Introduction
Osteoarthritis (OA) is a highly prevalent joint
disease, which is mainly characterised by the degradation of
articular cartilage, thickening of subchondral bone, synovial
inflammation and the formation of osteophytes (1). Data from the World Health Organisation
ascertains that worldwide, ~10% of men and 18% of women, as well as
~65% of all those aged >60 years, have symptomatic OA (2). Despite the high prevalence of OA, the
aetiology and pathology of OA have not yet been fully elucidated.
The development and progression of OA are mediated by multiple
factors, including genetics, epigenetics, biochemistry and
mechanics (3–5). Several lines of research have
demonstrated that a disturbance in the balance of anabolic and
catabolic factors, which maintain the homeostasis of cartilage,
plays a vital role in OA (6).
Understanding the specific role of each factor is important for the
treatment of OA, and is also vital for creating effective
therapeutic interventions targeting the development and progression
of OA. Since numerous molecular events occur in OA chondrocytes
during hyaline cartilage degeneration, several studies have been
conducted to investigate the role of osteopontin (OPN) and
caveolin-1 in OA (7,8).
OPN is an acidic phosphorylated matrix protein
secreted by numerous cell types, such as osteoclasts, macrophages,
lymphocytes and epithelial cells (9). OPN is present in the extracellular
matrix (ECM) of mineralised tissues and the extracellular fluids at
sites of inflammation (10).
Previous studies have demonstrated that OPN plays a significant
role in cell adhesion, migration and metastasis (11,12). In
the last decade, the vast majority of research has indicated that
the mRNA expression levels of OPN in OA cartilage and synovium
fluid are clearly higher compared with normal cartilage, and that
the expression levels of OPN protein and OPN mRNA are positively
associated with the severity of the disease (13–15). By
contrast, a previous study reported that an OPN deficiency resulted
in aging-associated and instability-induced OA in OPN-deficient
mice, suggesting that OPN may defer the pathogenesis in OA
(16). These observations strongly
indicate that OPN has complex roles in joint homeostasis and in the
pathogenesis of OA by modulating multiple targets of cells in the
joint; however, the role of OPN in OA remains poorly
understood.
Caveolin-1, the major structural component of
caveolae, is a small flask-shaped plasma membrane invagination
(17,18). The protein has been proposed to
function as a scaffolding protein, mediating numerous physiological
and pathological processes, including caveolae biogenesis,
vesicular transport, cholesterol homeostasis, signal transduction
and tumourigenesis (19,20). Previously, the expression of
caveolin-1 was analysed in normal and OA human knee joint cartilage
(21). Caveolin-1 has been reported
to play a role in the pathogenesis of OA through the involvement of
caveolin-1-induced downregulation of articular chondrocytes
(22).
Previous observations have revealed that caveolin-1
is associated with β1-integrin in human chondrocytes,
indicating that caveolin-1 and β1-integrin are part of
the same complex (21).
β1-integrin plays a direct role in the adhesion between
the cell and the ECM, in which OPN is involved through binding to
the OPN receptor (23). Since
β1-integrin mediates the role of OPN and colocalises
with caveolin-1, the expression of caveolin-1 was hypothesised to
correlate with OPN expression through the signalling pathway in
which β1-integrin mediates OPN. Therefore, to
investigate the hypothesis, the expression levels of OPN and
caveolin-1 in different degrees of impaired cartilage samples from
human subjects with OA were determined, and the correlation between
OPN and caveolin-1 was analysed. The aim of the present study was
to provide a more comprehensive understanding of OPN and caveolin-1
in OA.
Materials and methods
Patients and cartilage samples
A total of 40 patients (age, 52–71 years) with
primary knee OA and 10 normal healthy individuals were enrolled in
the study. Patients with OA were considered eligible if the
clinical and radiological data met the criteria of the American
College of Rheumatology (24) and
were carefully reviewed to exclude any forms of secondary OA or
other inflammatory joint diseases, including rheumatoid arthritis
and any other types of arthritis. All patients provided informed
consent, and this study was approved by the ethics committee of
Xiangya Hospital, Central South University (Changsha, China). In
total, 40 osteoarthritic cartilage samples were collected from the
40 patients with primary OA undergoing a total knee arthroplasty.
