Introduction
Matrix metalloproteinases (MMPs) belong to a family
of zinc-dependent endopeptidases that degrade proteins of the
extracellular matrix (ECM). MMPs regulate important biological
functions, including ECM integrity. The enzymes are expressed in
normal and diseased states and participate in vascular and cardiac
remodeling, wound healing, tissue resorption and tumor metastasis
(1,2). The activity of MMPs is tightly
controlled by several inhibitors, including the nonspecific
α2-macroglobulin and the locally produced specific
tissue inhibitors of metalloproteinases (TIMPs). Four human TIMPs
have been identified to date, which include TIMP-1, TIMP-2, TIMP-3
and TIMP-4. These proteins constitute a group of small molecules
with a mass of 21–28 kDa, which reversibly inhibit the activity of
MMPs, binding to them in a 1:1 stoichiometric ratio (3). TIMPs function in a tissue environment
to neutralize metalloproteinases that have already functioned,
thereby preventing excessive and uncontrolled degradation away from
the site of MMP production. The role of TIMPs is essential for the
homeostasis of the ECM. Although TIMPs share notable homology and
structural identity at the protein level, the different forms may
vary in their functions. TIMP-1 inhibits angiogenesis by
restraining the MMP-9-mediated release of vascular endothelial
growth factor (VEGF) from the matrix (4). TIMP-2 has also been shown to present
antiangiogenic properties by functioning through MMP inhibition or
through direct endothelial cell (EC) binding. In addition, TIMP-2
regulates MMP-14-induced MMP-2 activation by forming a tertiary
complex with pro-MMP-2 and its activator, MMP-14, on the cell
surface. TIMP-2 can either initiate or restrain the cleavage and
subsequent activation of MMP-2 (5).
Furthermore, TIMP-2 can bind to cells via interaction with integrin
α3β1 on the surface of human microvascular ECs. This interaction
mediates the suppression of VEGF or fibroblast growth
factor-mediated cell proliferation (6). TIMP-3 has been shown to promote
apoptosis (7).
The endothelium is composed of a single layer of
cells that constitute the inner surface of the blood vessels. The
endothelium functions not only as a barrier between the blood and
smooth muscle cells of the blood vessels, but synthesizes and
releases various substances with potent multidirectional biological
functions. Therefore, the endothelium plays a crucial role in the
regulation of vasomotorics and hemostasis. ECs can be a source of
the substances involved in angiogenesis and the inflammatory
process.
Atherosclerosis is a systemic multiform condition
that leads to various diseases depending on the vascular site where
the condition is most pronounced. For example, coronary heart
disease (CHD), stroke or intermittent claudication may be caused by
ischemia in the lower limbs. Furthermore, the aortic wall may be a
site of a different dangerous pathology, namely an aortic aneurysm.
The American National Cholesterol Education Program, Adult
Treatment Panel III, indicates that an abdominal aortic aneurysm
should be considered as an equivalent of CHD, which is one of the
manifestations of atherosclerosis (8). Endothelium dysfunction, independent of
the underlying causes, is the first step leading to the development
of atherosclerosis, CHD and other cardiovascular diseases (CVDs)
(9).
Elastin-derived peptides (EDPs) are generated as a
result of the degradation of elastin fibers. Elastin is a
long-lived macromolecule with a very slow turn-over. However, the
metabolism of elastin is accelerated in various disease states,
such as atherosclerosis, lung emphysema, neoplasm and arthritis
(10). Cell responses to EDPs are
attributed to the binding of a VGVAPG hexapeptide sequence, which
can be detected in insoluble elastin or EDPs. This sequence binds
to the elastin receptor and exerts numerous biological effects
(11). An experimental study
undertaken in our Department has revealed that rabbits receiving
EDP injections develop atherosclerosis (12). In addition, disturbances in elastin
metabolism, leading to increased serum levels of EDPs, are
considered as one of the risk factors of atherosclerosis. Since
κ-elastin is an acknowledged EDP, numerous experiments have
evaluated the effect of κ-elastin on the aorta and EC lines
(13–15). In a previous study, the influence of
κ-elastin on the production of MMP-1 and MMP-2 in cultured human EC
lines derived from coronary arteries, the iliac artery and aorta
was evaluated (16). These
localizations in the vascular system are clinically important and
present various conditions of blood flow.
The aim of the present study was to compare the
production of three TIMPs, TIMP-1, TIMP-2 and TIMP-3, and their
concentration ratios in cultured human endothelium derived from
three clinically important vascular localizations, namely the
coronary arteries, aorta and iliac artery. In addition, the present
study evaluated the effect of κ-elastin on the production of the
three TIMPs and their proportions in the three studied endothelial
cell lines.
