Introduction
Skin wound healing is a complex multifactorial
process, involving inflammation, cell proliferation, cell
migration, re-epithelialization, angiogenesis, extracellular matrix
deposition and remodeling (1,2). Burn
wounds have a long healing period, and are the most representative
persistent wounds for use in the study of skin wound healing. Deep
second-degree burn wounds exhibit spontaneous epithelial
regeneration through the proliferation and migration of the skin
appendages under the wound and the epidermal cells around the wound
margin (3). The healing period is
relatively long; thus, deep second-degree burns are useful for the
experimental study of burn wounds. Animal models of burn wounds
have been used to study various pathological changes and healing
mechanisms involved in the burn wound healing process (4).
Burn-induced tissue ischemia and inflammation can
increase the number of mast cells in burn tissues (5) and stimulate mast cells to release
vasoactive substances by degranulation (6). Mast cells play a role in the acute
inflammatory phase and participate in wound healing together with
fibroblasts (7,8). Once activated, mast cells degranulate
and release histamine, heparin and a variety of enzymes, such as
chymase, cathepsin G and hydroxy peptidase A (9). Chymase is closely associated with
tissue fibrosis.
Chymase is an α-chymase-like serine protease that is
involved in numerous physiological and pathophysiological
processes. One of the most important functions of chymase is the
regulation of angiotensin (Ang)II generation. In addition, chymase
promotes myocardial and skin fibroblast proliferation (10,11), the
release of transforming growth factor-β1 bound to the extracellular
matrix and the degradation of procollagen protein that participate
in tissue remodeling (12) and
inflammation (13). Previous studies
have shown that mast cell chymase is active during the healing
process in burn wounds (14,15). However, the changes in chymase
expression levels and activity in the tissues during burn wound
healing remain unclear. Human chymase differs to the chymase in the
majority of other animals with regard to substrate specificity,
with the exceptions of monkeys, dogs and hamsters (16–18). To
the best of our knowledge, there have been no previous studies
reporting the use of a hamster model in the study of burn wounds.
In the present study, a hamster model of deep second-degree burn
wound was established in order to study the association between
mast cell chymase and the burn wound healing process.
Materials and methods
Hamster model for deep second-degree
burn wound
A total of 24 hamsters (weight, 40–60 g; age, 8
weeks) were used, obtained from the Ürümqi Municipal Center for
Disease Control and Prevention (Ürümqi, China). All animals were