The normal samples were obtained from the knees of 10 post-mortem
donors (age, 32–41 years), with no history of joint pain. Full
ethical consent was obtained from all the donors and families.
Osteoarthritic changes were classified histomorphologically using
the Mankin grading system (25) as
follows: Mankin score 0, normal cartilage with a smooth surface and
a regular zonal distribution of chondrocytes; Mankin score 1–4,
cartilage surface shows fibrillations and a superficial loss of
proteoglycans (safranin-O staining), but the zonal structure is
intact; Mankin score 5–8, cartilage samples have clefts reaching
down to the middle cartilage zone, and clusters of proliferating
chondrocytes are present; Mankin score ≥9, severely affected
cartilage samples with clefts reaching down to the deep zone, in
which the tangential zone is lost and chondrocyte clusters are
present. Of the patients included in the study, 9 samples were
classified as Mankin score 0, 10 samples were determined to be
Mankin score 1–4, 15 samples were classified as Mankin score 5–8
and 13 samples were classified with a Mankin score of ≥9. Biopsies
(cartilage/bone samples) were obtained from the lateral and medial
sides of the tibia plateau, including the loading zone.
Cartilage/bone samples (1.0 cm thick) with a cartilage surface of
~2.0×0.5 cm were incubated in freshly prepared 4% (w/v)
paraformaldehyde, buffered with 0.01 M sodium phosphate (pH 7.4)
containing 0.14 M NaCl (phosphate-buffered saline; PBS), for 12 h
at 4°C. The tissue samples were decalcified in
diethylpyrocarbonate-treated 0.2 M EDTA (pH 8.0) for 8 weeks at
4°C. The buffer was changed twice a week. The tissue specimens were
subsequently dehydrated in a grading concentration of ethanol and
xylene, and finally embedded in paraffin.
Immunohistochemistry
OPN protein and caveolin-1 expression levels were
examined by immunohistochemistry using an OPN antibody (ab8488;
Abcam, Cambridge, UK) and caveolin-1 antibody (clone N-20; Santa
Cruz Biotechnology, Inc., Wembley, UK), respectively, according to
the manufacturer's instructions. Following deparaffinisation with
xylene and rehydration through a series of graded ethanol
solutions, the sections were treated with 3% hydrogen peroxide for
10 min and then microwaved in 10 mM citrate buffer (pH 6.0) to
unmask the epitopes. Subsequently, the sections were incubated with
the OPN antibody at a dilution of 1:150 for 1 h. After washing, a
horseradish peroxidase/Fab polymer conjugate (PicTure™-Plus kit;
Zymed Life Technologies, Carlsbad, CA, USA) was applied to the
sections for 30 min. Finally, the sections were incubated with
diaminobenzidine for 5 min to develop the signals. A negative
control was simultaneously prepared by omitting the primary
antibody. The sections were assessed by a pathologist who was blind
to the clinical data. To evaluate the expression of OPN, the
sections were examined under an Olympus microscope (magnification,
x100; Olympus Corporation, Tokyo, Japan). Positive OPN
immunostaining was defined as detectable immunoreactivity in the
perinuclear or other cytoplasmic regions in the chondrocytes. The
relative OPN distribution in the cartilage tissue was visualised
and quantified as average grey values. Semi-quantitative assessment
of the mean average grey values for OPN expression was performed on
scanned autoradiograms using medical image analysis software
(MIAS)-4400 and Image J software. Grey-scale images were captured
and converted to absorbance units, and a region from the cartilage
surface to the cartilage-bone junction was analysed. Densities were
normalised against those with PBS, and the experiment was repeated
three times. To reduce the error arising from the small variation
in section thickness, a total of three sections per sample were
measured and averaged. Therefore, the final data, applied in all
analyses, consisted of the mean value of three independent
measurements representing the average levels of OPN in the
articular cartilage. The coefficient of variation of OPN expression
in the articular cartilage was <2%. The aforementioned procedure
was conducted in the same manner to assess caveolin-1
expression.
Statistical analysis
Static grey analysis from the MIAS was applied to
measure the average grey values in 10 randomly selected regions of
the OPN and caveolin-1 immunohistochemical slices. SPSS software
for Windows (version 19.0; IBM SPSS, Armonk, NY, USA) was used for
data management and statistical analysis. One way analysis of
variance was employed to examine the differences in the mean values
between multiple groups. In addition, Spearman's correlation and
linear regression were conducted to determine the correlations
between the average grey values for OPN and caveolin-1 in the
articular cartilage and the Mankin score of OA. Results are
presented as the mean ± standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference.
