Introduction
Acute kidney injury involves the abrupt loss of
renal function, which is strongly associated with increased
mortality rates and the subsequent development of chronic kidney
disease (1). While acute kidney
injury has a number of etiologies, a major cause is ischemia, which
can occur during kidney transplantation, renal artery angioplasty,
sepsis, partial nephrectomy, accidental or iatrogenic trauma,
hydronephrosis, elective urological surgery, aortic bypass surgery
and cardiopulmonary bypass, or by the action of vasoconstrictor
drugs and certain hypotensive states (2,3).
Previous studies have demonstrated that ischemic
preconditioning (IPC) creates resistance against organ ischemia and
reperfusion (I/R) injury through ‘organ conditioning’ (4,5).
Although IPC has protective effects and may reduce I/R injury,
clinical application is limited and IPC is not suitable for
clinical implementation since the onset of an ischemic insult is
unpredictable. Ischemic postconditioning (IPoC) involves the
application of a series of brief rapid intermittent ischemic
episodes at the onset of reperfusion in the previously ischemic
tissue or organ (6). IPoC has
improved feasibility and operability; thus, is more clinically
applicable and attracts greater attention compared with IPC.
Recent studies have indicated that IPoC
significantly reduces the inflammatory response in cerebral and
lung I/R injury (7,8). An excessive inflammatory reaction
frequently resides in the tissue subjected to the I/R injury and
leads to damage (9). Therefore,
reducing the inflammatory injury is regarded as a major technique
for the attenuation of I/R injury. In a previous study, IPoC was
demonstrated to attenuate renal damage following I/R injury
(10). However, the ability of IPoC
to reduce the inflammatory response induced by renal I/R injury has
not yet been investigated. Therefore, the aim of the present study
was to determine whether IPoC inhibits inflammation following renal
I/R injury.
Materials and methods
Animal model of I/R
In total, 56 adult healthy male Wistar rats
(specific-pathogen-free grade; weight, 210–250 g) were supplied by
the Animal Experimental Center of the Medical College of Wuhan
University (Wuhan, China). The study was approved by the Committee
of the Animal Experimental Center of Wuhan University, and the
procedures were conducted according to the routine animal-care
guidelines. All the experimental procedures complied with the
Guidelines for the Care and Use of Laboratory Animals. Briefly, the
rats were anesthetized using pentobarbital (45 mg/kg) and placed on
a homeothermic table in order to maintain a core body temperature
of 37°C. A midline laparotomy incision was made and a right
nephrectomy was performed. Next, the left kidney was subjected to
45 min of ischemia, followed by reperfusion.
The animals were randomly divided into three groups
of eight rats each, including the sham-operated (sham), I/R and
IPoC groups. In the I/R and IPoC groups, the reperfusion period was
24 h, 72 h and 120 h and the number of rats in each group were as
follows: Sham (8 rats), I/R 24 h (8 rats), I/R 72 h (8 rats), I/R
120 h (8 rats), IPoC 24 h (8 rats), IPoC 72 h (8 rats) and IPoC 120
h (8 rats). In the sham group, the rats were subjected to a
resection of the right kidney. In the I/R group, after the right
kidney was removed, the left kidney vessels were subjected to
ischemia for 45 min, followed by reperfusion. In the IPoC group,
the rats were subjected to 45 min of ischemia, after which the left
kidney was immediately subjected to six cycles of reperfusion for
10 sec and a 10-sec ischemic episode, followed by reperfusion. All
the ischemic kidneys were harvested following a reperfusion period
of 24, 72 or 120 h.
Preservation of the kidneys
The left kidney was removed under fully maintained
anesthesia. Following removal, the kidney was fixed in 10%
phosphate-buffered formalin or immediately frozen, and stored at
−80°C for the following experiments.
Serum assays
At 24 h following the initiation of I/R injury, in
every group, 1-ml blood samples were collected and analyzed
according to directions of the Creatinine and Urea Assay kits
(Nanjing Jiancheng Chemical Industrial Co., Ltd, Nanjing, China).
The absorbance was measured using a spectrophotometer (Shimadzu
UV-1700; Shimadzu Corporation, Kyoto, Japan) and the concentrations
of blood urea nitrogen (BUN) and creatinine (Cr) were
calculated.