individually housed in stainless steel cages with a 12-h light-dark
cycle and a controlled temperature. The hamsters received food and
water ad libitum and were acclimatized for at least two
weeks prior to thermal injury. All animal care and experimental
procedures were approved by the Animal Ethics Committee of the
First Affiliated Hospital of Xinjiang Medical University (Ürümqi,
China).
The body surface areas of the hamsters (equivalent
to 0.0913 × weight2/3) ranged between 106.8 and 139.9
cm2 (19). The burn wound
was circular, with a diameter of ~3 cm, accounting for 5–6.5% of
their body surface area. The hamsters were anesthetized with
ketamine (0.7 g/kg), and diazepam and atropine were used to
maintain adequate anesthesia. Once anesthetized, the hair on the
back of each hamster was removed to reveal a bare region with a
diameter of ~3 cm. There are a variety of methods for producing
deep second-degree burns in animal models (20–24);
however, the majority of these methods are adapted to large animals
and may be complex. The burn wound depth may be affected by the
contact temperature, heat duration, burn area and pressure. Since
hamsters are small in size, there is a risk of accidental mortality
if the burn is not inflicted correctly. The end of a 50-ml syringe,
with no plunger, was used to administer the burn. While the hamster
was anesthetized, the end of the syringe was gently pressed onto
the bare region on the back of the hamster to keep the hot water
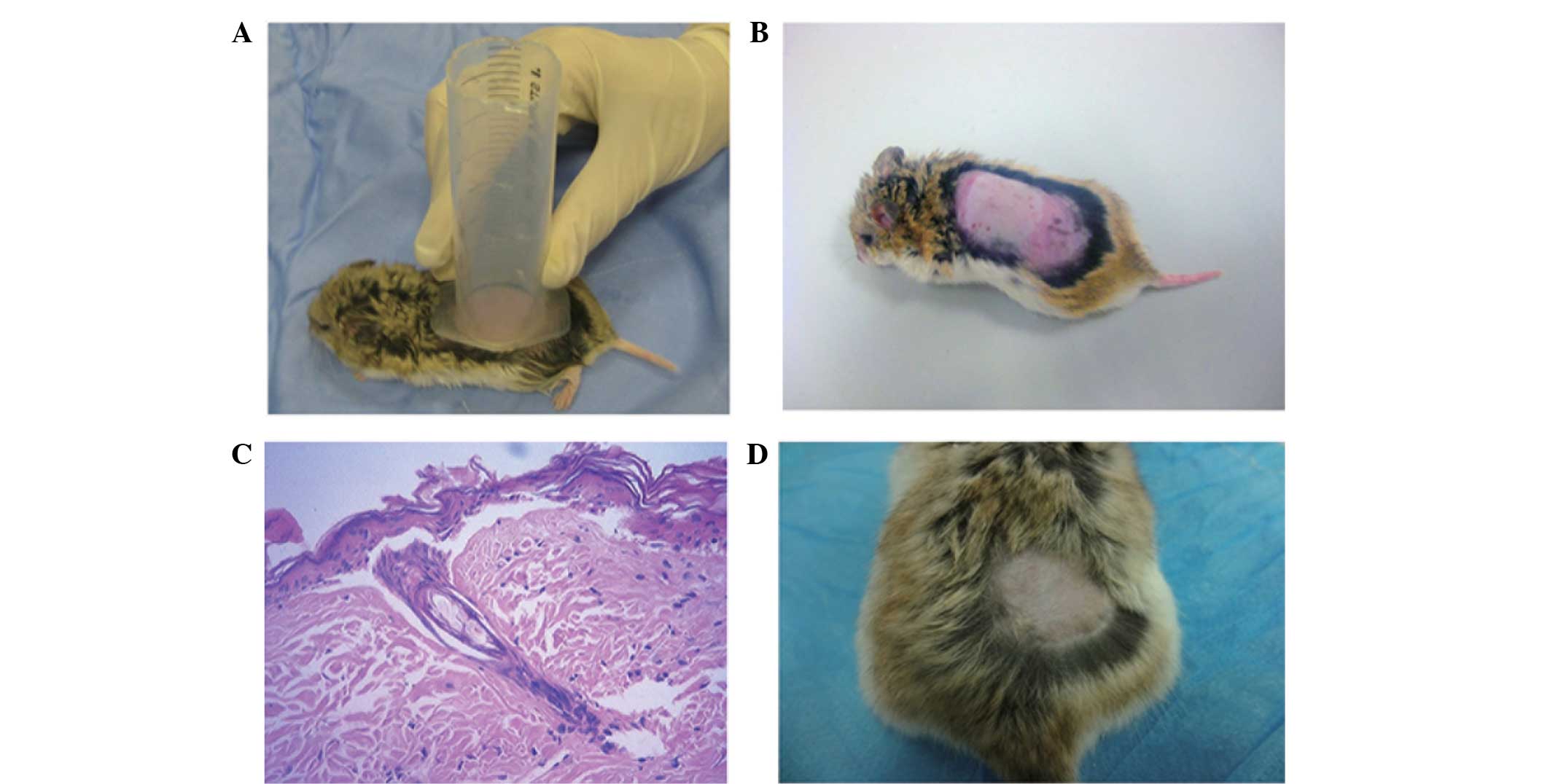
inside the syringe (Fig. 1A). Next,
20 ml water (75°C) was poured into the syringe, producing a
circular burn wound with a diameter of ~3 cm. The durations of
water contact with the back skin of the hamsters were 0, 6, 8, 10
and 12 sec, for the five groups of four hamsters. The wounds of all
the hamsters were was hed with running water for 1 min and 1.5–1.8
ml isotonic saline was injected intraperitoneally for fluid
resuscitation. The hamsters were insulated and received 700 mg/kg
morphine when awake. The hamsters stopped experiencing any pain by
day 2 after the burn was administered which was evident by their
return to normal behavior. At 24 h after the burn, the wound
tissues in the superficial layer of the deep fascia were removed,
with the hamsters under ketamine anesthesia. The dissected tissues
were immersed in 10% formaldehyde solution, with a volume ten times
the volume of the tissues. The dissected tissues were divided into
sections and stained with hematoxylin and eosin (H&E). The
optimum duration of hot water contact for producing a deep
second-degree burn was determined by H&E staining. According to
the histologically determined durations of contact for deep
second-degree burn, the wounds were created on the remaining four
hamsters to observe wound healing durations.