Materials and methods
Cell culture
EC lines isolated from the human coronary artery,
iliac artery and aorta were purchased from Lonza (Basel,
Switzerland). The three EC lines were cultured according to the
manufacturer's recommendations. Briefly, the cells were maintained
in EBM-2 medium with 5% fetal bovine serum and endothelial
cell-specific supplements (insulin-like growth factor, VEGF and
heparin; all purchased from Lonza) in a 95% CO2
atmosphere at 37°C. ECs were used in the experiments after 3–4
passages. Following trypsinization, the cells were grown to
confluence in 24-well plates. Subsequently, after serum starvation,
the cells were incubated with κ-elastin (Elastin Products Company,
Owensville, MO, USA) at concentrations of 0.1, 0.4, 1.0, 2.5 or 5.0
µg/ml for 24 h. The cell culture medium was removed, centrifuged
for 15 min at 1,500 × g and stored at −70°C for subsequent
analyses.
ELISA
TIMP-1, TIMP-2 and TIMP-3 concentrations were
determined using commercially available kits (R&D Systems,
Inc., Minneapolis, MN, USA) with antibodies against human TIMPs
(R&D Systems, Inc.). The experiment was performed four times
with two repeats at each time, producing eight sets of samples for
the determination of the evaluated parameters. Each measurement was
performed in duplicate and a mean value of the two determinations
was calculated.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Prior to the parametric analyses, normal distribution
was verified with the Shapiro-Wilk test. In addition, the
homogeneity of variance was analyzed using Bartlett's test. The
statistical significance of the differences between groups was
calculated using a parametric one-way analysis of variance test for
data with a normal distribution, assuming homogeneity and
heterogeneity of variances, followed by the Tukey-Kramer test. The
Kruskal-Wallis rank test was applied in the case of non-normality
of distribution, which was followed by the Steel-D was s test.
Dunnett's test or Steel's test was used to compare each group with
the control. P≤0.05 was considered to indicate a statistically
significant difference. The statistical software Statistica 10.0
(Statsoft, Tulsa, Oklahoma, USA) was used in this analysis.
Results
Concentration of TIMPs in the various
EC lines
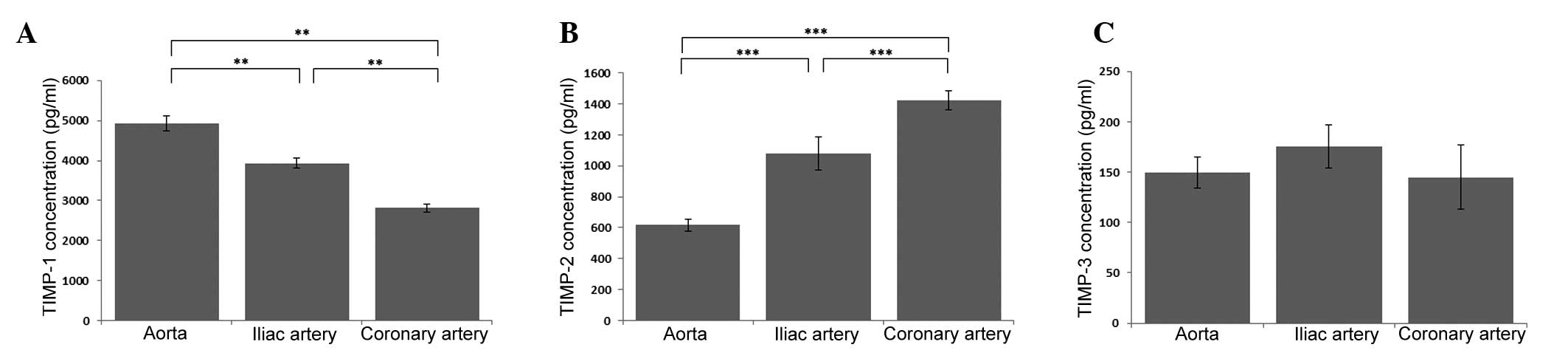
The highest concentration of TIMP-1 was observed in
the aortic endothelium, while the lowest concentration was detected
in the endothelium derived from the coronary artery (P<0.01). In
the endothelium derived from the iliac artery, a statistically
significantly decrease was observed in the TIMP-1 concentration
when compared with the aortic ECs (P<0.01), while a
statistically significant increase was observed when compared with
the coronary artery ECs (P<0.01; Fig.
1A). The opposite pattern of dependence was observed in the
case of TIMP-2. TIMP-2 reached the highest concentration in the
cell culture medium from the coronary artery endothelium, while the
lowest concentration was observed in the aortic ECs (P<0.001).