Histological examinations
The kidneys were fixed in 10% phosphate-buffered
formalin, embedded in paraffin and cut into 4-µm sections. The
sections were deparaffinized and hydrated gradually, followed by
staining with hematoxylin and eosin. Morphological assessments were
performed by an experienced renal pathologist who was unaware of
the assigned treatments. An established grading scale of 0–4,
outlined by Jablonski et al (11), was used in the histopathological
assessment of I/R-induced damage.
Immunohistochemistry
Immunohistochemical staining was used to analyze the
expression of nuclear factor-κB (NF-κB). Briefly, the 4-µm sections
were deparaffinized, and endogenous peroxidase activity was blocked
with 3% hydrogen peroxide for 10 min at 37°C. Next, the sections
were treated with 10% normal goat serum in Tris-buffered saline
(TBS) for 30 min at 37°C. Subsequently, the samples were incubated
overnight at 4°C with a polyclonal rabbit anti-NF-κB antibody (no.
ab16502; 1:1,000; Abcam, Cambridge, UK). After washing three times
with phosphate-buffered saline (PBS), the sections were incubated
with a goat anti-rabbit secondary antibody (1:2,000; ZSGB-BIO
Corporation, Beijing, China) for 30 min at room temperature,
followed by the addition of the color reagent,
3,3′-diaminobenzidine. The aforementioned experiments were
routinely performed in the negative control group although PBS was
used instead of incubation with the primary antibody.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), and the RNA
concentration was determined using a spectrophotometer (Shimadzu
UV-1700; Shimadzu Corporation). Single-stranded cDNA was
synthesized with a RevertAid First Strand cDNA synthesis kit
(Takara Bio, Inc., Kyoto, Japan), according to the manufacturer's
instructions. Reverse transcription PCR was performed using the
SYBR® Green mix kit (Applied Biosystems Life Technologies, Foster
City, CA, USA). The primers used were as follows: Tumor necrosis
factor-α (TNF-α) forward, 5′-GCCACCACGCTCTTCTGTC-3′ and reverse,
5′-GCTACGGGCTTGTCACTCG-3′ (GenBank accession no. NM_012675.3);
intercellular adhesion molecule-1 (ICAM-1) forward,
5′-GGGATGGTGAAGTCTGTCAA-3′ and reverse, 5′-GGCGGTAATAGGTGTAAATGG-3′
(GenBank accession no. NM_012967); and interleukin-6 (IL-6)
forward, 5′-TTGCCTTCTTGGGACTGATGT-3′ and reverse,
5′-TACTGGTCTGTTGTGGGTGGT-3′ (GenBank accession no. NM_012589.1).
β-actin was used as a housekeeping gene and the data were presented
as the ratio of gene expression against that of β-actin. The
β-actin sense primer used was 5′-TGCTATGTTGCCCTAGACTTCG-3′ and the
antisense primer was 5′-GTTGGCATAGAGGTCTTTACGG-3′ (GenBank
accession no. NM_031144). The initial activation was at 95°C for 15
sec, followed by 58°C for 20 sec and 72°C for 20 sec for 40 cycles.
SLAN®-96s Real-Time PCR system (Shanghai Hongshi Medical Technology
Co., Ltd, Shanghai, China) was used to carry out the analysis.
There were three samples per assay.
Western blot analysis
Total proteins were extracted and quantified using a
bicinchoninic acid assay. Next, equivalent protein samples (40
µg/lane) were separated using 10% SDS-PAGE and transferred to
nitrocellulose membranes. The membranes were blocked with 5%
non-fat milk in TBS/Tween-20, and incubated with polyclonal rabbit
anti-NF-κB primary antibodies against NF-κB (no. ab16502; 1:1,000;
Abcam). Following two washes with PBS, the membranes were incubated
with a goat anti-rabbit secondary antibody conjugated to
horseradish peroxidase (1:2,000; ZSGB-BIO Corporation). Specific
bands were visualized using an enhanced chemiluminescence detection
kit (Pierce Biotechnology, Inc., Rockford, IL, USA), and the
optical densities were detected using Quantity One software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error of
mean. The mean values of the groups were compared using one-way
analysis of variance, followed by the Student-Newman-Keuls test.
