Introduction
Endometriosis, a condition characterized by the
presence and proliferation of endometrial tissue outside the
uterine cavity, is a cause of pain and reduced fertility worldwide.
The etiology of the condition is believed to involve the retrograde
flow of menstrual fluid through the fallopian tubes, leading to the
deposition of viable endometrial tissue and the subsequent
implantation of this tissue onto the peritoneal surface (1). Although retrograde menstruation appears
in 90% of women of a reproductive age, only 10% of cases are
characterized by the presence of endometrial tissue outside the
uterine cavity (2). It has
previously been found that women with endometriosis exhibit
aberrant endometrial gene expression (3). Furthermore, the endometrial cells from
women with endometriosis have a predisposition to adhere and grow
outside of the uterus (4).
Macrophage migration inhibitory factor (MIF) is a
cytokine that is secreted by the immune cells and the anterior
pituitary gland (5). MIF has been
found to be a key mediator of systemic inflammatory responses and
is thus a critical regulator of inflammatory pathways (6), which have an essential role in the
pathogenesis of endometriosis (7).
Compared with the MIF expression in normal fertile women, MIF
expression has been found to be elevated in early, active
endometriotic lesions and in the intrauterine human endometrium of
women with endometriosis (8), which
suggests that MIF has a possible role in endometriosis-related pain
and infertility. Although the mechanism underlying the regulation
of MIF is important, it has yet to be elucidated whether the mRNA
level of MIF is altered in endometrial tissues from women with
endometriosis. Endometriosis can be considered to be an
estrogen-dependent disease (9) that
is rarely observed prior to menarche and typically disappears
following menopause. The aims of the present study, therefore, were
as follows: i) To examine whether the MIF mRNA level is altered in
endometrial tissues from women with endometriosis; ii) to
investigate whether the MIF expression level in endometrial tissues
is associated with the serum 17β-estradiol (E2) level;
and iii) to observe whether E2 treatment induces a
change in MIF expression in endometrial cells.
Materials and methods
Patients and sample collection
This study was approved by the Ethics Committee of
the School of Medicine, Zhejiang University (Hangzhou, China).
Written informed consent was obtained from each subject prior to
tissue collection. A total of 102 women of a reproductive age
volunteered for this study. All subjects had a normal menstrual
cycle (28–32 days) and had not received any anti-inflammatory or
hormonal treatment for ≥6 months before inclusion in the study. At
the time of surgery, the pelvic organs of the women were examined
carefully for the presence and extent of endometriosis.
Among the patients, 55 women (aged 25–39 years) were
diagnosed with an ovarian endometriotic cyst, and each of these
women underwent a uterus-preserved cystectomy by laparoscopy. A
final diagnosis of endometriosis was confirmed and supported by the
subsequent histology. The endometriosis staging was in accordance
with the revised American Fertility Society classification
(10). Among the 55 women with
endometriosis, 12 were classed as fertile with stage I (n=5), stage
II (n=4), stage III (n=2) or stage IV (n=1) disease. The other 43
women were infertile with stage I (n=13), stage II (n=16), stage
III (n=9) or stage IV (n=5) disease. Information about pelvic pain,
dysmenorrhea and average ovarian endometriotic cyst diameter was
taken from the patients' clinical records. The control subjects
were 47 women (aged 28–38 years), undergoing surgery for tubal
ligation (36 cases) or hysterectomy for benign indications (11
cases), who had no visible evidence of endometriosis. The mean age
of the women with endometriosis and control women was 31.7±3.8 and
30.9±2.9 years, respectively, and no difference in age was observed
between the two groups (P>0.05).
Blood samples were obtained for the measurement of
the serum E2 and progesterone (P) concentrations on the
morning of the day of surgery, and endometrial tissues were
simultaneously obtained via Pipelle® endometrial curettage (CCD
Laboratories, Paris, France) on days 6–10 (proliferative phase) or
18–26 (secretory phase) of the menstrual cycle. Samples at were
collected different phases of the menstrual cycle from different
patients in order to avoid the possible effects of the first sample
on subsequent samples. Following collection, the endometrial
tissues were rapidly either snap-frozen in liquid nitrogen and
stored at −80°C for the subsequent extraction of protein and mRNA.
