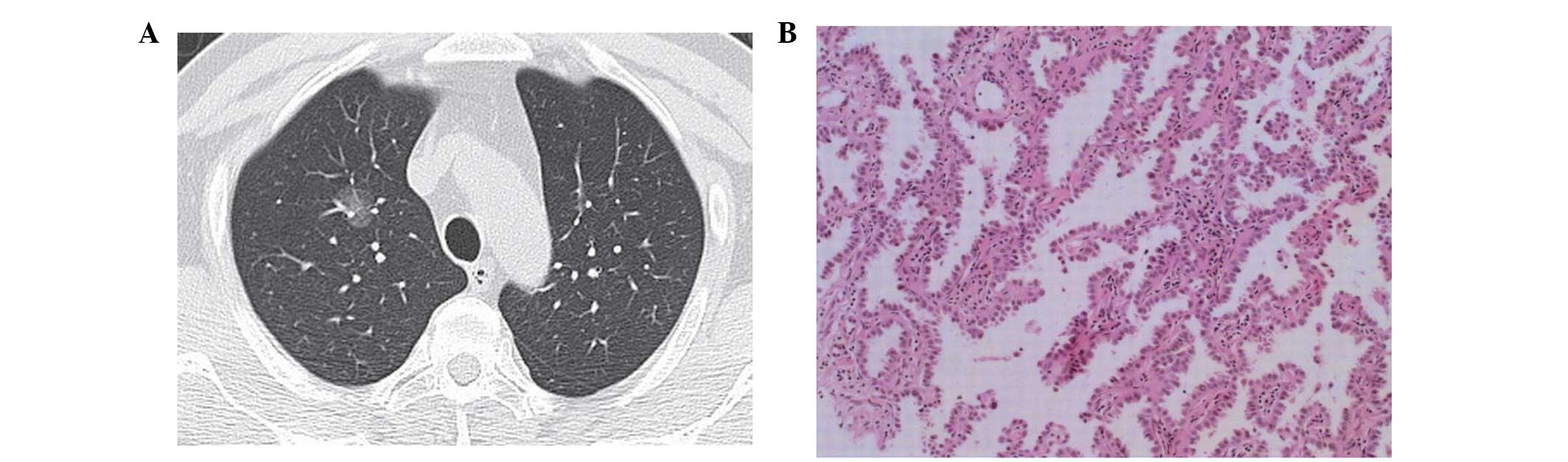
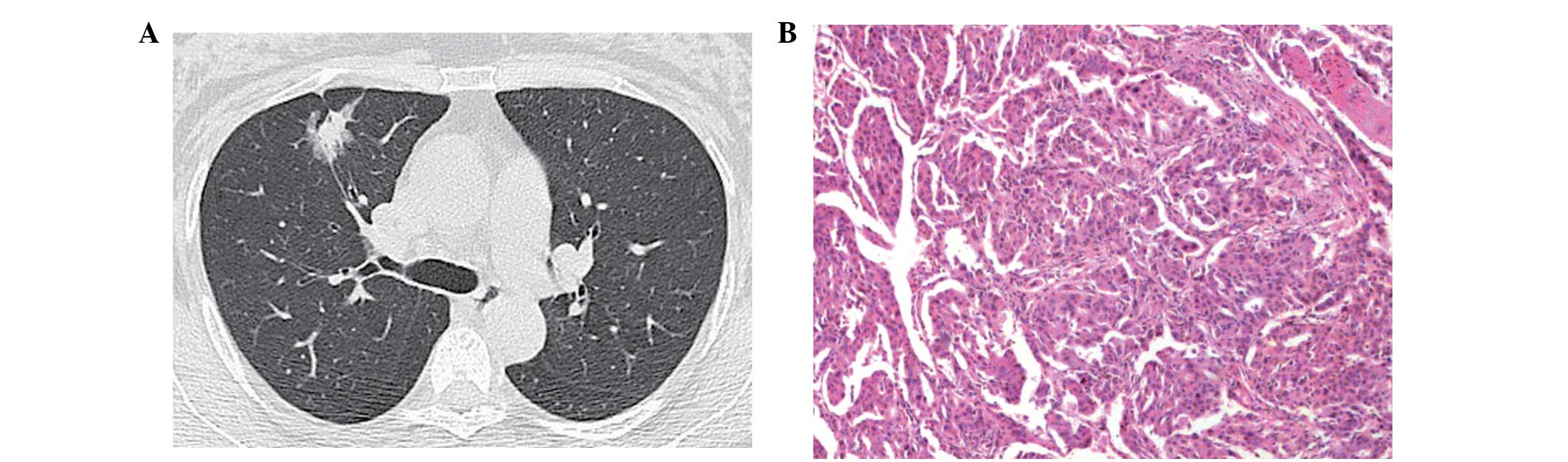
histopathological invasion type may be well-matched (Figs. 1–3).