Results
Mankin scoring system in each
group
A total of 50 biopsies, which were obtained from 40
patients and 10 normal individuals, were ascribed respectively to
four groups (normal, minor, moderate and severe), according to the
Mankin grading system. Three biopsies were excluded due to
unsuccessful cutting from the cartilage and a failure in the
procedure of immunohistochemistry. Ultimately, 47 samples were
analysed. Grouping of the cartilage tissue samples according to the
Mankin scoring system are shown in Table
I.
 | Table I.Mankin scoring system of cartilage in
each group. |
Table I.
Mankin scoring system of cartilage in
each group.
| Group | Sample (n) | Mankin score |
|---|
| Normal | 9 | 0.55±0.53 |
| Minor | 10 | 3.90±1.12 |
| Moderate | 15 | 7.77±1.09 |
| Severe | 13 | 11.93±1.44 |
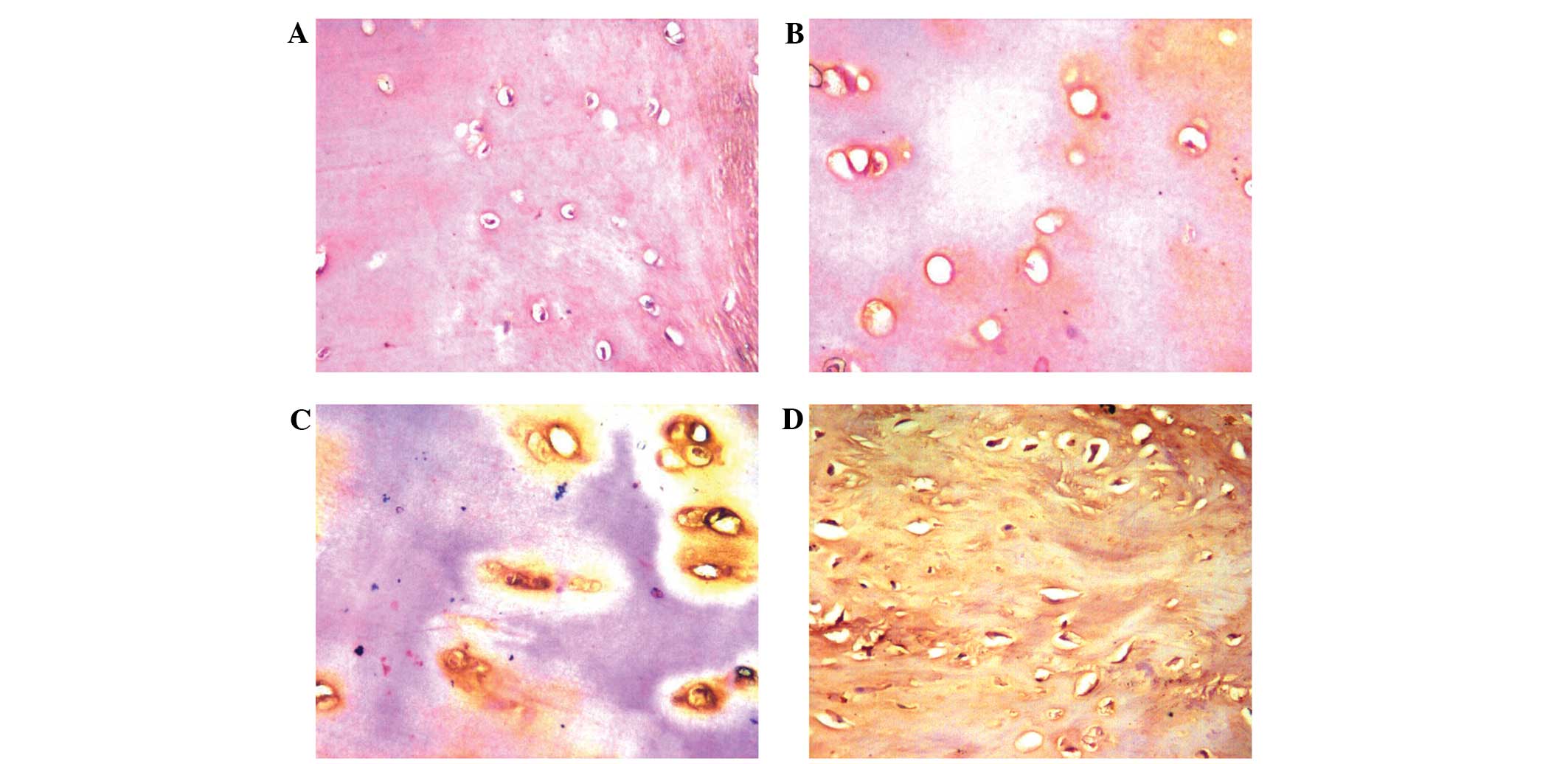
Enhanced expression of OPN protein in
OA cartilage tissues
OPN expression levels in the various degrees of
impaired cartilage tissues were compared, as shown in Fig. 1. OPN, which is located in the ECM,
was shown to be expressed in the normal and OA groups. The severe
group exhibited a higher OPN expression level compared with the
moderate, minor and normal groups (average grey value, 97.98±12.46
vs. 114.14±10.85, 125.33±8.02 and 140.06±12.17, respectively;
Table II). A low grey value
indicates higher expression levels. The mutual comparisons between
these groups revealed statistically significant differences
(P<0.05). Thus, the results indicated that expression of the OPN