H&E staining
Tissue samples were cut into slices for H&E
staining. The sections were dewaxed with xylene for 15 min and
soaked with 100% ethanol for 3 min, 95% ethanol for 3 min and 70%
ethanol for 3 min. The sections were then was hed with water,
air-dried, soaked with hematoxylin for 15 min and was hed with
water a further three times. Subsequently, the sections were
stained with eosin for 3 min and soaked with 95% ethanol I for 3
min, 95% ethanol II for 3 min, 100% ethanol I for 3 min and 100%
ethanol II for 3 min. Once the ethanol had dried, the sections were
soaked in xylene for 2 h and subsequently mounted using
Permount™ Mounting Medium purchased from Multi Sciences
(Lianke) Biotech Co., Ltd. (Hangzhou, China).
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted with TRIzol®(Invitrogen Life
Technologies, Carlsbad, CA, USA), chloroform, isopropanol and 75%
ethylene glycol, and stored in a refrigerator at −80°C. Reverse
transcription was conducted by adding 1.5 µg sample RNA to a 20-µl
reaction system that included 1 µl Oligo (dT) Primer, 1 µl reverse
transcriptase and 4 µl buffer (Takara Biotechnology Co., Ltd.,
Dalian, China). The reaction mixture was incubated for 1 h at 37°C
and then heated to 85°C for 5 min to terminate the reaction. For
qPCR amplification, 5 µl reacted solution was extracted and added
to a 20-µl reaction system that included 10 µl SYBR Premix Ex Taq,
0.25 µl target primer/0.25 µl β-actin primer (Takara Biotechnology
Co., Ltd.) and 4.5 µl ddH2O. The reaction was conducted
on a Thermal Cycler Dice Real Time System (Takara Bio, Inc., Otsu,
Japan). For the amplification of chymase, the following PCR
procedure was used: 95°C for 3 min, 35 cycles of 95°C for 45 sec,
55°C for 45 sec and 72°C for 45 sec, and a final extension at 72°C
for 5 min. For the amplification of β-actin, the following PCR
procedure was used: 95°C for 4 min, 35 cycles of 95°C for 30 sec,
60°C for 30 sec and 72°C for 2 min, and a final extension at 72°C
for 7 min. The PCR product was examined by dissociation curve
analysis, and the relative quantification of gene expression was
normalized against the internal standard, β-actin.
The primers were designed as follows: Chymase
forward, 5-CTG AGA GGA TGC TTC TTC CTG C-3, and reverse, 5-AGA TCT
TAT TGA TCC AGG GCC G-3; β-actin forward, 5-AAC TCC ATC ATG AAG TGT
GA-3, and reverse, 5-ACT CCT GCT TGC TGA TCC AC-3.
Radioimmunoassay
Chymase activity in the burn tissue was determined
using a radioimmunoassay (25). Burn
tissue samples weighing 100 mg were repeatedly homogenized and
added to 50 mM NaH2PO4 buffer (10 w/v, pH
7.4) for 15 min at 4°C. The samples were centrifuged at 3,000 × g
for 20 sec at 4°C and the supernatants were discarded. The
sediments were added to 50 mM NaH2PO4 buffer
for ultrasonic homogenization (400 A, 5 sec/time with an interval
of 10 sec, repeated three to five times) and the homogenized
tissues were subsequently centrifuged at 30,000 × g for 20 min at
4°C. The homogenization and centrifugation steps were repeated
three times and the supernatants, which contained chymase, were
collected for each repeat. From each processed sample, 1,500 µl
supernatant was collected and divided into three tubes (500 µl
each) labeled A, B and C. For reaction system A, 6 ng AngI was
added, while 6 ng AngI and 50 µM lisinopril were added to reaction
system B and 6 ng AngI and 20 µM aprotinin were added to reaction
system C. The tubes were placed in a water bath (37°C) for 15 min,
and a 2.5-fold volume of precooled ethanol was added to terminate
the reaction. The resulting concentrations of AngII in reaction
systems A, B and C were determined using a radioimmunoassay kit
(Beijing North Institute of Biological Technology, Beijing, China),
according to the manufacturers instructions. The activity of
angiotensin-converting enzyme (ACE) was calculated by subtracting
the enzyme activity of reaction system B from that of A. The
activity of other serine proteases was calculated by subtracting
the enzyme activity of reaction system C from that of A. Finally,
the activity of chymase was calculated by subtracting the ACE
activity and the other serine protease activity from the enzyme
activity of reaction system A. The enzyme activity required for the
generation of 1 nmol AngII in 100 mg burn tissues per min was
defined as 1 enzyme activity unit (U). The activity of chymase was
expressed as U/mg.