According to the Noyes pathological diagnosis (hematoxylin and
eosin staining) the endometrial samples were assigned to one of
four groups: Proliferative phase of endometriosis (n=26), secretory
phase of endometriosis (n=29), proliferative phase of control
(n=24) and secretory phase of control (n=23).
Western blot analysis
Cells were lysed in Laemmli lysis buffer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) containing 1% Triton X-100
and scraped with a cell lifter. Equal quantities of protein (25 µl)
were separated using 8% SDS-PAGE and transferred to Immobilon™-P
membranes (Millipore, Billerica, MA, USA). Blocking was performed
with 5% skimmed dried milk at 4°C overnight, and the membranes were
then incubated with primary antibodies (sc-20121; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at room temperature for 2
h. Following incubation, the membranes were washed three times with
1X phosphate-buffered saline (PBS) in 0.1% Tween-20 and incubated
with the respective horseradish peroxidase-conjugated secondary
antibodies (1:10,000; P0488; Dako Cytomation, Inc., Carpinteria,
CA, USA). Signal visualization was conducted through enhanced
chemiluminescence (GE Healthcare Life Sciences, Little Chalfont,
UK). The bound antibody was detected by using an ECL detection
reagent (Santa Cruz Biotechnology, Inc.). The bands were scanned by
using Quantity One software (Bio-Rad Laboratories, Inc.).
Normalized densities were determined by using the ratio of the band
density of MIF to the band density of -actin.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated with TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and then reverse
transcribed using oligo(dT) primers and SuperScript® II reverse
transcriptase (Invitrogen Life Technologies). The PCR primers used
were as follows: MIF forward, 5′-TCA AGT CAG CAA CGT GGA AG-3′ and
reverse, 5′-TAT CGA GGC TGT GTC GAC TG-3′; GAPDH forward, 5′-AGC
CAT GTA CGT AGC CAT CC-3′ and reverse, 5′-CTC TCA GCT GTG GTG GTG
AA-3′. PCR was performed in a Bio-Rad DNA Engine Tetrad 2 Peltier
thermal cycler (Bio-Rad Laboratories, Inc.) under the following
conditions: One cycle of 95°C for 5 min; 40 cycles of 95°C for 30
sec, 54°C for 30 sec and 72°C for 30 sec; and finally one cycle of
72°C for 10 min. The PCR products (10 µl) were subsequently mixed
with 2 µl loading buffer and electrophoretically separated on 2%
agarose gel for visualization with ethidium bromide. GADPH was used
an endo-reference control.
Measurements of serum E2
and P levels
The concentrations of E2 and P in the
sera of the patients were measured with a double-antibody
radioimmunoassay (Diagnostic Products Corp., Los Angeles, CA,
USA).
Primary cell culture
All biopsies were collected in the operating room
under sterile conditions. The tissues were rinsed with PBS, and the
endometrium was dissected free from the underlying myometrium or
parenchyma. The tissue was sliced into ∼1-mm3 fragments,
which were subsequently incubated with collagenase (2 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) at 37°C in 5% CO2 for
60–90 min. DNase (2 mg/ml; Sigma-Aldrich) was then added. The
endometrial cells were separated from the debris by filtration
using narrow-gauge sieves with a 70-µm mesh filter, plated and
allowed to adhere to plastic dishes for ∼2 h. Any blood cells and
debris were subsequently removed by rinsing with PBS. All cells
were starved for 2 days prior to treatment with 1×10−7 M
E2. After 24 h, the cells were harvested and used for
western blotting and RT-PCR analysis.
Statistical analysis
All data were normally distributed. Statistical
analysis of the ratios of MIF to the end reference was performed
using one-way analysis of variance with SPSS 11.5 software (SPSS,
Inc., Chicago, IL, USA). Results are expressed as the mean ±
standard deviation. Multiple comparisons were performed using the
Bonferroni procedure, while single comparisons were conducted using
the Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Linear regression was used to
the analyze correlation between MIF expression and the serum
E2 and P levels.
