Introduction
Inhibitor of growth 4 (ING4) is located on
chromosome 12p13 and encodes a 249-amino acid protein containing a
novel conserved region in the N terminus, a nuclear localization
signal in the central region and a highly conserved plant
homeodomain in the C terminus (1).
Downregulation of ING4 has been reported in various types of
cancer, including hepatocellular and gastric carcinomas, and
breast, colon and lung cancers (2–6).
Previous studies have also demonstrated that ING4 is involved in a
variety of cellular processes, including cell proliferation,
apoptosis, migration, angiogenesis and the DNA damage response
(7–10).
In epithelial to mesenchymal transition (EMT),
epithelial cells transform into a mesenchymal phenotype, which
involves an increase in fibroid morphology, invasiveness and
resistance to apoptosis, as well as an increase in extracellular
matrix components (11). Increasing
evidence indicates that cancer cells acquire invasive properties by
EMT (12–15).
Thyroid cancer is a common endocrine malignancy that
has exhibited a rapid increase in global incidence in recent
decades (16). However, the roles of
recombinant ING4 protein in thyroid cancer remain unclear.
Therefore, in the present study, the effect of recombinant ING4 on
the growth, mobility and apoptosis of thyroid cancer cells was
investigated, as well as the association between ING4 and EMT.
Materials and methods
Cell culture
SW579, a human thyroid cancer cell line, was
purchased from the Shanghai Institutes for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured at 37°C with 5% (v/v) CO2 in Dulbecco's
modified Eagle's medium (DMEM; Life Technologies, Gaithersburg, MD,
USA), which was supplemented with 10% (v/v) fetal calf serum (FCS;
Life Technologies) and antibiotics (100 µM penicillin and 100 µM
streptomycin; Sigma-Aldrich, Carlsbad, CA, USA).
Colony formation assay
Cells were seeded at 200 cells per well in 24-well
tissue culture plates for 24 h under 5% CO2 at 37°C.
Subsequently, the cells were treated with various concentrations
(0, 50, 100, 150, 200 or 250 ng/ml) of recombinant ING4 protein
(Sino Biological, Inc., Beijing, China), and the plates were
incubated for 1 week in a humidified incubator at 37°C. Colonies
were stained with 0.05% crystal violet (Beyotime Institute of
Biotechnology, Shanghai, China) containing 50% methanol, and
counted. The colonies were counted in four or five random fields of
vision for each of the duplicate samples using a CX71 microscope
(Olympus Corporation, Tokyo, Japan) at a magnification of ×100. The
IC50 value of the recombinant ING4 protein was
determined and for use in later experiments.
Flow cytometric analysis of the
apoptosis rate
To detect the rate of apoptosis, an annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(KeyGen, Nanjing, China) was used in accordance with the
manufacturer's instructions. The samples were immediately analyzed
on a FACSCalibur flow cytometer (Becton-Dickinson Medical Devices,
Shanghai, China).
Transwell assay
A Transwell migration assay was performed using the
Boyden chamber (8 µM pore size; polycarbonate membrane; Cell
Biolabs, San Diego, CA, USA). The cells were resuspended in
FCS-free DMEM to a concentration of 3×105 cells/ml. The
upper chamber was loaded with 100 µl cell suspension, while the
lower chamber was loaded with 600 µl DMEM containing 10% FCS.
Following incubation for 12 h in normal culture conditions, the
filter was fixed in 4% paraformaldehyde (Sigma-Aldrich) and stained
with crystal violet. The cells on the upper side of the filter were
removed using a cotton swab, and the cells that had migrated to the
undersurface of the membrane were counted using a light microscope
(6XC; Shanghai Guangmai Instruments Ltd., Shanghai, China). In
total, 10 microscopic fields (magnification, ×400) were randomly
selected for the counting of the cells.
Gelatin zymography
A gelatin zymography assay was performed according
to the methods outlined by Song and Zhao (17). Briefly, 50 mg protein was applied to
10% polyacrylamide gels, with 1% gelatin incorporated as a
substrate for the gelatinolytic proteases. Subsequent to running
the gel, the sodium dodecyl sulfate (SDS) was removed by washing
twice in 2.5% Triton X-100 for 30 min. The gels were incubated
overnight in a zymography development buffer, which contained 50 mM
Tris-HCl (pH 7.4), 2 mM NaN3 and 5 mM CaCl2.