protein was strongly increased in the impaired cartilage.
 | Table II.Average grey value levels of OPN and
caveolin-1 in each group. |
Table II.
Average grey value levels of OPN and
caveolin-1 in each group.
| Group | Sample (n) | OPN value | Caveolin-1 value |
|---|
| Normal | 9 |
140.06±12.17 |
85.90±15.11 |
| Minor | 10 |
125.33±8.02a |
112.00±23.73a |
| Moderate | 15 |
114.14±10.85a, b |
131.72±19.32a |
| Severe | 13 |
97.98±12.46a, b,
c |
140.10±15.29a, b,
c |
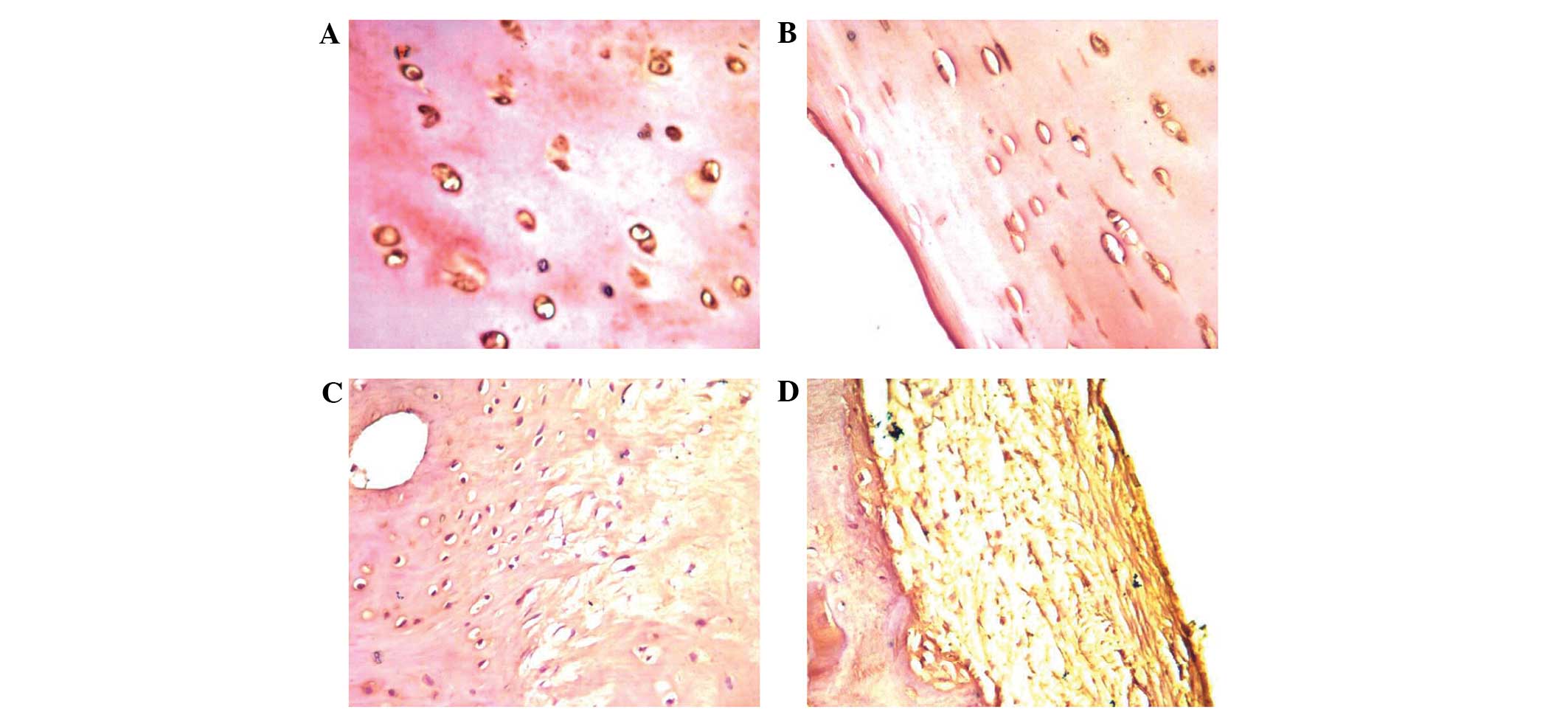
Decreased expression of caveolin-1
protein in OA cartilage tissues
To investigate the function of caveolin-1 in OA, the
expression levels in the various degrees of impaired cartilage
tissues were compared (Fig. 2).
Caveolin-1 was shown to be expressed in the normal and OA groups.
However, the severe group exhibited lower caveolin-1 expression
levels compared with the moderate, minor and normal groups (average
grey value, 140.10±15.29 vs. 131.72±19.32, 112.00±23.73 and
85.90±15.11, respectively; Table
II). The mutual comparisons between these groups were
demonstrated to be statistically significant (P<0.05), with the
exception of that between the moderate and severe groups
(P>0.05).
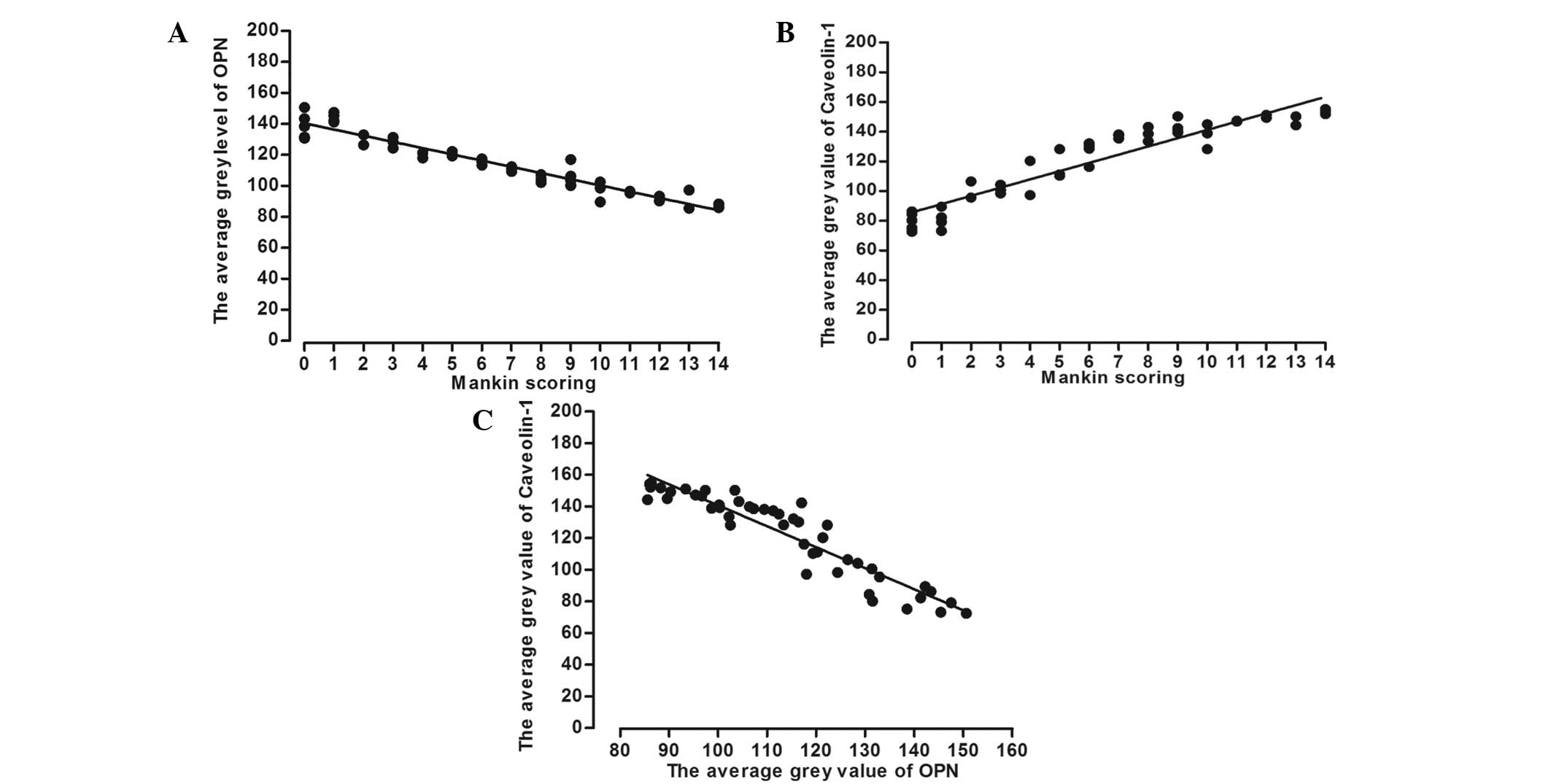
Correlations between OPN, caveolin-1
and Mankin scoring
To investigate the correlation between OPN and
caveolin-1 in OA, SPSS software was applied to assess the average
grey value for each protein and the Mankin scoring. Correlations