All statistical analyses were performed with the SPSS statistical
package (SPSS 13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
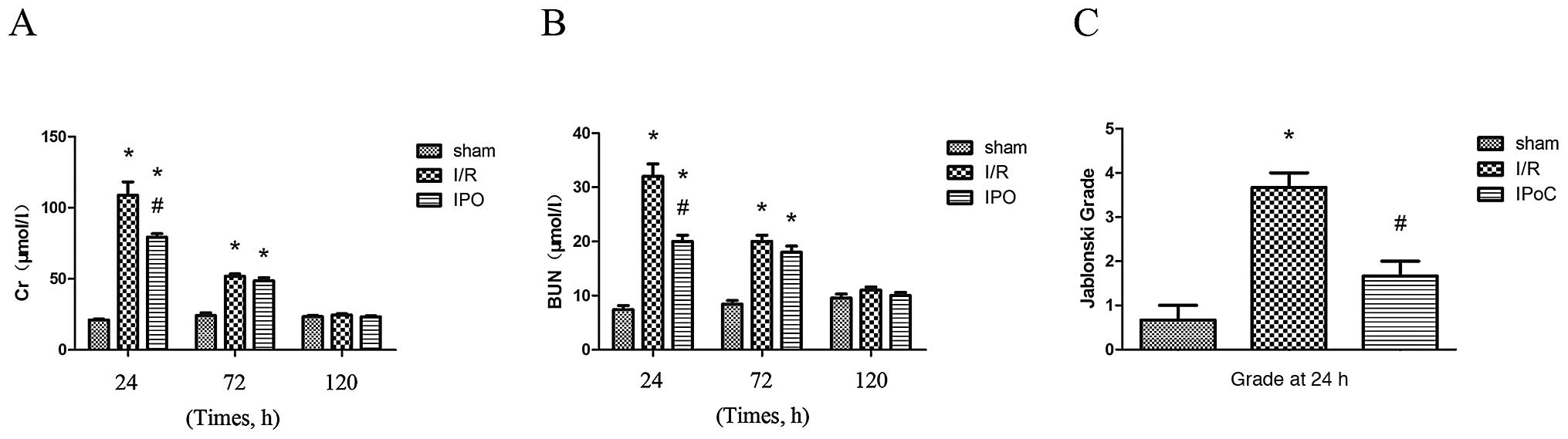
Renal function
Renal function was found to be different at each of
the three reperfusion time points (24, 72 and 120 h). The renal
functional parameters of the rats were significantly different at
24 and 72 h following I/R injury. Rats that were subjected to I/R
injury showed a significant increase in the levels of BUN and Cr
compared with the sham-operated rats at 24 and 72 h following I/R
injury. In addition, the renal function after I/R injury was
significantly improved following IPoC treatment (Fig. 1).