Following development, the gels were stained for 3 h in 45%
methanol/10% glacial acetic acid containing 1% (w/v) Coomassie
Brilliant Blue R-250 (Beyotime Institute of Biotechnology), and
subsequently partially destained with the same solution without
dye. The gelatinolytic activity of each matrix metalloproteinase
(MMP) was qualitatively evaluated as a clear band against the
blue-stained gelatin background.
Chick chorioallantoic membrane (CAM)
assay
A CAM assay was conducted according to the methods
outlined by Lokman et al (18). Chick eggs (Dezhou Food Imp & Exp
Co. Ltd., Dezhou, China) were incubated in a MultiQuip incubator
(MultiQuip, Austral, NSW, Australia) at 37°C with 60% humidity.
Under aseptic conditions, a small window was made in the shell on
day 3 of chick embryo development to observe the underlying
vasculature. The window was resealed with adhesive tape and the
eggs were returned to the incubator until day 11 of chick embryo
development. On day 11, all three groups of SW579 cells were
labeled with CellTracker™ Green 5-chloromethylfluorescein diacetate
(Invitrogen Life Technologies, Carlsbad, CA, USA) in suspensions
(1×106 cells/well) and mixed with growth factor reduced
Matrigel (8.9 mg/ml; BD Biosciences, Franklin Lakes, NJ, USA) to a
total volume of 30 µl. Subsequently, the Matrigel grafts were
placed on top of the CAM, and the eggs were resealed and returned
to the incubator for 72 h until day 14 (n=6 chicken embryos per
cell line; SW579, PBS and ING4). Matrigel grafts with the
surrounding CAM were harvested from each embryo, fixed with 4%
paraformaldehyde for 24 h and embedded in Optimum Cutting
Temperature compound (Tissue-Tek; Sakura Finetek USA, Inc.,
Torrance, CA, USA). The samples were stored at −80°C, and frozen
samples were cut into 5-µm sections using a Cryomicrotome CM 1850
(Leica Microsystems, Bannockburn, IL, USA). Images were acquired
using an Olympus fluorescence microscope (Olympus Corporation,
Osaka, Japan).
Preparation of nuclear and cytoplasmic
protein extracts
Nuclear and cytoplasmic protein fractions were
isolated using a CelLytic™ NuCLEAR™ Extraction kit (Sigma-Aldrich),
according to the manufacturer's instructions. Protein
concentrations were determined using a bicinchoninic acid protein
assay, with bovine serum albumin used as a standard (Pierce
Biotechnology, Inc., Rockford, IL, USA).
Western blot analysis
Protein extracts were resolved by SDS-PAGE, which
was followed by electrotransfer to nitrocellulose membranes
(Bio-Rad Laboratories, Inc., Philadelphia, PA, USA). Following a
blocking step using 5% milk in Tris-buffered saline with Tween® 20,
the membranes were incubated with primary antibodies at room
temperature overnight (Table I).
Subsequently, the membranes were developed and visualized with
enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA,
USA). Secondary monoclonal antibodies were purchased from the
Beyotime Institute of Biotechnology. The secondary monoclonal
antibodies included anti-mouse IgG (#A0216), anti-rabbit IgG
(#A0239) and anti-goat IgG (#A0181). The membranes were incubated
with secondary antibodies for 2 h at room temperature.
 | Table I.Antibodies used in western blot
analysis. |
Table I.