Statistical analysis
SPSS software, version 16.0 (SPSS, Inc., Madison,
WI, USA) was used for statistical analysis. The results are
presented as the mean ± standard deviation. Analysis of variance
and Dunnetts test were used to assess the differences between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
A hamster model of deep second-degree
burn is established by hot water contact for 12 sec
To evaluate the degree of the burn wounds, hamsters
were used as the animal model and H&E staining of the tissues
was performed. All hamsters used in the study survived. The degree
of paleness of the burnt skin varied at different time periods
after the injury was inflicted. At 24 h after the burn was
inflicted, the edge of the burn wound was red with swelling, while
the wound itself exhibited swelling and was white in color, with no
visible blisters (Fig. 1B). The burn
contact durations analyzed were 0, 6, 8, 10 and 12 sec.
Histological examination revealed that the epidermal cells were
partially detached from the burn area in the 12-sec burn duration
hamsters. H&E staining showed that the structure of the tissues
was unclear and that blisters had formed on the skin. In addition,
congestion had occurred in the dermal interstitial layer, collagen
fibers had integrated into sheets and residual hair follicles were
observed in the deep dermis. Subcutaneous vascular dilation,
congestion and edema were evident; however, the tissue structure
was not destroyed (Fig. 1C). Thus,
it was determined that the damage caused by 12 sec hot water
contact was equivalent to a deep second-degree burn. Deep
second-degree burns were produced on the four hamsters that
received hot water contact for 12 sec. There were no signs of
infection during wound healing and re-epithelialization was
complete after 18 days (Fig. 1D).
These results indicated that a hamster model of deep second-degree
burn was established by applying hot water contact for 12 sec.
Chymase activity and mRNA expression
levels increase in mast cells after burning and may be involved in
the process of burn wound healing
A radioimmunoassay and qPCR were used to investigate
the activity and mRNA expression levels of chymase in the burn
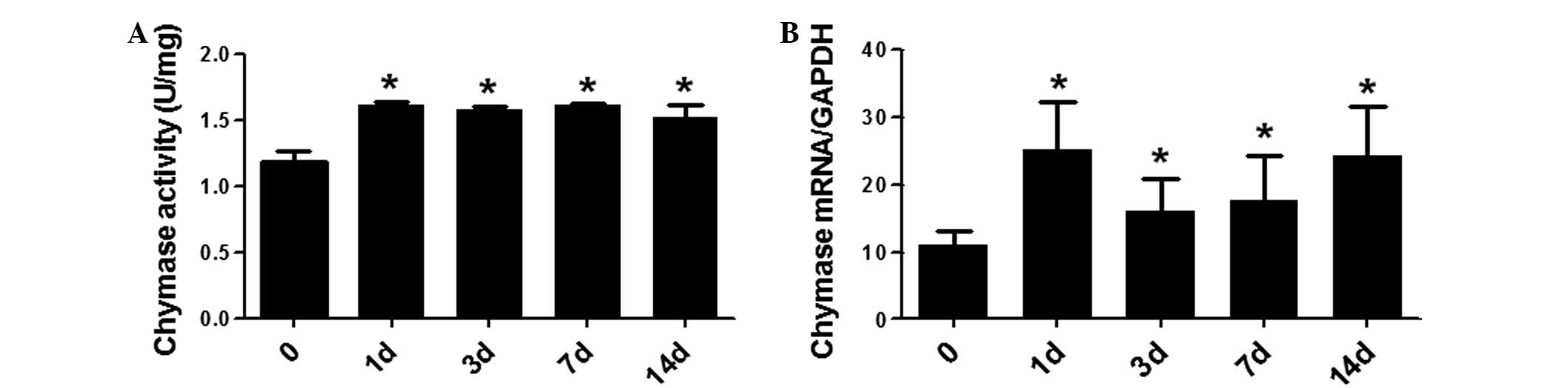
tissues. Chymase activity was observed in all the post-burn stages
and was significantly higher in the burnt tissue when compared with
the normal skin tissue (day 0). However, there were no
statistically significant differences in chymase activity amongst
the various post-burn stages (P>0.05; Fig. 2A). At days 1, 3, 7 and 14 after
burning, chymase mRNA expression levels were significantly higher
when compared with those in the normal skin tissue (day 0;
P<0.05). In addition, no statistically significant differences
were observed amongst the chymase mRNA expression levels of the
post-burn stages (P<0.05; Fig.