 | Table III.Image patterns and pathological
diagnosis. |
Table III.
Image patterns and pathological
diagnosis.
|
| Pathological
diagnosis |
|
|---|
|
|---|
| Image patterns | AIS, n (%) | MIA, n (%) | IAC, n (%) | Total |
|---|
| pGGO | 21 (25.3) | 30 (36.1) | 32 (38.6) | 83 |
| mGGO | 13 (21.3) | 18 (29.5) | 30 (49.2) | 61 |
| sGGO | 10 (14.7) | 14 (20.6) | 44 (64.7) | 68 |
| Total | 44 | 62 | 106 | 212 |
Clinical factors and gene mutation
status
Table IV shows the
patient characteristics and gene mutation status. L858R point
mutations and exon 19 deletions were more frequent in females than
in males, as compared with the wild-type EGFR (P<0.05). However,
no statistically significant difference was observed between
genders with regard to the KRAS mutation and wild type genotypes
(P=0.985). In patients with no history of smoking, L858R point
mutations were more frequently observed compared with
former/current smokers; however, smoking history was not shown to
correlate with an exon 19 deletion (P=0.067). In addition, in
patients with a history of smoking, KRAS mutations were observed
more frequently compared with non-smokers (P=0.010). The rate of an
L858R point mutation was 7/44 (15.9%) in patients with AIS, 8/62
(12.9%) in MIA patients and 19/106 (17.9%) in patients with IAC.
Furthermore, the rate of an exon 19 deletion was 4/44 (9.1%) in AIS
patients, 10/62 (16.1%) in patients with MIA and 25/106 (23.6%) in
IAC patients. The rate of a KRAS mutation was 2/44 (4.5%) in
patients with AIS, 5/62 (8.1%) in MIA patients and 11/106 (10.4%)
in patients with IAC. Histological diagnosis was shown to have an
association with an exon 19 deletion (P=0.041); however, the trend
association was not observed for the L858R point mutation or KRAS
mutation status (P=0.411 and 0.501, respectively), although there
was a tendency. A statistically significant association was
identified between the image pattern and gene mutation status. As
the proportion of GGO decreased (from pGGO to sGGO), the frequency
of L858R point mutations, exon 19 deletions and KRAS mutations
increased (P=0.029, 0.027 and 0.018, respectively).
 | Table IV.Clinical factors and gene mutation
status. |
Table IV.
Clinical factors and gene mutation
status.
|
| EGFR, n | KRAS, n |
|
|
|
|---|
|
|
|---|
| Clinical
factors | Wild type
(n=134) | L858R (n=34) | Exon 19 deletion
(n=39) | Wild type
(n=194) | Mutation
(n=18) | P1 | P2 | P3 |
|---|
| Gender |
|
|
|
|
|
|
|
|
|
Male | 100 | 13 | 15 | 119 | 11 | <0.001 | <0.001 | 0.985 |
|
Female | 34 | 21 | 24 | 75 | 7 |
|
|
|
| Smoking
history |
|
|
|
|
|
|
|
|
|
Never | 47 | 27 | 20 | 94 | 3 | <0.001 | 0.067 | 0.010 |
|
Formera/current | 87 | 7 | 19 | 100 | 15 |
|
|
|
| Histological
diagnosis |
|
|
|
|
|
|
|
|
|
AIS | 33 | 7 | 4 | 42 | 2 | 0.411 | 0.041 | 0.501 |
|
MIA | 44 | 8 | 10 | 57 | 5 |
|
|
|
|
IAC | 57 | 19 | 25 | 95 | 11 |
|
|
|
| Image pattern |
|
|
|
|
|
|
|
|
|
pGGO | 62 | 8 | 11 | 80 | 3 | 0.029 | 0.027 | 0.018 |
|
mGGO | 39 | 11 | 10 | 57 | 4 |
|
|
|
|
sGGO | 33 | 15 | 18 | 57 | 11 |
|
|
|
Discussion
Lung cancer remains the leading cause of cancer
mortality worldwide, with late diagnosis one of the major
challenges to improving outcomes (19,20).