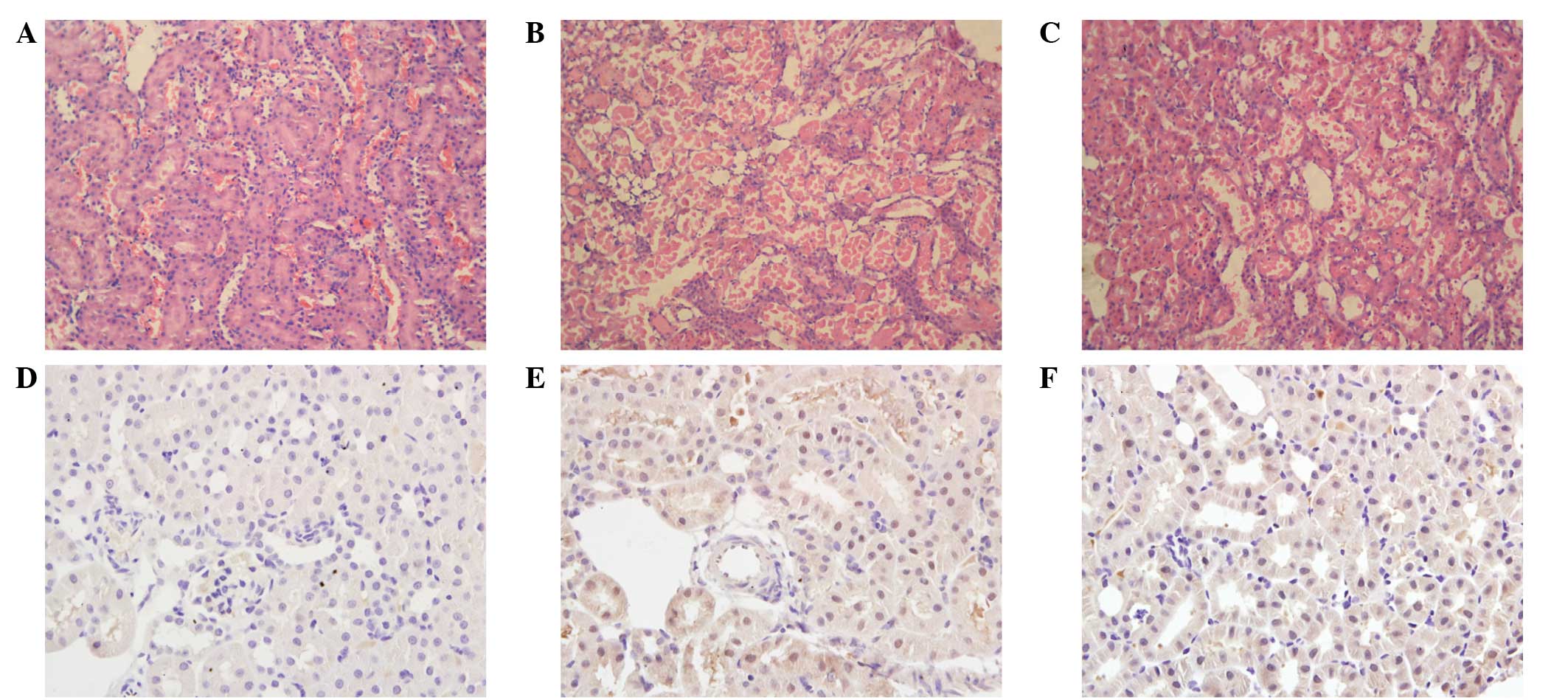
Histopathology
Compared with the sham group, the I/R group rats
suffered from significant tubular necrosis, medullary hemorrhage
and congestion. However, IPoC administration reduced these severe
renal damages (Fig. 2A-C).
Quantitative analysis revealed a markedly decreased Jablonski score
in the IPoC group rats compared with the I/R group (Fig. 1C).
Immunohistochemistry
NF-κB expression was detected using an
immunohistochemical technique. Staining revealed that the NF-κB
expression level was low in the sham group. However, the renal
tissues of the I/R group exhibited a strong positive expression for
NF-κB. Compared with the I/R group, the expression levels of NF-κB
were decreased in the IPoC group (Fig.
2D-F).
PCR analysis
The mRNA expression levels of ICAM-1, IL-6 and TNF-α
were calculated relative to β-actin. The expression levels were
found to vary following 24, 72 and 120 h of reperfusion, and were
significantly higher in the I/R group compared with the sham group.
However, IPoC was shown to significantly reduce the mRNA expression
levels of ICAM-1, IL-6 and TNF-α following I/R (Fig. 3).