In addition, in the ECs derived from the iliac artery, a
statistically significant decrease was observed in the
concentration of TIMP-2 when compared with the ECs derived from the
coronary artery (P<0.01), while a statistically significant
increase was observed when compared with the aortic ECs
(P<0.001; Fig. 1B). However, when
the TIMP-3 concentration was analyzed in the three EC lines, no
statistically significant differences were identified (Fig. 1C).
Effect of κ-elastin on TIMP-1
production
Following the addition of κ-elastin at
concentrations between 0.4 and 5.0 µg/ml to the cell culture of
aortic ECs, a statistically significant decrease in the TIMP-1
concentration was observed (P<0.001; Fig. 2A). However, in the cell culture of
the coronary artery-derived ECs, a higher concentration of
κ-elastin (1.0 and 2.5 µg/ml) was required to cause a statistically
significant reduction in the TIMP-1 concentration (Fig. 2C). The opposite effect of κ-elastin
on TIMP-1 production was observed in the ECs derived from the iliac
artery, where an increase in the TIMP-1 concentration was detected
at lower κ-elastin concentrations. The increase was most pronounced
at a κ-elastin concentration of 0.4 µg/ml, which resulted in ~23%
increase in the TIMP-1 concentration in the cell culture medium
(Fig. 2B).
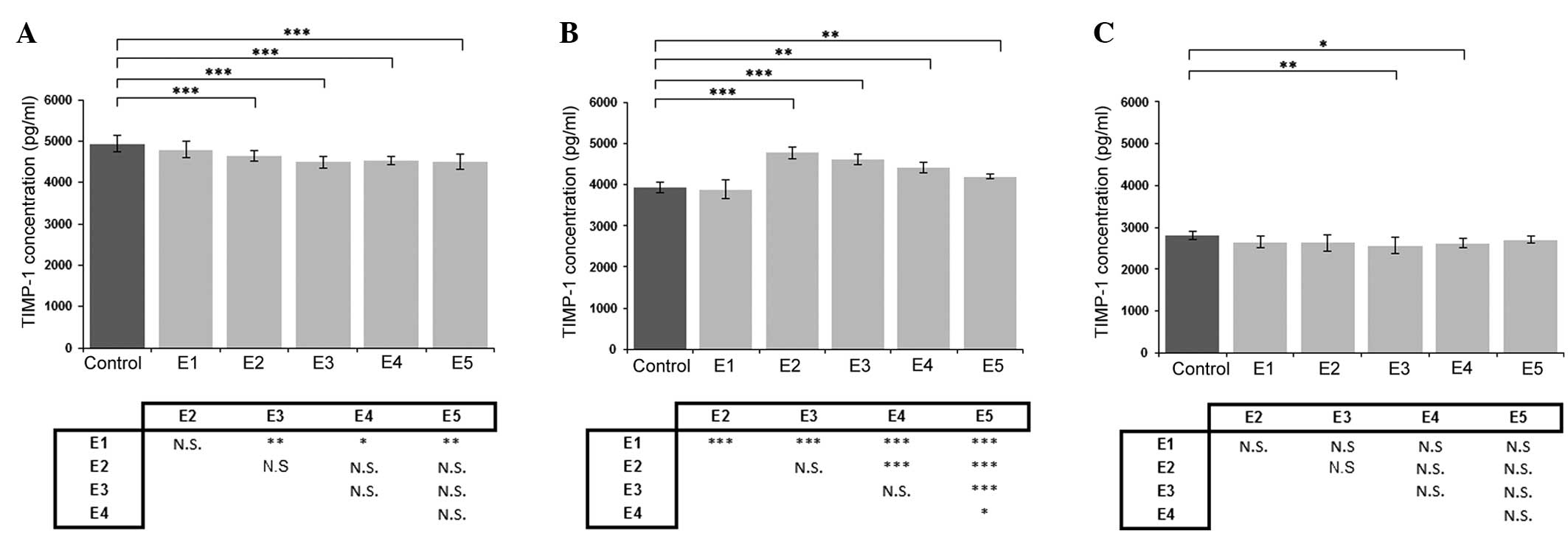 | Figure 2.Effects of various concentrations of
κ-elastin on the TIMP-1 concentration in human endothelial cells
derived from the (A) aorta, (B) iliac artery and (C) coronary
artery. E1, E2, E3, E4 and E5, κ-elastin concentrations of 0.1,
0.4, 1.0, 2.5 and 5.0 µg/ml, respectively. Values are presented as
the mean ± standard deviation. *P<0.05, **P<0.01 and
***P<0.001, compared between groups. N.S., not significant;
TIMP, tissue inhibitor of metalloproteinase. |
Effect of κ-elastin on TIMP-2
production
Evaluation of the effect of κ-elastin on TIMP-2
concentration revealed that a decrease in concentration was most
pronounced in the ECs obtained from the coronary arteries (Fig. 3C). With regard to the aortic ECs,
only a concentration of 1.0 µg/ml caused a statistically
significant reduction in TIMP-2 production (Fig. 3A). In addition, κ-elastin did not
cause any statistically significant changes in the TIMP-2
concentration in the ECs derived from the iliac artery (Fig. 3B).