Preliminary trials using spiral (helical) low-dose CT for lung
cancer screening have produced promising results, with stage I lung
cancer detected in >80% of newly diagnosed cases (21–23). In
recent years, the National Comprehensive Cancer Network have
recommended lung cancer screening using low-dose helical CT for
selected high-risk patients who are current or former smokers
(24). Increasingly, small nodules
have been identified. In the present study, tumors based on the
proportion of the solid section in the nodules were divided into
three groups, namely pGGO (non-solid part), mGGO (solid part of
<50%) and sGGO (solid part of ≥50%). The proportion of the solid
section is usually associated with disease progression (25). Previous studies have reported that
the solid proportion in advanced-stage lesions is significantly
larger compared with those in lesions at an earlier stage (25,26). In
addition, a number of studies have found that a low proportion of
solid matter in adenocarcinoma is a good prognostic indicator
(25–28). Therefore, the solid portion of a GGO
nodule in lung adenocarcinoma increases the biological invasion of
the tumor, which indicates that a solid section increases the level
of suspicion of invasive adenocarcinoma. In the present study, the
image pattern of GGO was shown to correlate with the IASLC/ATS/ERS
histological subtypes of adenocarcinoma. According to this
pathological-radiological correlation, the majority of AIS and MIA
cases corresponded to pGGO, while IAC cases corresponded to
sGGO.
EGFR and KRAS mutations are two of the most common
mutations in NSCLC (29,30). The presence of EGFR mutations is a
critical biological determinant for adequate therapy selection in
patients with lung cancer (31). A
significant association has been identified between EGFR mutations,
particularly exon 19 deletions and exon 21 (L858R) and exon 18
mutations, and a sensitivity to TKIs (4,32–34). In
addition, exon 20 insertion mutations have been hypothesized to be
a valuable predictor of resistance to clinically achievable levels
of TKIs (35,36). The prevalence of EGFR mutations in
Asian populations with an advanced stage of lung adenocarcinoma is
up to 51.4% (37). In the present
study, patients with stage IA lung adenocarcinoma, which is known
to have an improved prognosis compared with the more advanced
stages, were found to have a lower prevalence of EGFR mutation
(36.8%), as compared with advanced lung adenocarcinoma. The most
common EGFR mutations identified in patients with NSCLC are
deletions in exon 19 and mutations in exon 21 (38,39),
which concurs with the results of the current study. Furthermore,
the present study found that the E746_A750 deletion was the most
common exon 19 deletion. In the present study, the rate of a KRAS
mutation was 8.4%, which was lower than the KRAS mutation rate of
~25% reported in a North American population (8,9). All
types of KRAS mutations occurred in codons 12 and 13, with mutation
at codon 12 more frequent compared with codon 13.
In the present study, L858R point mutations and exon
19 deletions were more prevalent in females. In addition, L858R
point mutations were shown to correlate with a non-smoking history.