between the average grey value of OPN and caveolin-1 with the
Mankin scoring are shown in Fig. 3.
For the correlation between the average grey value of OPN and the
Mankin scoring, Pearson's correlation coefficient was determined to
be r=-0.824 (P<0.01). In addition, Pearson's correlation
coefficient for the correlation between the average grey value of
caveolin-1 and the Mankin scoring was r=0.725 (P<0.01).
Furthermore, the correlation between the average grey values of OPN
and caveolin-1 was determined to have a Pearson's correlation
coefficient of r=-0.676 (P<0.01).
Discussion
As an ECM protein, OPN plays a significant role in
the process of cell adhesion, migration and metastasis (9). OPN is expressed by various cells and
tissues, such as the kidney, placenta, neoplastic cells, lining
epithelial cells, islet cells and activated T cells; however, the
main source of OPN is bone cells, including chondrocytes and
osteoclasts. The present study demonstrated that OPN plays a
crucial role in the progression of mineralisation and absorption of
bone matrix. A previous study reported that OPN may function as an
inhibitor involved in the molecular pathogenesis of OA,
contributing to the progressive degeneration of articular cartilage
(26), whereas an additional study
drew the opposite conclusion (27).
However, these two studies did not investigate OPN overexpression.
In the present study, OPN expression was observed in the normal and
OA groups. However, the severe OA group were shown to have higher
OPN expression levels compared with the moderate, minor and normal
groups. Based on this observation, a statistically significant
difference was observed in the expression of OPN between OA and
normal cartilage, and the articular cartilage OPN expression was
shown to correlate with the severity level of cartilage
dysfunction. Thus, the results indicated that OPN may play a role
in the pathology and progression of OA as a destructive factor.
In addition to participating in the formation of
caveolae, caveolin-1 is known to directly interact with multiple
signalling proteins via its scaffolding domain in order to regulate
their activity. These proteins include important regulators of cell
transformation and growth (28).
Previously, caveolin-1 expression was demonstrated in normal human
knee joint cartilage by immunohistochemical analysis (21). The current findings also detected
caveolin-1 expression in the normal cartilage, as well as in the
different pathological levels of OA cartilage, with a lower
expression of caveolin-1 being accompanied by a higher regression
of cartilage. Therefore, the results of the present study
demonstrated that caveolin-1 may be involved in the degradation of
articular cartilage in OA, which differs from previous results that
hypothesised that caveolin-1 played a role in the pathogenesis of
OA through the promotion of chondrocyte downregulation. Further
research is required in order to elucidate the exact role of
caveolin-1 in OA.