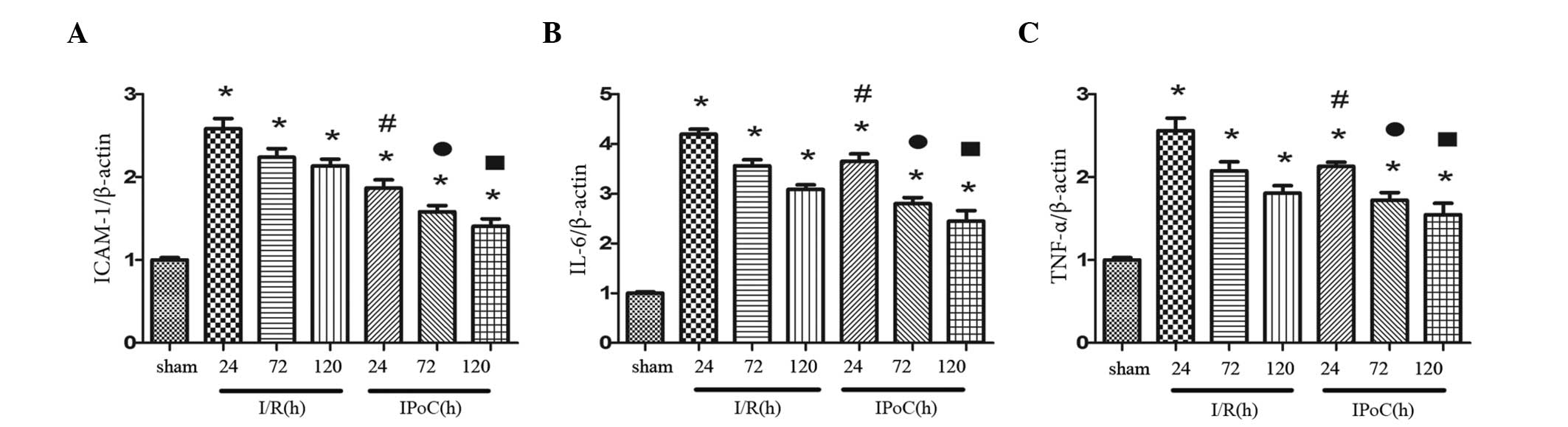 | Figure 3.Effect of IPoC on the mRNA expression
levels of (A) ICAM-1, (B) IL-6 and (C) TNF-α in the kidney after 45
min of ischemia, followed by 24, 72 and 120 h of reperfusion. The
mRNA expression levels were standardized against β-actin. The data
are presented as the mean ± standard error of mean. *P<0.05, vs.
sham; #P<0.05, vs. I/R at 24 h;
•P<0.05, vs. I/R at 72 h; P<0.05, vs. I/R at 120
h. TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; ICAM-1,
intercellular adhesion molecule-1; sham, sham-operated; I/R,
ischemia and reperfusion; IPoC, ischemic postconditioning. |
Western blot analysis
Compared with the sham group, the expression levels
of NF-κB were upregulated in the I/R group, and gradually decreased
with time. However, IPoC was found to attenuate the NF-κB
expression levels induced by I/R, as shown in Fig. 4.
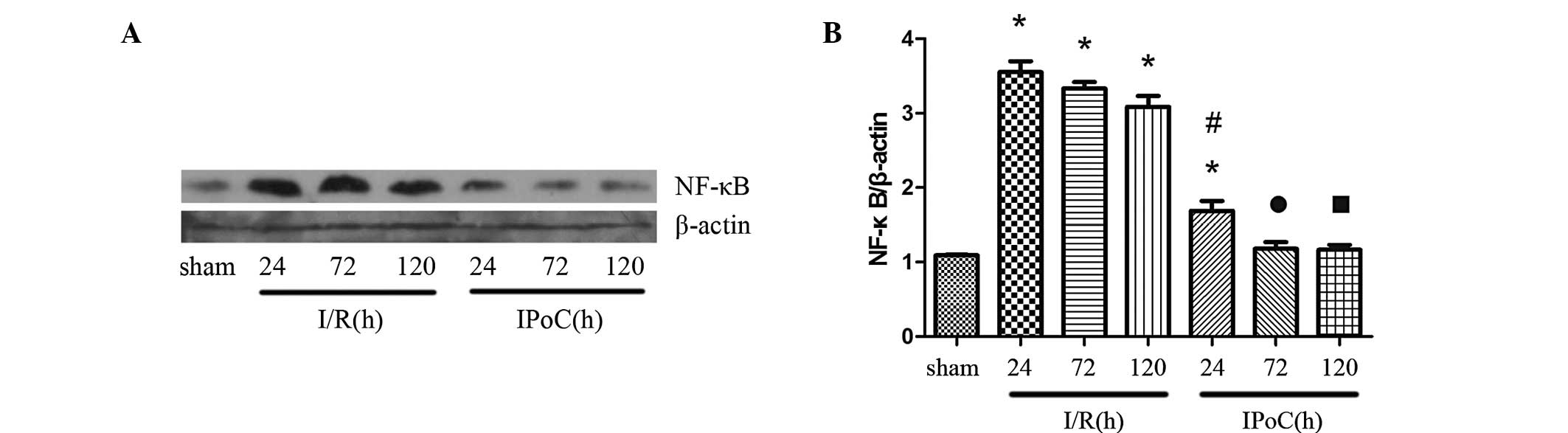 | Figure 4.(A) Representative western blot and
(B) quantitative analyses revealing the effects of IPoC on the
expression of NF-κB in the kidney after 45 min of ischemia,
followed by 24, 72 and 120 h of reperfusion. β-actin was used to
ensure an equal amount of protein was loaded in each lane. The data
are presented as the mean ± standard error of mean. *P<0.05, vs.