Although the difference in smoking history between individuals with
exon 19 deletion and wild type genotypes was not statistically
significant (P=0.067), a greater number of exon 19 deletions
occurred in those who had never smoked. Tam et al (40) reported that the rate of KRAS mutation
was 5.3% in women, which was lower compared with the 16.7% rate in
men (P=0.009); however, Marks et al (41) reported KRAS mutation rates of 15.3%
in women and 19.2% in men, and observed no differences between
genders (P=0.581). In the present study, KRAS mutations were not
found to correlate with gender, although a correlation was observed
with the smoking status. Furthermore, the correlation between
IASLC/ATS/ERS histological subtypes of adenocarcinoma and EGFR/KRAS
mutations was analyzed. The rates of EGFR mutations in patients
diagnosed with AIS, MIA and IAC were 25.0, 29.0 and 63.6%,
respectively. Thus, EGFR mutations were more frequently observed in
patients with IAC compared with those diagnosed with AIS or MIA
(P=0.047). Although EGFR mutations have been implicated in the
early stages of lung adenocarcinoma development, not all cases of
AIS, MIA and IAC have an EGFR mutation. Further analysis of the
L858R point mutation and exon 19 deletion indicated that exon 19
deletions were significantly associated with histological diagnosis
(P=0.041); however, no such association was observed for L858R
point mutations. Exon 19 deletions were found to be significantly
associated with the histological diagnosis (P=0.041); however, an
association was not observed for L858R point mutations. Thus, the
results of the present study demonstrated that the frequency of
exon 19 deletions increased with different histologies from AIS to
MIA to IAC. Histologically, it may be inferred that early
adenocarcinoma with an exon 19 deletion may have an aggressive
behavior.
A number of studies have reported an association
between EGFR and KRAS mutations with image patterns. As early as
2006, Yano et al (42)
reported that EGFR mutations were more frequently detected in small
peripheral adenocarcinomas with a high ratio of GGO components, as
compared with those with a low ratio of GGO components.
Subsequently, in 2007, Yoshida et al (43) reported that EGFR mutations exhibited
no correlation with the appearance or increase in consolidation
within pGGO. In 2009, Chung et al (44) examined 56 pulmonary nodules presented
with GGO from 24 patients to analyze the mutation status of the
EGFR and KRAS genes and any pathological-radiological correlations.
The authors found that the rate of EGFR mutation was 38.4% in pGGO
cases, 41.6% in mGGO cases and 50% in sGGO cases, while only two
KRAS mutations were identified in sGGO cases. However, in 2010,
Glynn et al (45) reported
that the high proportion of GGO on a CT image was not significantly
associated with the presence of an EGFR mutation or with the
presence of a KRAS mutation. In 2009, Park et al (46) examined the EGFR gene copy number
status in 132 patients using fluorescence in situ
hybridization. The authors reported that a low EGFR gene copy
number was more commonly observed in adenocarcinoma with a GGO of
>50%. In 2013, Lee et al (47) reported that tumors with a GGO
proportion of >50% were less frequent among tumors with
EGFR-overexpression compared with tumors without
EGFR-overexpression in 214 patients with stage I NSCLC.
Furthermore, in 2011, Hsu et al (48) retrospectively surveyed 162 patients
with stage I lung adenocarcinoma with a tumor size of <3 cm, and
found that the part-solid and solid pattern tumors had more typical
EGFR mutations compared with the pGGO tumors. In 2012, Aoki et
al (49) analyzed 25 lung
adenocarcinomas of <3 cm in diameter and found a high incidence
of EGFR mutations in the GGO-dominant lung adenocarcinomas;
however, no correlation was observed between EGFR mutations and
changes in the CT patterns. Prior to 2010, the reported
correlations between EGFR and KRAS mutations with GGO image
patterns were tentative. With the emergence of large sample
research (48), the majority of
studies have reported that a low GGO component is associated with
EGFR mutations or overexpression, which is consistent with the
results of the present study; however, the association between KRAS
mutations and radiological findings remains unclear.
In the present study, the results demonstrated that
the lower the proportion of the GGO component, the higher the
frequency of EGFR or KRAS mutations in patients with stage IA
adenocarcinoma of the lung. This association may be significant in
diagnosis, since a low proportion of GGO correlates with a poor
survival rate (25–28), and EGFR amplification or KRAS
mutation are associated with a worse prognosis (2,8). In
addition, these observations indicate that such mutations have an
association with the progressive behavior of GGO. However, it is
well known that EGFR and KRAS mutations are mutually exclusive in
patients with lung cancer (10,50).