Signal transduction is the major function of
caveolin-1. Previous studies have demonstrated that caveolin-1 and
β1-integrin are part of the same complex in human
chondrocytes (29,30). Integrin, which is one of the
transmembrane glycoproteins, is located throughout the cell
membrane. Integrins interact with a variety of cellular adhesive
molecules and are regarded as a family of adhesion molecules. As
the main receptor of the ECM, integrin induces the adhesion process
of the cell. Schwab et al (21) detected the colocalisation between
caveolin-1 and β1-integrin using double staining methods
and immune electron microscopy in human samples of knee articular
cartilage, and drew the conclusion that β1-integrin had
a close association with the three subtypes of caveolin, which may
be different parts of the same complex. Based on these
observations, all the caveolin subtypes were hypothesised to be
indirectly involved in processes of the cell and ECM through
participating in the signalling pathway induced by
β1-integrins in chondrocytes. OPN induces adhesion
between the cell and ECM by binding to the OPN receptor, and
β1-integrin may directly play a role in this
process.
In the present study, a correlation was observed
between cartilage OPN expression and the severity level of
cartilage dysfunction; however, caveolin-1 expression was shown to
exhibit the opposite trend. In addition, the correlation between
OPN and caveolin-1 expression was statistically significant,
indicating that caveolin-1 may correlate with OPN through the
signalling pathway in which β1-integrin mediates
OPN.
However, the present study had several limitations.
Firstly, the sample size was not sufficiently large to arrive at
any definitive conclusions. Secondly, the true pathogenesis of OA
may not be fully reflected when representing the progression of OA
with the articular cartilage degeneration degree.
In conclusion, the correlation between OPN and
caveolin-1 may play a substantial role in the pathogenesis and
progression of OA. However, further research is required to
elucidate the contribution of OPN and caveolin-1 to the
pathogenesis of the degenerative process of OA.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81272034) and the
Fundamental Research Funds for the Central Universities of Central
South University (2013 zzts081).
Glossary
Abbreviations
Abbreviations:
|
OA
|
osteoarthritis
|
|
OPN
|
osteopontin
|
|
ECM
|
extracellular matrix
|
|
PBS
|
phosphate-buffered saline
|
|
MIAS
|
medical image analysis software
|
References
|
1
|
Poole AR: An introduction to the
pathophysiology of osteoarthritis. Front Biosci. 4:D662–D670. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woolf AD and Pfleger B: Burden of major
musculoskeletal conditions. Bull World Health Organ. 81:646–656.
2003.PubMed/NCBI
|
|
3
|
Loughlin J: Genetics of osteoarthritis and
potential for drug development. Curr Opin Pharmacol. 3:295–299.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Im GI and Choi YJ: Epigenetics in
osteoarthritis and its implication for future therapeutics. Expert
Opin Biol Ther. 13:713–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murray RC, Zhu CF, Goodship AE, Lakhani
KH, Agrawal CM and Athanasiou KA: Exercise affects the mechanical
properties and histological appearance of equine articular
cartilage. Journal of orthopaedic research. J Orthop Res.
17:725–731. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aigner T, Soeder S and Haag J: Il-1 beta
and BMPS-interactive players of cartilage matrix degradation and
regeneration. Eur Cell Mater. 12:49–56. 2006.PubMed/NCBI
|
|
7
|
Dai SM, Shan ZZ, Nakamura H, et al:
Catabolic stress induces features of chondrocyte senescence through
overexpression of caveolin 1: Possible involvement of caveolin
1-induced down-regulation of articular chondrocytes in the
pathogenesis of osteoarthritis. Arthritis Rheum. 54:818–831. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao SG, Cheng L, Zeng C, et al: Usefulness
of specific OA biomarkers, thrombin-cleaved osteopontin, in the
posterior cruciate ligament OA rabbit model. Osteoarthritis
Cartilage. 21:144–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Denhardt DT and Guo X: Osteopontin: a
protein with diverse functions. FASEB J. 7:1475–1482.