2B). These results demonstrated that chymase activity and mRNA
expression levels were increased in the mast cells of the burned
tissue and may be involved in the process of burn wound
healing.
Discussion
Hamsters were selected as experimental animals to
study chymase activity, as the AngII generated by chymase differs
between species, but is similar in humans and hamsters. The burns
may easily have been fatal to the hamsters; however, the
temperature of the water was controlled and the burn area selected
was easy to manipulate. Thus, hot water at 75°C was used to produce
deep second-degree burn wounds on the hamsters.
Kim et al (26) used continuous scan laser Doppler
vibrometry to observe the microvascular perfusion conditions of
large-area deep burn wounds in mice and determined the degrees of
the burn wounds according to the recovery of the mice.
Second-degree burns developed into third-degree burns if the fluid
resuscitation rate was ≤4 ml/kg/%burn. The optimal fluid
resuscitation rate was 4–8 ml/kg/%burn and the perfusion peak was 6
ml/kg/%burn. In the present study, the burn wounds were was hed
with water for 5 min to reduce the residual heat. In addition,
1.5–1.8 ml normal saline (6 ml/kg/%burn) was administered via
intraperitoneal injection for fluid resuscitation in order to
reduce the exacerbation of the depth of the wounds.
In the present study, the burn area was 7.1
cm2, accounting for 5–6.5% of the body surface area,
which was large enough for the requirements of the study. All
hamsters used in the study survived. In addition, no wound
infection or other complications were found during the healing
process of the deep second-degree wounds. The wounds produced by
contact with 20 ml hot water (75°C) in a 50-ml syringe on the skin
of the hamsters for 12 sec satisfied the requirements of a deep
second-degree burn wound.
Chen et al (27) used qPCR to detect the mRNA expression
levels of cardiac chymase in a hamster model of heart failure,
while Guo et al (28) did the
same in ovalbumin-stimulated atherosclerosis hamsters. Matsumoto
et al (29) used qPCR to
detect the mRNA expression levels of chymase in dogs with
tachycardia-induced heart failure following the administration of
the chymase inhibitor, SUNC8257. Notably, the present study
observed increased chymase activity and mRNA expression levels in
the hamsters during the process of burn wound healing when compared
with the levels prior to the burns. Chymase activity and mRNA
expression levels during the healing process were significantly
increased when compared with the levels prior to the burn model
establishment (P<0.05), while those in different post-burn
stages showed no statistically significant differences (P>0.05).
The increased chymase activity in the burn tissue may have been
caused by a burn-induced stress response in the hamsters, in which
the burns directly acted on the mast cells, activating them to
increase degranulation and release chymase. In addition, the burns
caused increased vascular permeability, and inflammatory responses
increased the numbers and sustained activation of mast cells in the
burn wounds.
In conclusion, the results of the present study
indicate that chymase is involved in the process of burn wound
healing. These results may provide experimental evidence for
elucidating the process of burn wound healing and aid the
development of new treatment methods for burn wounds.
Acknowledgements
This study was supported by the Youth Science
Foundation of the First Affiliated Hospital of Xinjiang Medical
University (no. 2012QN02).
References
|
1
|
Martin P: Wound healing - Aiming for
perfect skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akbari H, Fatemi MJ, Iranpour M, et al:
The healing effect of nettle extract on second degree burn wounds.
World J Plast Surg. 4:23–28. 2015.PubMed/NCBI
|
|
4
|
Domergue S, Jorgensen C and Noël D:
Advances in research in animal models of burn-related hypertrophic
scarring. J Burn Care Res. Oct 29–2014.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rantfors J and Cassuto J: Role of
histamine receptors in the regulation of edema and circulation
postburn. Burns. 29:769–777. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santos FX, Arroyo C, Garcia I, et al: Role
of mast cells in the pathogenesis of postburn inflammatory
response: reactive oxygen species as mast cell stimulators. Burns.
26:145–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
el Sayed SO and Dyson M: Responses of
dermal mast cells to injury. J Anat. 182:369–376. 1993.PubMed/NCBI
|
|
8
|
Hebda PA, Collins MA and Tharp MD: Mast
cell and myofibroblast in wound healing. Dermatol Clin. 11:685–696.