Therefore, there can be correlation without causation between the
two mutations and a low GGO proportion. Consolidation of GGO
lesions may be due to unmeasured or unknown factors. Two previous
studies reported that the inactivation of p53 may be associated
with the appearance of central consolidation within pGGO (43,49).
In conclusion, in stage IA lung adenocarcinoma
patients, EGFR mutations were shown to have a relatively low
incidence, as compared with more advanced stages of the disease.
According to the IASLC/ATS/ERS classification, image patterns of
GGO were shown to correlate with subtypes of adenocarcinomas,
including AIS, MIA and IAC. Furthermore, exon 19 deletions were
more frequently observed in IAC cases when compared with AIS or MIA
cases; however, no association was observed with L858R point
mutations. In addition, KRAS mutations were not shown to have a
statistically significant association with the subtypes of
adenocarcinoma. EGFR and KRAS mutations were associated with a low
GGO proportion in the lesions, although further research is
required to determine whether this association is causal.
Therefore, evaluating the GGO image patterns of stage IA lung
adenocarcinoma patients may provide important information for the
subtypes of lung adenocarcinoma and the status of EGFR and KRAS
mutations.
References
|
1
|
Cappuzzo F, Gregorc V, Rossi E, et al:
Gefitinib in pretreated non-small-cell lung cancer (NSCLC):
Analysis of efficacy and correlation with HER2 and epidermal growth
factor receptor expression in locally advanced or metastatic NSCLC.
J Clin Oncol. 21:2658–2663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cappuzzo F, Hirsch FR, Rossi E, et al:
Epidermal growth factor receptor gene and protein and gefitinib
sensitivity in non-small-cell lung cancer. J Natl Cancer Inst.
97:643–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pao W and Miller VA: Epidermal growth
factor receptor mutations, small-molecule kinase inhibitors, and
non-small-cell lung cancer: current knowledge and future
directions. J Clin Oncol. 23:2556–2568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kosaka T, Yatabe Y, Endoh H, Kuwano H,
Takahashi T and Mitsudomi T: Mutations of the epidermal growth
factor receptor gene in lung cancer: biological and clinical
implications. Cancer Res. 64:8919–8923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slebos RJ, Hruban RH, Dalesio O, Mooi WJ,
Offerhaus GJ and Rodenhuis S: Relationship between K-ras oncogene
activation and smoking in adenocarcinoma of the human lung. J Natl
Cancer Inst. 83:1024–1027. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsao MS, Aviel-Ronen S, Ding K, et al:
Prognostic and predictive importance of p53 and RAS for adjuvant
chemotherapy in non small-cell lung cancer. J Clin Oncol.
25:5240–5247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eberhard DA, Johnson BE, Amler LC, et al:
Mutations in the epidermal growth factor receptor and in KRAS are
predictive and prognostic indicators in patients with
non-small-cell lung cancer treated with chemotherapy alone and in
combination with erlotinib. J Clin Oncol. 23:5900–5909. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Febbo PG, Ladanyi M, Aldape KD, et al:
NCCN Task Force report: Evaluating the clinical utility of tumor
markers in oncology. J Natl Compr Canc Netw. 9 (Suppl 5):S1–S32.
2011.
|
|
11
|
Sequist LV, Heist RS, Shaw AT, et al:
Implementing multiplexed genotyping of non-small-cell lung cancers
into routine clinical practice. Ann Oncol. 22:2616–2624. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janne PA, Shaw AT, Pereira JR, et al:
Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell
lung cancer: a randomised, multicentre, placebo-controlled, phase 2
study. Lancet Oncol. 14:38–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin HR, Masuyer E, Ferlay J and Curado
MP: Asian Contributors to CI5 IX4: Cancer in Asia - Incidence rates
based on data in cancer incidence in five continents IX
(1998–2002). Asian Pac J Cancer Prev. 11 (Suppl 2):11–16.