1993.PubMed/NCBI
|
|
10
|
Sodek J, Chen J, Nagata T, et al:
Regulation of osteopontin expression in osteoblasts. Ann N Y Acad
Sci. 760:223–241. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rittling SR and Chambers AF: Role of
osteopontin in tumour progression. Br J Cancer. 90:1877–1881. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao SG, Li KH, Zeng KB, Tu M, Xu M and Lei
GH: Elevated osteopontin level of synovial fluid and articular
cartilage is associated with disease severity in knee
osteoarthritis patients. Osteoarthritis Cartilage. 18:82–87. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hasegawa M, Segawa T, Maeda M, Yoshida T
and Sudo A: Thrombin-cleaved osteopontin levels in synovial fluid
correlate with disease severity of knee osteoarthritis. J
Rheumatol. 38:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Honsawek S, Tanavalee A, Sakdinakiattikoon
M, Chayanupatkul M and Yuktanandana P: Correlation of plasma and
synovial fluid osteopontin with disease severity in knee
osteoarthritis. Clin Biochem. 42:808–812. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsui Y, Iwasaki N, Kon S, et al:
Accelerated development of aging-associated and instability-induced
osteoarthritis in osteopontin-deficient mice. Arthritis Rheum.
60:2362–2371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lisanti MP, Scherer PE, Tang Z and
Sargiacomo M: Caveolae, caveolin and caveolin-rich membrane
domains: a signalling hypothesis. Trends Cell Biol. 4:231–235.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu P, Rudick M and Anderson RG: Multiple
functions of caveolin-1. J Biol Chem. 277:41295–41298. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Engelman JA, Chu C, Lin A, et al:
Caveolin-mediated regulation of signaling along the p42/44 MAP
kinase cascade in vivo. A role for the caveolin-scaffolding domain.
FEBS Lett. 428:205–211. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okamoto T, Schlegel A, Scherer PE and
Lisanti MP: Caveolins, a family of scaffolding proteins for
organizing ‘preassembled signaling complexes’ at the plasma
membrane. J Biol Chem. 273:5419–5422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwab W, Kasper M, Gavlik JM, Schulze E,
Funk RH and Shakibaei M: Characterization of caveolins from human
knee joint cartilage: expression of caveolin-1, −2 and −3 in
chondrocytes and association with integrin beta1. Histochem Cell
Biol. 113:221–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai SM, Shan ZZ, Nakamura H, et al:
Catabolic stress induces features of chondrocyte senescence through
overexpression of caveolin 1: possible involvement of caveolin
1-induced down-regulation of articular chondrocytes in the
pathogenesis of osteoarthritis. Arthritis Rheum. 54:818–831. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ross FP, Chappel J, Alvarez JI, et al:
Interactions between the bone matrix proteins osteopontin and bone
sialoprotein and the osteoclast integrin alpha v beta 3 potentiate
bone resorption. J Biol Chem. 268:9901–9907. 1993.PubMed/NCBI
|
|
24
|
Aletaha D, Neogi T, Silman AJ, et al: 2010
rheumatoid arthritis classification criteria: An American College
of Rheumatology/European League Against Rheumatism collaborative
initiative. Ann Rheum Dis. 69:1580–1588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Sluijs JA, Geesink RG, van der
Linden AJ, Bulstra SK, Kuyer R and Drukker J: The reliability of
the Mankin score for osteoarthritis. J Orthop Res. 10:58–61. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Attur MG, Dave MN, Stuchin S, et al:
Osteopontin: an intrinsic inhibitor of inflammation in cartilage.
Arthritis Rheum. 44:578–584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsui Y, Iwasaki N, Kon S, et al:
Accelerated development of aging-associated and instability-induced
osteoarthritis in osteopontin-deficient mice. Arthritis Rheum.
60:2362–2371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Golen KL: Is caveolin-1 a viable
therapeutic target to reduce cancer metastasis? Expert Opin Ther
Targets. 10:709–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jokhadar SZ, Majhenc J, Svetina S and
Batista U: Positioning of integrin β1, caveolin-1 and focal
adhesion kinase on the adhered membrane of spreading cells. Cell
Biol Int. 37:1276–1284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Radel C, Carlile-Klusacek M and Rizzo V:
Participation of caveolae in beta1 integrin-mediated
mechanotransduction. Biochem Biophys Res Commun. 358:626–631. 2007.
View Article : Google Scholar : PubMed/NCBI
|