sham; #P<0.05, vs. I/R at 24 h;
•P<0.05, vs. I/R at 72 h; P<0.05, vs. I/R at 120
h. NF-κB, nuclear factor-κB; sham, sham-operated; I/R, ischemia and
reperfusion; IPoC, ischemic postconditioning. |
Discussion
In recent years, I/R injury has elicited an
increasing interest due to the impact on organs, such as the
kidney, liver and heart. In addition, research into the protective
effects of ‘organ conditioning’ against I/R injury has received
increasing interest. IPC has been demonstrated to protect organs
against the tissue damage induced by I/R, and the underlying
mechanisms were shown to involve a complex set of signaling
transduction pathways (12).
However, clinical application of IPC is often restricted since the
onset of ischemic injury is difficult to predict (13). A more clinically suitable approach is
IPoC, performed at the onset of reperfusion. IPoC was first
reported by Zhao et al (6) as
an effective strategy against cardiac I/R injury. The protective
effects of IPoC have also been demonstrated in several animal
models of non-cardiac I/R injury (14). Our previous study revealed that IPoC
attenuated oxidative stress and protected rats against renal I/R
injury; however, a protective mechanism directly involving the
kidney was not elucidated. Our study supported and further
demonstrated the aforementioned findings, revealing that IPoC was
able to reduce the expression levels of BUN and Cr and improve
renal morphology following I/R injury (10). In addition, IPoC was shown to inhibit
inflammation following renal I/R injury in rats, for the first
time.
Inflammation is a key factor in the occurrence and
development of ischemic damage. The activation of monocytes and
macrophages contributes to the synthesis and release of a variety
of proinflammatory cytokines following I/R injury (15). Within hours after an ischemic
episode, a large number of proinflammatory mediators are released,
leading to the development of tissue damage. Among the pathological
processes involved in I/R injury, TNF-α plays a key role in the
development and maintenance of an inflammatory response. The
infiltration of leukocytes into the kidney, assisted by TNF-α, may
aggravate the ischemic injury (16).
In addition, ICAM-1, an adhesion molecule, facilitates leukocyte
infiltration and adhesion, aggravating the injuries caused by I/R.
A previous study demonstrated that the functional inhibition of
TNF-α, which was associated with the decreased expression of
ICAM-1, was able to reduce the extent of I/R injury (17). IL-6 is a pleiotropic cytokine
involved in the regulation of immune and inflammatory responses. In
the present study, the increased expression levels of IL-6, TNF-α
and ICAM-1, which are markers of inflammation, were reduced by
IPoC. Thus, IPoC was found to reduce the inflammatory responses
following renal I/R injury. The mRNA expression levels of TNF-α,
IL-6 and ICAM-1 in the I/R group increased significantly in
response to the I/R-induced renal damage, peaking after 24 h of
reperfusion, followed by a gradual decrease. However, IPoC was
shown to significantly protect the renal tissue from I/R injury,
since the increased expression levels of the inflammatory markers
were reduced markedly in the IPoC group when compared with the I/R
group (Fig. 3). Therefore, the
results demonstrated that IPoC reduces the inflammatory response
following renal I/R injury.
NF-κB, an important nuclear transcription factor,
regulates the expression of a large number of genes that play a key
role in the regulation of apoptosis, inflammation, viral
replication and tumorigenesis (18).