2010.PubMed/NCBI
|
|
14
|
Alberg AJ, Ford JG and Samet JM: American
College of Chest Physicians: Epidemiology of lung cancer: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132 (Suppl 3):29S–55S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ainbinder DJ, Esmaeli B, Groo SC, Finger
PT and Brooks JP: Introduction of the 7th edition eyelid carcinoma
classification system from the American Joint Committee on
Cancer-International Union Against Cancer staging manual. Arch
Pathol Lab Med. 133:1256–1261. 2009.PubMed/NCBI
|
|
16
|
Beasley MB, Brambilla E and Travis WD: The
2004 World Health Organization classification of lung tumors. Semin
Roentgenol. 40:90–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Travis WD, Brambilla E, Noguchi M, et al:
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society international
multidisciplinary classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takano T, Ohe Y, Sakamoto H, et al:
Epidermal growth factor receptor gene mutations and increased copy
numbers predict gefitinib sensitivity in patients with recurrent
non-small-cell lung cancer. J Clin Oncol. 23:6829–6837. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carney DN: Lung cancer - time to move on
from chemotherapy. N Engl J Med. 346:126–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chute JP, Chen T, Feigal E, Simon R and
Johnson BE: Twenty years of phase III trials for patients with
extensive-stage small-cell lung cancer: perceptible progress. J
Clin Oncol. 17:1794–1801. 1999.PubMed/NCBI
|
|
21
|
Henschke CI, McCauley DI, Yankelevitz DF,
et al: Early lung cancer action project: Overall design and
findings from baseline screening. Lancet. 354:99–105. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henschke CI, Naidich DP, Yankelevitz DF,
et al: Early lung cancer action project: Initial findings on repeat
screenings. Cancer. 92:153–159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaneko M, Kusumoto M, Kobayashi T, et al:
Computed tomography screening for lung carcinoma in Japan. Cancer.
89 (Suppl):2485–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Comprehensive Cancer Network,
Inc. (NCCN), . Lung Cancer Screening. Version 1. 2012, NCCN; Fort
Washington, USA: 2011, http://www.lungcanceralliance.org/assets/docs/news/NCCN%20Screening%20Guidelines%2010_11.pdf
|
|
25
|
Takashima S, Maruyama Y, Hasegawa M, et
al: CT findings and progression of small peripheral lung neoplasms
having a replacement growth pattern. AJR Am J Roentgenol.
180:817–826. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuriyama K, Seto M, Kasugai T, et al:
Ground-glass opacity on thin-section CT: value in differentiating
subtypes of adenocarcinoma of the lung. AJR Am J Roentgenol.
173:465–469. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aoki T, Tomoda Y, Watanabe H, et al:
Peripheral lung adenocarcinoma: correlation of thin-section CT
findings with histologic prognostic factors and survival.
Radiology. 220:803–809. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kodama K, Higashiyama M, Yokouchi H, et
al: Prognostic value of ground-glass opacity found in small lung
adenocarcinoma on high-resolution CT scanning. Lung Cancer.
33:17–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carneiro JG, Couto PG, Bastos-Rodrigues L,
et al: Spectrum of somatic EGFR, KRAS, BRAF, PTEN mutations and
TTF-1 expression in Brazilian lung cancer patients. Genet Res
(Camb). 96:e0022014.PubMed/NCBI
|
|
30
|
Das BR, Bhaumik S, Ahmad F, et al:
Molecular spectrum of somatic EGFR and KRAS gene mutations in non
small cell lung carcinoma: Determination of frequency, distribution
pattern and identification of novel variations in Indian patients.
Pathol Oncol Res. Jan 31–2015.(Epub ahead of print). View Article : Google Scholar
|
|
31
|
National Comprehensive Cancer Network,
Inc. (NCCN), . Non-Small Cell Lung Cancer. Version 2. 2013, NCCN;
Fort Washington, USA: 2013, http://www.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf
|
|
32
|
Cappuzzo F, Finocchiaro G, Metro G, et al:
Clinical experience with gefitinib: an update. Crit Rev Oncol
Hematol. 58:31–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sequist LV, Joshi VA, Janne PA, et al:
Response to treatment and survival of patients with non-small cell
lung cancer undergoing somatic EGFR mutation testing. Oncologist.
12:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji H, Li D, Chen L, et al: The impact of
human EGFR kinase domain mutations on lung tumorigenesis and in
vivo sensitivity to EGFR-targeted therapies. Cancer Cell.
9:485–495. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lund-Iversen M, Kleinberg L,
Fjellbirkeland L, Helland A and Brustugun OT: Clinicopathological
characteristics of 11 NSCLC patients with EGFR-exon 20 mutations. J
Thorac Oncol. 7:1471–1473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yasuda H, Kobayashi S and Costa DB: EGFR
exon 20 insertion mutations in non-small-cell lung cancer:
preclinical data and clinical implications. Lancet Oncol.
13:e23–e31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi Y, Au JS, Thongprasert S, et al: A
prospective, molecular epidemiology study of EGFR mutations in
Asian patients with advanced non-small-cell lung cancer of
adenocarcinoma histology (PIONEER). J Thorac Oncol. 9:154–162.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Riely GJ, Pao W, Pham D, et al: Clinical
course of patients with non-small cell lung cancer and epidermal
growth factor receptor exon 19 and exon 21 mutations treated with
gefitinib or erlotinib. Clin Cancer Res. 12:839–844. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun JM, Won YW, Kim ST, et al: The
different efficacy of gefitinib or erlotinib according to epidermal
growth factor receptor exon 19 and exon 21 mutations in Korean
non-small cell lung cancer patients. J Cancer Res Clin Oncol.
137:687–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tam IY, Chung LP, Suen WS, et al: Distinct
epidermal growth factor receptor and KRAS mutation patterns in
non-small cell lung cancer patients with different tobacco exposure
and clinicopathologic features. Clin Cancer Res. 12:1647–1653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marks JL, Broderick S, Zhou Q, et al:
Prognostic and therapeutic implications of EGFR and KRAS mutations
in resected lung adenocarcinoma. J Thorac Oncol. 3:111–116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yano M, Sasaki H, Kobayashi Y, et al:
Epidermal growth factor receptor gene mutation and computed
tomographic findings in peripheral pulmonary adenocarcinoma. J
Thorac Oncol. 1:413–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshida Y, Kokubu A, Suzuki K, et al:
Molecular markers and changes of computed tomography appearance in
lung adenocarcinoma with ground-glass opacity. Jpn J Clin Oncol.
37:907–912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chung JH, Choe G, Jheon S, et al:
Epidermal growth factor receptor mutation and pathologic-radiologic
correlation between multiple lung nodules with ground-glass opacity
differentiates multicentric origin from intrapulmonary spread. J
Thorac Oncol. 4:1490–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Glynn C, Zakowski MF and Ginsberg MS: Are
there imaging characteristics associated with epidermal growth
factor receptor and KRAS mutations in patients with adenocarcinoma
of the lung with bronchioloalveolar features? J Thorac Oncol.
5:344–348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park EA, Lee HJ, Kim YT, et al: EGFR gene
copy number in adenocarcinoma of the lung by FISH analysis:
investigation of significantly related factors on CT, FDG-PET, and
histopathology. Lung Cancer. 64:179–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee Y, Lee HJ, Kim YT, et al: Imaging
characteristics of stage I non-small cell lung cancer on CT and
FDG-PET: relationship with epidermal growth factor receptor protein
expression status and survival. Korean J Radiol. 14:375–383. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hsu KH, Chen KC, Yang TY, et al: Epidermal
growth factor receptor mutation status in stage I lung
adenocarcinoma with different image patterns. J Thorac Oncol.
6:1066–1072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Aoki T, Hanamiya M, Uramoto H, Hisaoka M,
Yamashita Y and Korogi Y: Adenocarcinomas with predominant
ground-glass opacity: correlation of morphology and molecular
biomarkers. Radiology. 264:590–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Riely GJ, Politi KA, Miller VA and Pao W:
Update on epidermal growth factor receptor mutations in non-small
cell lung cancer. Clin Cancer Res. 12:7232–7241. 2006. View Article : Google Scholar : PubMed/NCBI
|