1993.PubMed/NCBI
|
|
9
|
Kovanen PT: Mast cells: multipotent local
effector cells in atherothrombosis. Immunol Rev. 217:105–122. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao XY, Zhao LY, Zheng QS, et al: Chymase
induces profibrotic response via transforming growth
factor-beta1/Smad activation in rat cardiac fibroblasts. Mol Cell
Biochem. 310:159–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maruichi M, Takai S, Sugiyama T, et al:
Role of chymase on growth of cultured canine Tenons capsule
fibroblasts and scarring in a canine conjunctival flap model. Exp
Eye Res. 79:111–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saarinen J, Kalkkinen N, Welgus HG and
Kovanen PT: Activation of human interstitial procollagenase through
direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J
Biol Chem. 269:18134–18140. 1994.PubMed/NCBI
|
|
13
|
Doggrell SA and Wanstall JC: Vascular
chymase: pathophysiological role and therapeutic potential of
inhibition. Cardiovasc Res. 61:653–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishikori Y, Kakizoe E, Kobayashi Y, et
al: Skin mast cell promotion of matrix remodeling in burn wound
healing in mice: relevance of chymase. Arch Dermatol Res.
290:553–560. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong X, Chen J, Zhang Y and Cen Y: Mast
cell chymase promotes cell proliferation and expression of certain
cytokines in a dose-dependent manner. Mol Med Rep. 5:1487–1490.
2012.PubMed/NCBI
|
|
16
|
Wintroub BU, Schechter NB, Lazarus GS,
Kaempfer CE and Schwartz LB: Angiotensin I conversion by human and
rat chymotryptic proteinases. J Invest Dermatol. 83:336–339. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takai S, Shiota N, Jin D and Miyazaki M:
Functional role of chymase in angiotensin II formation in human
vascular tissue. J Cardiovasc Pharmacol. 32:826–833. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caughey GH, Raymond WW and Wolters PJ:
Angiotensin II generation by mast cell alpha- and beta-chymases.
Biochim Biophys Acta. 1480:245–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schildt B and Nilsson A: Standardized
burns in mice. Eur Surg Res. 2:23–33. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Somboonwong J, Kankaisre M, Tantisira B
and Tantisira MH: Wound healing activities of different extracts of
Centella asiatica in incision and burn wound models: an
experimental animal study. BMC Complement Altern Med. 12:1032012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramos-Gallardo G, Ambriz-Plascencia AR and
Gonzalez-Reynoso L: Systemic steroids use in second-degree burn
using an animal model. Rev Med Inst Mex Seguro Soc. 50:9122012.(In
Spanish). PubMed/NCBI
|
|
22
|
Gaines C, Poranki D, Du W, Clark RA and
Van Dyke M: Development of a porcine deep partial thickness burn
model. Burns. 39:311–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kempf M, Cuttle L, Liu PY, Wang XQ and
Kimble RM: Important improvements to porcine skin burn models, in
search of the perfect burn. Burns. 35:454–455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cuttle L, Kempf M, Phillips GE, et al: A
porcine deep dermal partial thickness burn model with hypertrophic
scarring. Burns. 32:806–820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cribbs RK, Luquette MH and Besner GE: A
standardized model of partial thickness scald burns in mice. J Surg
Res. 80:69–74. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim DE, Phillips TM, Jeng JC, et al:
Microvascular assessment of burn depth conversion during varying
resuscitation conditions. J Burn Care Rehabil. 22:406–416. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen W, Yu MH, Li YM, Chen WJ and Xia YP:
Beneficial effects of astragalus polysaccharides treatment on
cardiac chymase activities and cardiomyopathy in diabetic hamsters.
Acta Diabetol. 47 (Suppl 1):35–46. 2010. View Article : Google Scholar
|
|
28
|
Guo T, Chen WQ, Zhang C, Zhao YX and Zhang
Y: Chymase activity is closely related With plaque vulnerability in
a hamster model of atherosclerosis. Atherosclerosis. 207:59–67.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsumoto T, Wada A, Tsutamoto T, Ohnishi
M, Isono T and Kinoshita M: Chymase inhibition prevents cardiac
fibrosis and improves diastolic dysfunction in the progression of
heart failure. Circulation. 107:2555–2558. 2003. View Article : Google Scholar : PubMed/NCBI
|