Antibodies used in western blot
analysis.
| Protein | Manufacturer | Catalog number | Dilution |
|---|
| E-cadherin | Santa Cruz
Biotechnologya | sc-7870 | 1:200 |
| Vimentin | Santa Cruz
Biotechnologya | sc-6260 | 1:200 |
| N-cadherin | Santa Cruz
Biotechnologya | sc-7939 | 1:200 |
| Anti-Wnt5b | Abcamb | ab94914 | 1:200 |
| LRP6 | Santa Cruz
Biotechnologya | sc-17982 | 1:200 |
| p-LRP6 (Ser1490) | Cell Signaling
Technologyc | 3395 | 1:200 |
| Axin2 | Cell Signaling
Technologyc | 2151 | 1:200 |
| GSK-3β | Santa Cruz
Biotechnologya | sc-81462 | 1:200 |
| β-catenin | Santa Cruz
Biotechnologya | sc-1496 | 1:200 |
| β-actin | Santa Cruz
Biotechnologya | sc-130656 | 1:1,000 |
| β-tubulin | Santa Cruz
Biotechnologya | sc-55526 | 1:500 |
Statistical analysis
Numerical data are expressed as the mean ± standard
deviation, and differences among the mean values were evaluated
using the Student's t-test. Statistical analyses were conducted
using SPSS software (version 11.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Antitumor effects of recombinant ING4
protein on SW579 cells
Cell viability was assessed using the colony
formation assay. As shown in Fig.
1A, the proliferative rate of the cells in the recombinant
ING4-treated group was inhibited significantly in a dose-dependent
manner (P<0.05). The IC50 value of the recombinant
ING4 protein was determined to be 192.5 ng/ml. When compared with
the untreated SW579 cells or the phosphate-buffered saline-treated