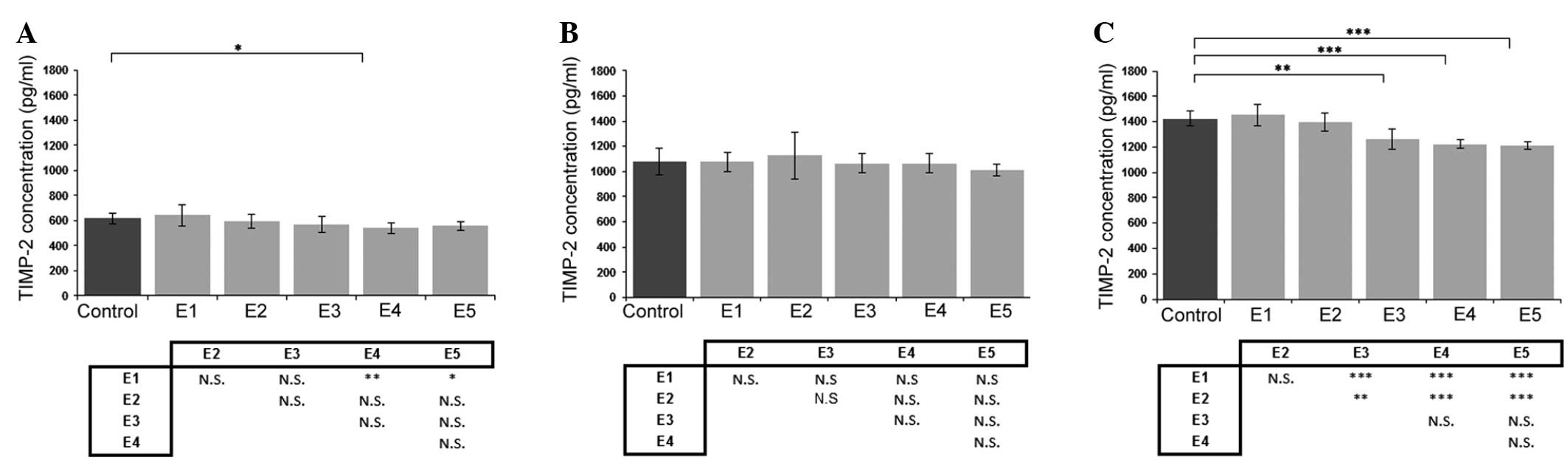 | Figure 3.Effects of various concentrations of
κ-elastin on the TIMP-2 concentration in human endothelial cells
derived from the (A) aorta, (B) iliac artery and (C) coronary
artery. E1, E2, E3, E4 and E5, κ-elastin concentrations of 0.1,
0.4, 1.0, 2.5 and 5.0 µg/ml, respectively. Values are presented as
the mean ± standard deviation. *P<0.05, **P<0.01 and
***P<0.001, compared between groups. N.S., not significant;
TIMP, tissue inhibitor of metalloproteinase. |
Effect of κ-elastin on TIMP-3
production
When the effect of κ-elastin on the concentration of
TIMP-3 was analyzed, no statistically significant differences were
detected in the cell lines derived from the aorta or iliac artery
(Fig. 4A and B). However, the
highest studied concentration of κ-elastin caused a statistically
significant increase in TIMP-3 production in the ECs derived from
the coronary arteries (Fig. 4C).