Normally, an inactive form of NF-κB is sequestered in the cytoplasm
bound to IκB proteins, which regulates its activity (19). Numerous stimuli, including I/R
injury, can activate NF-κB signaling through the degradation of IκB
and the release of the NF-κB p65-p50 dimer. The dimer translocates
to the nucleus, binds to the κB binding sites of DNA and regulates
the transcriptional activation of target genes (20). NF-κB is crucial in the regulation of
genes encoding proinflammatory cytokines, such as IL-6, TNF-α and
ICAM-1. A previous study demonstrated that NF-κB may be a vital
regulator of inflammation following kidney damage, with
inflammatory mechanisms shown to be closely associated with
increased expression levels of NF-κB (21). In the present study, the expression
of NF-κB was investigated following 24, 72 and 120 h of reperfusion
in the I/R and IPoC groups. The results indicated that the
expression levels of NF-κB were upregulated in the I/R group after
24 h of reperfusion and then gradually decreased. By contrast, IPoC
was found to significantly attenuate the expression levels induced
by I/R injury, which was consistent with the changes observed in
renal function.
However, a number of limitations exist in the
present study. A recent clinical study demonstrated that IPoC did
not reduce the delayed graft function or improve renal function
following kidney transplantation, although IPoC application was
found to be feasible and safe (22).
A possible explanation for the conflicting results may be that
healthy young animals are used in the majority of animal
experiments, while in the aforementioned clinical study, the
transplant donors were older and suffered from a number of
comorbidities. Therefore, future studies should investigate aged or
diseased rats. Furthermore, in vitro studies are required to
confirm the results, since an inherent interconnection of the
effects of IPoC treatment on tissue salvage and protein signals was
observed. In addition, only a short-term period of survival was
assessed in the present study, while a previous study demonstrated
that IPoC protected rats against I/R damage after 12 weeks and had
beneficial effects on renal fibrosis (23). The anti-inflammatory properties of
IPoC may possibly lead to the long-term protection of renal
fibrosis; therefore, the long-term consequences of IPoC shoulwd be
investigated in a further study. Furthermore, only six cycles of
reperfusion for 10 sec followed by 10 sec of ischemia were applied
for IPoC. Thus, the current study did not reveal whether IPoC plays
an ‘on-off’ or ‘dose-dependent’ role. In the case that IPoC is
‘dose-dependent’, the ischemic episode period of 10 sec may not
afford the maximal protective effect against renal I/R injury.
Thus, the optimal interval length and number of cycles require
further investigation.
In conclusion, IPoC was demonstrated to protect rats
against inflammation following renal I/R injury, and the underlying
mechanism of IPoC was found to be associated with the decreased
expression of NF-κB. Therefore, inhibiting the activation of NF-κB
may develop smaller impairments following renal I/R injury.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 30901494).
Abbreviations:
|
I/R
|
ischemia and reperfusion
|
|
IPoC
|
ischemic postconditioning
|
|
IPC
|
ischemic preconditioning
|
|
BUN
|
blood urea nitrogen
|
|
Cr
|
creatinine
|
|
NF-κB
|
nuclear factor-κB
|
|
ICAM
|
intercellular adhesion molecule
|
|
qPCR
|
quantitative polymerase chain
reaction
|
|
TNF
|
tumor necrosis factor
|
|
IL
|
interleukin
|
|
PBS
|
phosphate-buffered saline
|
|
TBS
|
Tris-buffered saline
|
References
|
1
|
Kam Tao Li P, Burdmann EA and Mehta
RLWorld Kidney Day Steering Committee 2013: Acute kidney injury:
Global health alert. J Nephropathol. 2:90–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yun Y, Duan WG, Chen P, Wu HX, Shen ZQ,
Qian ZY and Wang DH: Ischemic postconditioning modified renal
oxidative stress and lipid peroxidation caused by ischemic
reperfusion injury in rats. Transplant Proc. 41:3597–3602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barri YM, Sanchez EQ, Jennings LW, Melton
LB, Hays S, Levy MF and Klintmalm GB: Acute kidney injury following
liver transplantation: definition and outcome. Liver Transpl.