cells, the apoptotic ratio of the cells following treatment with
recombinant ING4 protein was observed to increase significantly
using annexin V-FITC and propidium iodide double staining
(P<0.05; Fig. 1B). In addition,
the Transwell assay revealed that cell motility was significantly
decreased in the recombinant ING4 protein-treated group, as
compared with the untreated cells (P<0.05; Fig. 1C). The activity levels of MMP-2 and
−9 were shown to be inhibited by ING4 protein in SW579 cells
(Fig. 1D). Additionally, using the
CAM model, ING4 protein was demonstrated to inhibit SW579 cells
from escaping primary tumor sites and scattering among blood
vessels (Fig. 1E).
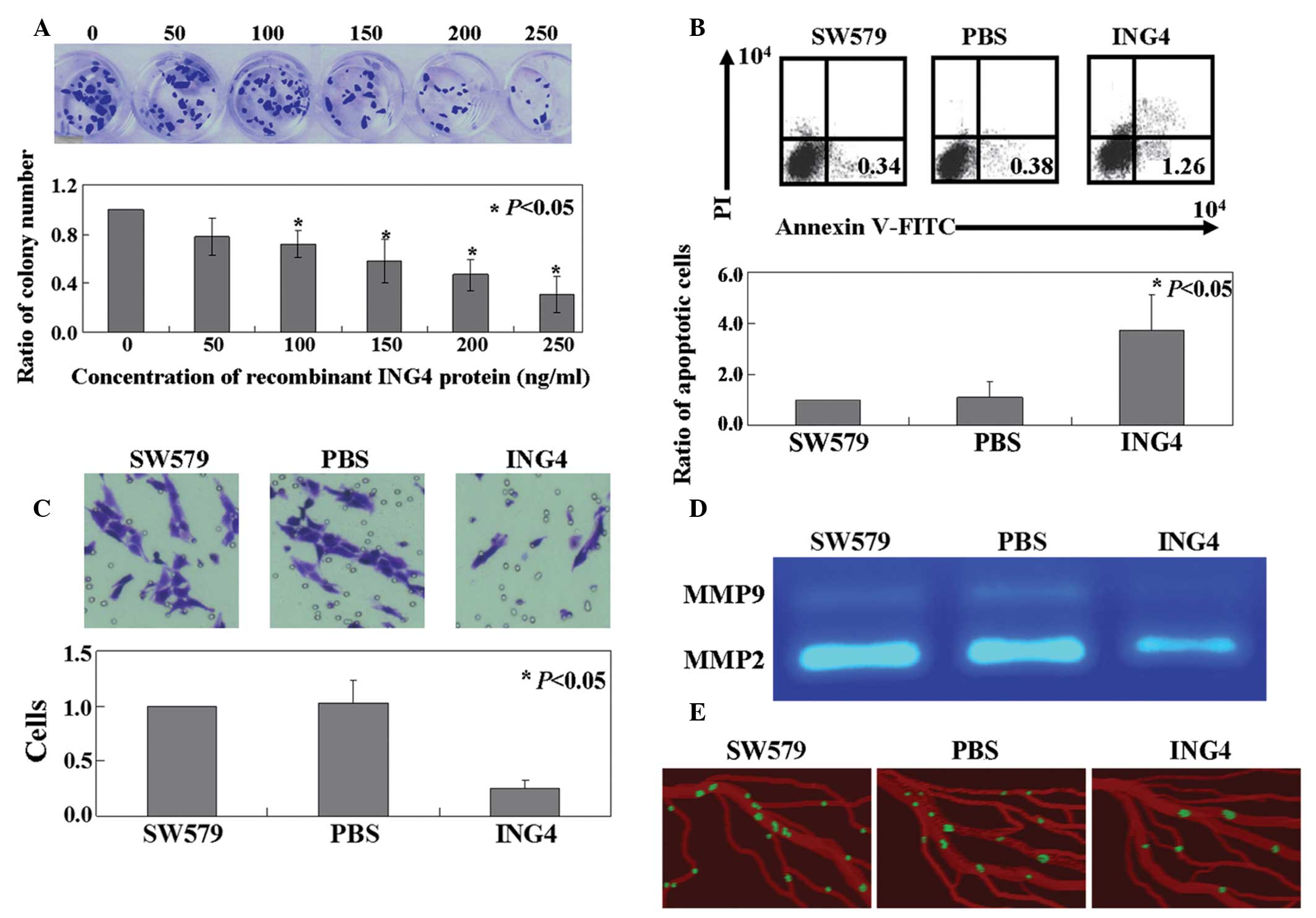 | Figure 1.Antitumor activities of ING4 in SW579
cells. (A) Proliferation ratio of SW579 cells treated with
recombinant ING4 protein was measured using the colony formation
assay. (B) Apoptotic ratio of cells was analyzed by double staining
with annexin-V FITC/PI. (C) Transwell assays were performed to
detect the mobility of the SW579 cells treated with recombinant
ING4 protein. (D) Gelatinolytic activity levels of MMP-2 and -9,
secreted from the SW579 cells, were analyzed by zymography. (E)
Invasive GFP-labeled SW579 cells were visualized intravascularly.
SW579, untreated SW579 cells; PBS, PBS treated SW579 cells; ING4,
recombinant ING4 protein treated SW579 cells; ING, inhibitor of