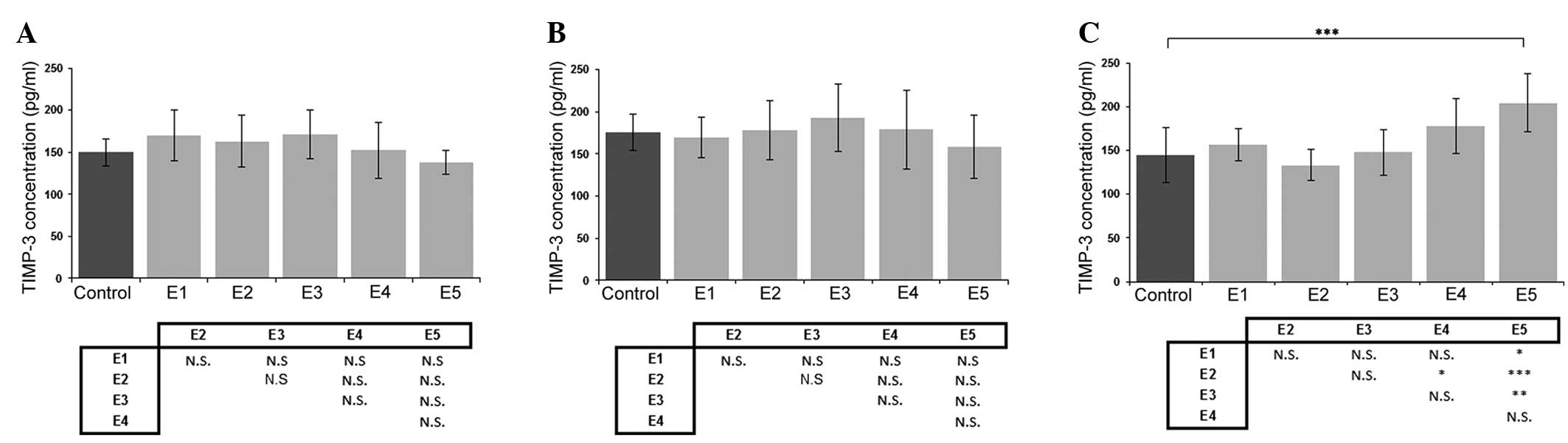 | Figure 4.Effects of various concentrations of
κ-elastin on the TIMP-3 concentration in human endothelial cells
derived from the (A) aorta, (B) iliac artery and (C) coronary
artery. E1, E2, E3, E4 and E5, κ-elastin concentrations of 0.1,
0.4, 1.0, 2.5 and 5.0 µg/ml, respectively. Values are presented as
the mean ± standard deviation. *P<0.05, **P<0.01 and
***P<0.001, compared between groups. N.S., not significant;
TIMP, tissue inhibitor of metalloproteinase. |
Effect of κ-elastin on the
concentration of TIMPs in the various EC lines
Comparisons of the effect of different
concentrations of κ-elastin revealed no statistically significant
differences in the TIMP-1 concentration in the ECs derived from the
coronary arteries, in the TIMP-2 concentration in the ECs derived
from the iliac artery and in the TIMP-3 concentration in the aortic
ECs and ECs derived from the iliac artery (Figs. 2C, 3B,
4A and B).
The most pronounced effect of κ-elastin on the
production of TIMP-1 was observed in the ECs derived from the aorta
and iliac artery. However, with regard to TIMP-2, the most
pronounced effect was observed in the ECs derived from the coronary
artery.
TIMP concentration ratios
The following ratios of TIMP concentrations were
calculated: TIMP-1/TIMP-2, TIMP-1/TIMP-3 and TIMP-2/TIMP-3
(Table I). Each ratio presented a
different value in the ECs obtained from the various localizations.
For example, the TIMP-1/TIMP-2 ratio was equal to seven in the
unstimulated aortic ECs, while the ratio was determined to be ~3.6
in the iliac artery ECs and ~1.9 in the coronary artery ECs. In the
majority of cases, the addition of κ-elastin did not significantly
change these proportions. The preservation of the magnitude of
these TIMP ratios was observed for all three TIMP ratios and for
all three studied EC lines. In addition, the stimulatory and
inhibitory effects of κ-elastin were not found to significantly
alter or reverse these proportions.
 | Table I.Concentration ratios of TIMPs in the
various EC lines. |
Table I.
Concentration ratios of TIMPs in the
various EC lines.
| EC line | TIMP-1/TIMP-2 | TIMP-2/TIMP-3 | TIMP-1/TIMP-3 |
|---|
| Control |
|
|
|
|
Aorta | 7.0 | 4.0 | 33.0 |
| Iliac
artery | 3.6 | 6.1 | 22.0 |
| Coronary
artery | 1.9 | 9.8 | 19.5 |
| E1 |
|
|
|
|
Aorta | 1.9 | 3.7 | 28.2 |
| Iliac
artery | 3.5 | 6.4 | 22.9 |
| Coronary
artery | 1.8 | 9.3 | 17.0 |
| E2 |
|
|
|
|
Aorta | 8.6 | 3.6 | 28.0 |
| Iliac
artery | 4.2 | 6.3 | 27.0 |
| Coronary
artery | 1.8 | 10.4 | 19.7 |
| E3 |
|
|
|
|
Aorta | 7.9 | 3.3 | 26.2 |
| Iliac
artery | 4.3 | 5.5 | 24.0 |
| Coronary
artery | 2.0 | 8.5 | 17.3 |
| E4 |
|
|
|
|
Aorta | 8.3 | 3.5 | 29.8 |
| Iliac
artery | 4.1 | 5.9 | 25.0 |
|
Coronary artery | 2.0 | 6.9 | 14.7 |
| E5 |
|
|
|
|
Aorta | 8.0 | 4.0 | 33.0 |
| Iliac
artery | 4.0 | 6.4 | 26.6 |
|
Coronary artery | 2.2 | 6.0 | 13.2 |
Discussion
EDPs have been demonstrated to be risk factors for
atherosclerosis. Robinet et al (17) observed that EDPs containing the
VGVAPG motif accelerated angiogenesis by inducing the angiogenic
phenotype of ECs.