15:475–483. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma ZF, Chen W, Cao CC and Chen X: Ischemic
preconditioning attenuates brain injury induced by
ischemia/reperfusion during moderate hypothermia low-flow
procedures. Int J Neurosci. Jan 24–2014.(Epub ahead of print).
View Article : Google Scholar
|
|
5
|
Ma J, Qiao Z and Xu B: Effects of ischemic
preconditioning on myocardium Caspase-3, SOCS-1, SOCS-3, TNF-α and
IL-6 mRNA expression levels in myocardium IR rats. Mol Biol Rep.
40:5741–5748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong Y, Rogers MR and Qin X: Effective
neuroprotection by ischemic postconditioning is associated with a
decreased expression of RGMa and inflammation mediators in ischemic
rats. Neurochem Res. 38:815–825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Lei S, Yuan J, Liu X, Zhang D, Gu X,
Zhang L and Xia Z: Ischemic postconditioning downregulates Egr-1
expression and attenuates postischemic pulmonary inflammatory
cytokine release and tissue injury in rats. J Surg Res.
181:204–212. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu
H and Chen Z: Ozone oxidative preconditioning inhibits inflammation
and apoptosis in a rat model of renal ischemia/reperfusion injury.
Eur J Pharmacol. 581:306–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu
H and Chen Z: Ischemic postconditioning inhibits apoptosis after
renal ischemia/reperfusion injury in rat. Transpl Int. 21:364–371.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jablonski P, Howden BO, Rae DA, Birrell
CS, Marshall VC and Tange J: An experimental model for assessment
of renal recovery from warm ischemia. Transplantation. 35:198–204.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Granfeldt A, Lefer DJ and Vinten-Johansen
J: Protective ischaemia in patients: preconditioning and
postconditioning. Cardiovasc Res. 83:234–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Venugopal V, Ludman A, Yellon DM and
Hausenloy DJ: ‘Conditioning’ the heart during surgery. Eur J
Cardiothorac Surg. 35:977–987. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao ZQ: Postconditioning in reperfusion
injury: a status report. Cardiovasc Drugs Ther. 24:265–279. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang JQ, Chen X, Zhang C, Tao L, Zhang ZH,
Liu XQ, Xu YB, Wang H, Li J and Xu DX: Phenylbutyric acid protects
against carbon tetrachloride-induced hepatic fibrogenesis in mice.
Toxicol Appl Pharmacol. 266:307–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Ge P and Zhu Y: TLR2 and TLR4 in
the brain injury caused by cerebral ischemia and reperfusion.
Mediators Inflamm. 2013:1246142013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma JQ, Liu CM, Qin ZH, Jiang JH and Sun
YZ: Ganoderma applanatum terpenes protect mouse liver
against benzo(α)pyren-induced oxidative stress and inflammation.
Environ Toxicol Pharmacol. 31:460–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YW, Zhang Y, Zhang L, Li X, Yu JB,
Zhang HT, Tan BB, Jiang LH, Wang YX, Liang Y, et al: Protective
effect of tea polyphenols on renal ischemia/reperfusion injury via
suppressing the activation of TLR4/NF-κB p65 signal pathway. Gene.
542:46–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong ET and Tergaonkar V: Roles of
NF-kappaB in health and disease: mechanisms and therapeutic
potential. Clin Sci (Lond). 116:451–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Liu X, Zhu Y, Chen S, Zhou D and
Wang Y: Honokiol inhibits the inflammatory reaction during cerebral
ischemia reperfusion by suppressing NF-κB activation and cytokine
production of glial cells. Neurosci Lett. 534:123–127. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van den Akker EK, Hesselink DA, Manintveld
OC, et al: Ischemic postconditioning in human DCD kidney
transplantation is feasible and appears safe. Transpl Int.
27:226–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weng X, Shen H, Kuang Y, Liu X, Chen Z,
Zhu H, Jiang B, Zhu G and Chen H: Ischemic postconditioning
inhibits the renal fibrosis induced by ischemia-reperfusion injury
in rats. Urology. 80:484.e1–484.e7. 2012. View Article : Google Scholar
|