growth; FITC, fluorescein isothiocyanate; PI propidium iodide; MMP,
matrix metalloproteinase; PBS, phosphate-buffered saline; GFP,
green fluorescent protein. |
Mechanisms of recombinant ING4
protein-induced apoptosis and inhibited mobility in SW579
cells
To identify the mechanisms underlying the effects of
recombinant ING4 protein in SW579 cells, the expression levels of
various proteins were detected by western blot analysis. When
compared with the untreated cells, the ING4-treated SW579 cells
exhibited a higher expression level of the epithelial marker,
E-cadherin, while lower expression levels of the mesenchymal
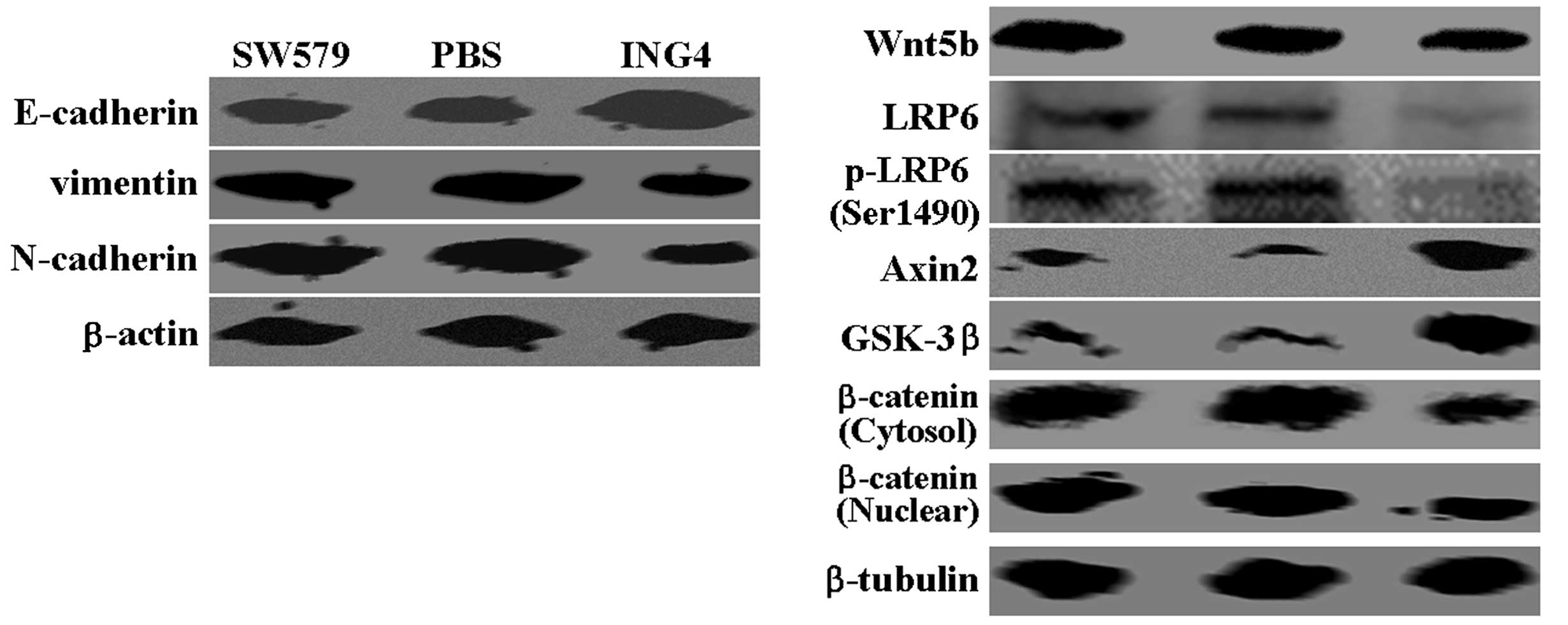
markers, vimentin and N-cadherin, were detected (Fig. 2). Notably, changes in Wnt5b
expression were observed in the SW579 cells following treatment
with ING4. Furthermore, the western blot analysis assays detected a
significant inhibition of low-density lipoprotein receptor-related
protein 6 expression and phosphorylation following treatment with
ING4 (Fig. 2). A concomitant
increase in Axin2 and glycogen synthase kinase-3β expression was
detected in the ING-treated SW579 cells, as compared with the
control groups, while decreased expression levels of nuclear and
cytosolic β-catenin were observed (Fig.
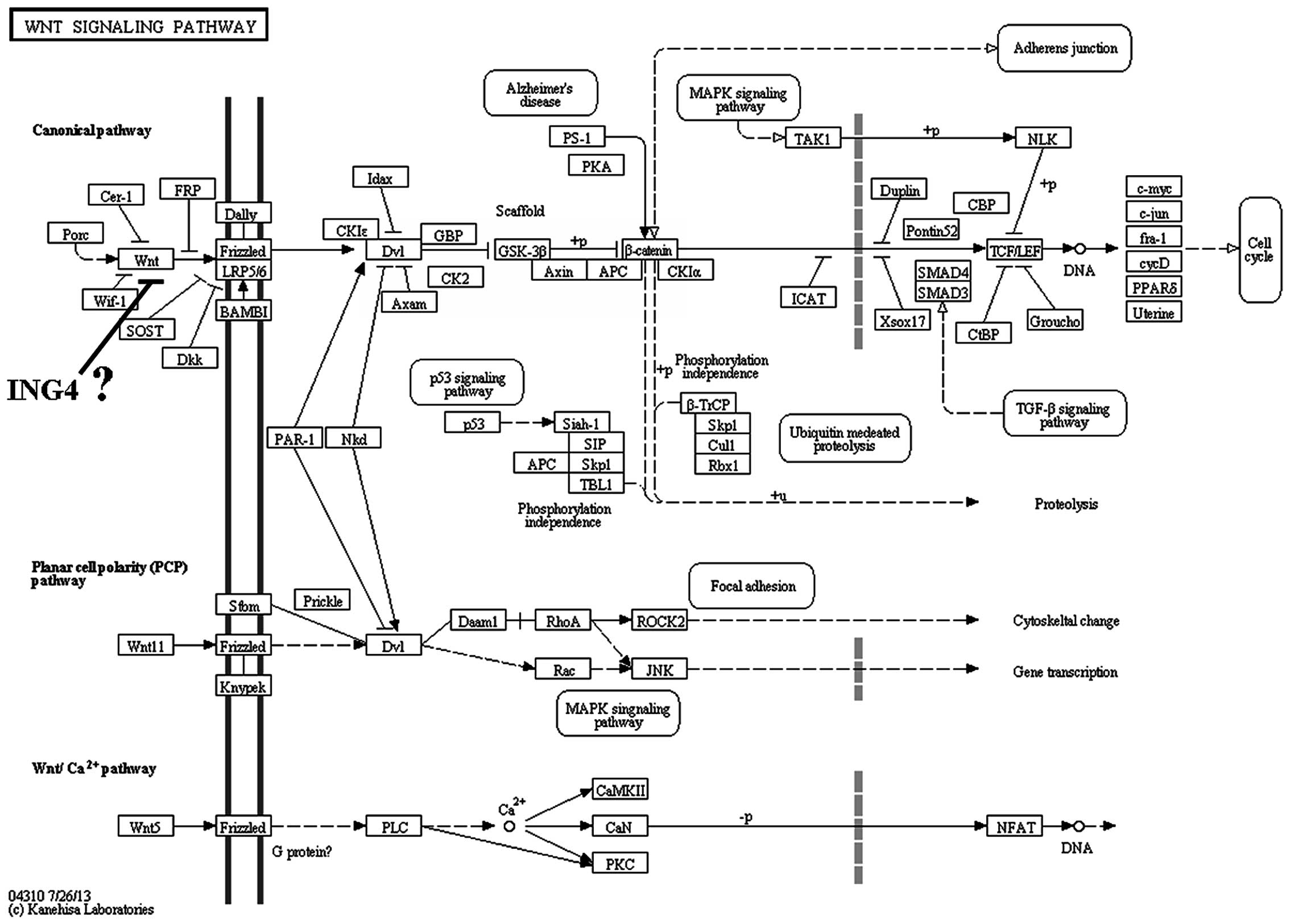
2). Pathway enrichment analysis was performed in the Kyoto
Encyclopedia of Genes and Genomes database (Institute for Chemical