TIMP-1 has attracted increasing attention as a
possible novel marker of endothelium dysfunction. A number of
studies have focused on evaluating the level of TIMP-1 in blood
samples obtained from patients with various risk factors of
atherosclerosis and CVD. Mieczkowska et al (18) observed that females with metabolic
syndrome had statistically higher blood levels of TIMP-1. In
addition, TIMP-1 was found to positively correlate with a variety
of risk factors, such as body mass index, waist to hip ratio, waist
circumference, fasting glucose level and triglyceride
concentration, while negatively correlating with the high-density
lipoprotein cholesterol level. Furthermore, de la Sierra and
Larrousse (19) found that TIMP-1
may be a marker of endothelial dysfunction in high-risk
hypertensive patients. TIMP-1 was shown to have a significant
inverse correlation with maximal endothelium-dependent
vasodilatation. Ragino et al (20) compared the blood levels of TIMP-1 in
patients with unstable and stable atherosclerotic plaques in the
coronary arteries. Circulating TIMP-1 levels were revealed to be
lower in the individuals with unstable plaques. The aforementioned
studies do not present convergent results, which may be due to the
methodology of these studies. Serum levels of TIMP-1 are a sum of
the production by ECs in the whole vascular system. The results of
the present study indicated that the same stimulator, κ-elastin,
may have a divergent influence on the endothelium depending on the
localization in the circulatory system. For example, a stimulatory
effect on TIMP-1 production was observed in the ECs derived from
the iliac artery, while an inhibitory effect was observed in the EC
lines obtained from the aorta and coronary arteries. In the case of
TIMP-2 production, in the ECs obtained from the aorta and iliac
artery, the effect of κ-elastin appeared to be neutral.
In an experimental model, the induction of a murine
femoral artery injury caused an initial decrease in TIMP-1 and
TIMP-2 expression and activity levels during the first five days,
with subsequent restoration of the expression and activity levels
(21). However, in a similar
preceding experiment, no changes in TIMP-1 and TIMP-2 expression
and activity levels were observed (22). Moore et al (23) observed that in rats subjected to
oxidative stress, plasma levels of TIMP-1 may serve as an early
marker of endothelium dysfunction.
Using an experimental model, Misra et al
observed that murine fibroblasts cultured in hypoxemic conditions
exhibited increased expression levels of TIMP-1 and TIMP-2;
however, these changes were not simultaneous. An augmentation in
TIMP-1 expression preceded the increased expression of TIMP-2
(24). Furthermore, Mammi et
al observed that in patients with heart failure, serum levels
of TIMP-1 can be decreased by physical exercise (25). Notably, Ramirez Correa et al
(26) used local TIMP-1 gene
transfer and achieved a significant reduction of restenosis in
human coronary smooth muscle cells.
EDPs have been shown to promote angiogenesis
(17), a process that is involved in
a variety of pathological and physiological conditions.
Angiogenesis plays a pivotal role in postischemic
neovascularization of the myocardium. The results of the present
study indicated that EDPs may increase the production of TIMPs. The
results of these two studies (24,25) and
the present study can make one raise a question about an influence
of EDPs on coronary arteries, whether EDPs may be deleterious or
beneficial in certain circumstances. TIMP-2 also exhibits
angioinhibitory properties, which comprise several mechanisms,
including MMP inhibition or direct EC binding (27). In addition, the results of the
present study indicated that TIMP-1 and TIMP-2 presented various
production profiles in unstimulated ECs, and their production
differed depending on the vasculature localization. However, the
effect of κ-elastin preserved the production proportion in each
cell line from the various localizations in the vascular bed.
Therefore, the TIMP concentrations proportion may be a
characteristic feature of ECs in various arteries. The calculated
TIMP ratios support the observation that the endothelium does not
constitute a uniform pool of cells with the same biological
characteristics. The biochemical properties can vary depending on
their localization in the cardiovascular system and the different
characteristics of blood flow in these places. Therefore, the
observations of the present study have an important implication.