Research, Kyoto University, Kyoto, Japan; Institute of Medical
Science, University of Tokyo, Tokyo, Japan), and the analysis
results revealed that the Wnt signaling pathway was involved in the
antitumor effects of ING4 (Fig.
3).
Discussion
As previously discussed, ING4 is involved in cell
proliferation, apoptosis, migration, angiogenesis and the DNA
damage response (7–10). Consistent with these previous
studies, the present study confirmed the ability of ING4 to inhibit
cell proliferation and mobility, and induce apoptosis in SW579
cells. To the best of our knowledge, although ING4 is not observed
in a number of cancer types, this is the first study to demonstrate
the antitumor roles of ING4 in thyroid cancer.
The primary finding of the present study was the
involvement of the Wnt signaling pathway in the mechanism
underlying the effects of ING4. Canonical Wnt/β-catenin signaling
directly alters gene expression, and has been shown to be a key
regulator of cell proliferation, differentiation and apoptosis in a
variety of cancer types (19,20).
Abnormal activation of the Wnt/β-catenin signaling pathway and the
subsequent upregulation of β-catenin has also been associated with
the development of breast cancer (21). In the present study, ING4 was
demonstrated to suppress Wnt5b expression in SW579 cells. The
inhibition of Wnt5b expression results in decreased cytosolic
accumulation of β-catenin, followed by reduced β-catenin
translocation to the nucleus (22).