While studying EC biology, biochemistry and their response to
various stimuli, the localization of the obtained ECs should be
taken into consideration.
TIMP-3 is considered to be a major regulator of
angiogenesis. The protein can block the binding of VEGF to its
receptor, VEGFR-2, and this function is independent of the MMP
inhibitory effect (28). In
addition, TIMP-3 can induce vascular cell apoptosis. Vacek et
al (29) used a mouse
experimental model and found that a mechanical injury of the
carotid artery led to an increase in the TIMP-3 level in the
injured artery. Similar results were obtained by Basu et al
(30). In mice with chronic
hyperhomocysteinemia, an increase in TIMP-3 gene expression was
observed in the aorta. Furthermore, in an additional study, when a
cigarette smoke extract was used as an evaluated risk factor, a
decreased secretion of TIMP-3 was detected in the rabbit
endothelium (31). TIMP-3 has been
analyzed as a factor for gene therapy, and overexpression of TIMP-3
has been shown to result in a sustained retardation of vein graft
intimal thickening (32). In the
present study, TIMP-3 production appeared to be the most resistant
to the influence of κ-elastin as a modulator.
In conclusion, the present study demonstrated that
ECs from various parts of the vascular system present a different
production with regard to the three analyzed TIMPs. These cells
also differ in their response to κ-elastin, applied as a modulator.
Therefore, the observations of the current study indicate that the
endothelium can adapt to various conditions of blood flow in
different sections of the vascular system and modulate the
production of important molecules involved in ECM homeostasis.
A number of studies have calculated and analyzed
various TIMP/MMP ratios (21,25).
However, the present study was the first to calculate the ratios
for three TIMP indicators: TIMP-1/TIMP-2, TIMP-1/TIMP-3 and
TIMP-2/TIMP-3. The results demonstrated that ECs in various
localizations had their own specific values of these ratios that
are preserved when a risk factor, such as κ-elastin, influences the
endothelium. Therefore, these ratio indicators may be
characteristic features used to describe ECs in various clinically
important vascular localizations. Subsequently, the biological
differences of ECs derived from various localizations in the
cardiovascular system should be taken into consideration in further
studies evaluating the function of ECs and their response to
various stimuli.
References
|
1
|
Ganea E, Trifan M, Laslo AC, Putina G and
Cristescu C: Matrix metalloproteinases: useful and deleterious.
Biochem Soc Trans. 35:689–691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raffetto JD and Khalil RA: Matrix
metalloproteinases and their inhibitors in vascular remodeling and
vascular disease. Biochem Pharmacol. 75:346–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Troeberg L and Nagase H: Analysis of TIMP
expression and activity. Methods Mol Med. 135:251–267. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stetler-Stevenson WG: The tumor
microenvironment: regulation by MMP-independent effects of tissue
inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 27:57–66.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen Q, Lee ES, Pitts RL, Wu MH and Yuan
SY: Tissue inhibitor of metalloproteinase-2 regulates matrix
metalloproteinase-2-mediated endothelial barrier dysfunction and
breast cancer cell transmigration through lung microvascular
endothelial cells. Mol Cancer Res. 8:939–951. 2010.PubMed/NCBI
|
|
6
|
Seo DW, Li H, Guedez L, Wingfield PT, et
al: TIMP-2 mediated inhibition of angiogenesis: an MMP-independent
mechanism. Cell. 114:171–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kallio JP, Hopkins-Donaldson S, Baker AH
and Kahari VM: TIMP-3 promotes apoptosis in nonadherent small cell
lung carcinoma cells lacking functional death receptor pathway. Int
J Cancer. 128:991–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grundy SM: National Cholesterol Education
Program (NCEP) - The National Cholesterol Guidelines in 2001, Adult
Treatment Panel (ATP) III: Approach to lipoprotein management in
2001 National Cholesterol Guidelines. Am J Cardiol. 90:11i–21i.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hornebeck W and Robert L: Elastase-like
enzymes in aortas and human breast carcinomas: quantitative
variations with age and pathology. Adv Exp Med Biol. 79:145–164.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gayral S, Garnotel R, Castaing-Berthou A,
et al: Elastin-derived peptides potentiate atherosclerosis through
the immune Neu1-PI3K൧ pathway. Cardiovasc Res. 102:118–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gminski J and Drozdz M: Succinyl
trialanine p-nitroanilide hydrolytic activities in plasma and the
aorta of rabbits experimentally immunized with soluble elastin. Exp
Pathol. 43:37–40. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faury G, Ristori MT, Verdetti J, Jacob MP
and Robert L: Effect of elastin peptides on vascular tone. J Vasc
Res. 32:112–119. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Faury G, Garnier S, Weiss AS, et al:
Action of tropoelastin and synthetic elastin sequences on vascular
tone and on free Ca2+ level in human vascular
endothelial cells. Circ Res. 82:328–336. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robert L, Labat-Robert J and Robert AM:
Genetic, epigenetic and posttranslational mechanisms of aging.