Furthermore, the present results indicated that targeting the
β-catenin pathways may suppress the mobility of SW579 cells, which
is associated with EMT. E-cadherin is known to anchor and sequester
β-catenin in the membrane to prevent activation; thus, the
activation of β-catenin signaling may result from the
downregulation of E-cadherin at EMT (22). E-cadherin expression has also been
shown to be upregulated following decreased β-catenin expression
(22).
In conclusion, the results of the present study have
confirmed the antitumor activities and mechanisms of ING4-induced
apoptosis in SW579 cells. These results provide initial evidence
indicating the potential of ING4 as a therapeutic target for
thyroid cancer.
Acknowledgements
The authors thank Miss. Wei Wang for the provision
of valuable comments.
References
|
1
|
Shiseki M, Nagashima M, Pedeux RM, et al:
p29ING4 and p28ING5 bind to p53 and p300 and enhance p53 activity.
Cancer Res. 63:2373–2378. 2003.PubMed/NCBI
|
|
2
|
Byron SA, Min E, Thal TS, et al: Negative
regulation of NF-κB by the ING4 tumor suppressor in breast cancer.
PLoS One. 7:e468232012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng ZL, Li FJ, Gao F, Sun DS and Yao L:
Upregulation of miR-650 is correlated with downregulation of ING4
and progression of hepatocellular carcinoma. J Surg Oncol.
107:105–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Jin Y, Sun WJ, et al: Reduced
expression and novel splice variants of ING4 in human gastric
adenocarcinoma. J Pathol. 219:87–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lou C, Jiang S, Guo X and Dong XS: ING4 is
negatively correlated with microvessel density in colon cancer.
Tumour Biol. 33:2357–2364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang QS, Li M, Zhang LY, et al:
Down-regulation of ING4 is associated with initiation and
progression of lung cancer. Histopathology. 57:271–281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zhang Q, Cai L, et al: Inhibitor of
growth 4 induces apoptosis in human lung adenocarcinoma cell line
A549 via Bcl-2 family proteins and mitochondria apoptosis pathway.
J Cancer Res Clin Oncol. 135:829–835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen JC, Unoki M, Ythier D, et al:
Inhibitor of growth 4 suppresses cell spreading and cell migration
by interacting with a novel binding partner, liprin alpha1. Cancer
Res. 67:2552–2558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colla S, Tagliaferri S, Morandi F, et al:
The new tumor-suppressor gene inhibitor of growth family member 4
(ING4) regulates the production of proangiogenic molecules by
myeloma cells and suppresses hypoxia-inducible factor-1 alpha
(HIF-1alpha) activity: Involvement in myeloma-induced angiogenesis.
Blood. 110:4464–4475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J and Li G: Cell cycle regulator ING4
is a suppressor of melanoma angiogenesis that is regulated by the
metastasis suppressor BRMS1. Cancer Res. 70:10445–10453. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santisteban M, Reiman JM, Asiedu MK, et
al: Immune-induced epithelial to mesenchymal transition n
vivo generates breast cancer stem cells. Cancer Res.
69:2887–2895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song GQ and Zhao Y: Different therapeutic
effects of distinct KISS1 fragments on breast cancer n vitro
and n vivo. Int J Oncol. 43:1219–1227. 2013.PubMed/NCBI
|
|
18
|
Lokman NA, Elder AS, Ricciardelli C and
Oehler MK: Chick chorioallantoic membrane (CAM) assay as an n
vivo model to study the effect of newly identified molecules on
ovarian cancer invasion and metastasis. Int J Mol Sci.
13:9959–9970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Apte U, Zeng G, Thompson MD, et al:
beta-Catenin is critical for early postnatal liver growth. Am J
Physiol Gastrointest Liver Physiol. 292:G1578–G1585. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nejak-Bowen K and Monga SP:
Wnt/beta-catenin signaling in hepatic organogenesis. Organogenesis.
4:92–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown AM: Wnt signaling in breast cancer:
Have we come full circle? Breast Cancer Res. 3:351–355. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar : PubMed/NCBI
|