Biogerontology. 11:387–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siemianowicz K, Gminski J, Goss M, Francuz
T, Likus W, Jurczak T and Garczorz W: Influence of elastin-derived
peptides on metalloprotease production in endothelial cells. Exp
Ther Med. 1:1057–1060. 2010.PubMed/NCBI
|
|
17
|
Robinet A, Fahem A, Cauchard J-H, et al:
Elastin-derived peptides enhance angiogenesis by promoting
endothelial cell migration and tubulogenesis through upregulation
of MT1-MMP. J Cell Sci. 118:343–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mieczkowska J, Mosiewicz J, Barud W and
Kwasniewski W: Changes in the activity of connective tissue matrix
enzymes in the metabolic syndrome. Arch Med Sci. 7:634–641. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de la Sierra A and Larrousse M:
Endothelial dysfunction is associated with increased levels of
biomarkers in essential hypertension. J Hum Hypertens. 24:373–379.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ragino IuI, Cherniavskiĭ AM, Polonskaia
IaV, Volkov AM, Kashtanova EV, Tsymbal SIu and Polovnikova EM:
Inflammatory-destructive biomarkers of atherosclerotic plaques
instability. Study of arterial wall and blood. Kardiologiia.
52:37412012.(In Russian). PubMed/NCBI
|
|
21
|
Zou Y, Qi Y, Roztocil E and Davies MG:
Patterns of gelatinase activation induced by injury in the murine
femoral artery. J Surg Res. 154:135–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou Y, Fu Y and Davies MG: Role for Gᵝᵧ
G-proteins in protease regulation during remodeling of the murine
femoral artery. J Surg Res. 178:40–47. 2013. View Article : Google Scholar
|
|
23
|
Moore R, Hawley A, Sigler R, Farris D,
Wrobleski S, Ramacciotti E and Myers D: Tissue inhibitor of
metalloproteinase-1 is an early marker of acute endothelial
dysfunction in a rodent model of venous oxidative injury. Ann Vasc
Surg. 23:498–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Misra S, Fu AA, Misra KD, Shergill UM,
Leof EB and Mukhopadhyay D: Hypoxia-induced phenotypic switch of
fibroblasts to myofibroblasts through a matrix metalloproteinase
2/tissue inhibitor of metalloproteinase-mediated pathway:
implications for venous neointimal hyperplasia in hemodialysis
access. J Vasc Interv Radiol. 21:896–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mammi C, la Sala A, Volterrani M, et al:
Exercise training reduces serum capacity to induce endothelial cell
death in patients with chronic heart failure. Eur J Heart Fail.
13:642–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramirez Correa GA, Zacchigna S, Arsic N,
Zentilin L, Salvi A, Sinagra G and Giacca M: Potent inhibition of
arterial intimal hyperplasia by TIMP1 gene transfer using AAV
vectors. Mol Ther. 9:876–884. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seo DW, Saxinger WC, Guedez L, Cantelmo
AR, Albini A and Stetler-Stevenson WG: An integrin-binding
N-terminal peptide region of TIMP-2 retains potent angio-inhibitory
and anti-tumorigenic activity in vivo. Peptides. 32:1840–1848.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi JH, Ebrahem Q, Ali M, et al: Tissue
inhibitor of metalloproteinases-3 peptides inhibit angiogenesis and
choroidal neovascularization in mice. PLoS One. 8:e556672013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vacek TP, Gillespie W, Tyagi N, Vacek JC
and Tyagi SC: Hydrogen sulfide protects against vascular remodeling
from endothelial damage. Amino Acids. 39:1161–1169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Basu P, Qipshidze N, Sen U, Givvimani S,
Munjal C, Mishra PK and Tyagi SC: Chronic hyperhomocysteinemia
causes vascular remodelling by instigating vein phenotype in
artery. Arch Physiol Biochem. 117:270–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lemaitre V, Dabo AJ and D'Armiento J:
Cigarette smoke components induce matrix metalloproteinases-1 in
aortic endothelial cells through inhibition of mTOR signaling.
Toxicol Sci. 123:542–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
George SJ, Wan S, Hu J, MacDonald R,
Johnson JL and Baker AH: Sustained reduction of vein graft
neointima formation by ex vivo TIMP-3 gene therapy. Circulation.
124:(Suppl). S135–S142. 2011. View Article : Google Scholar : PubMed/NCBI
|