Results
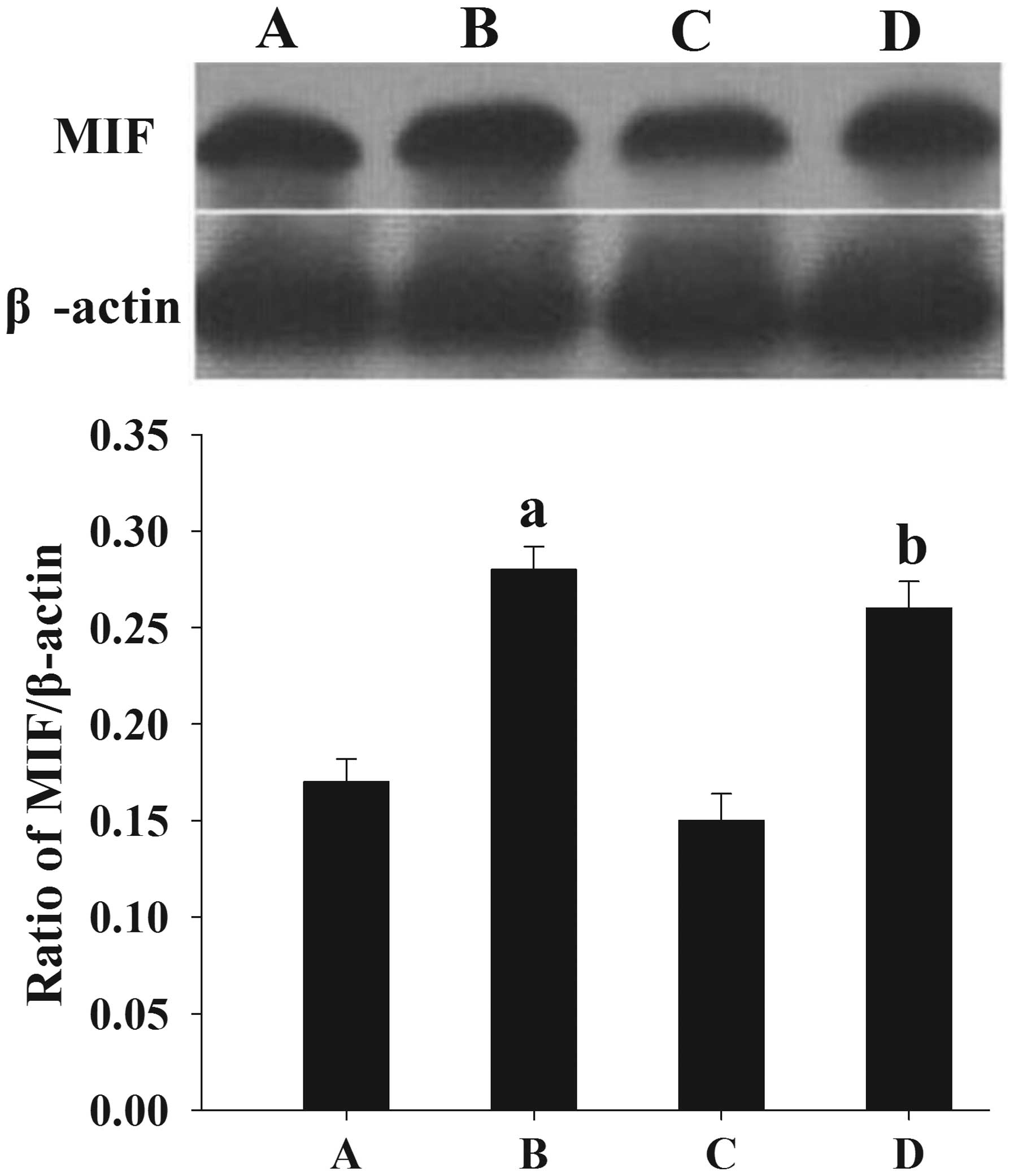
A difference in human endometrial MIF
expression can be found between women with and without
endometriosis
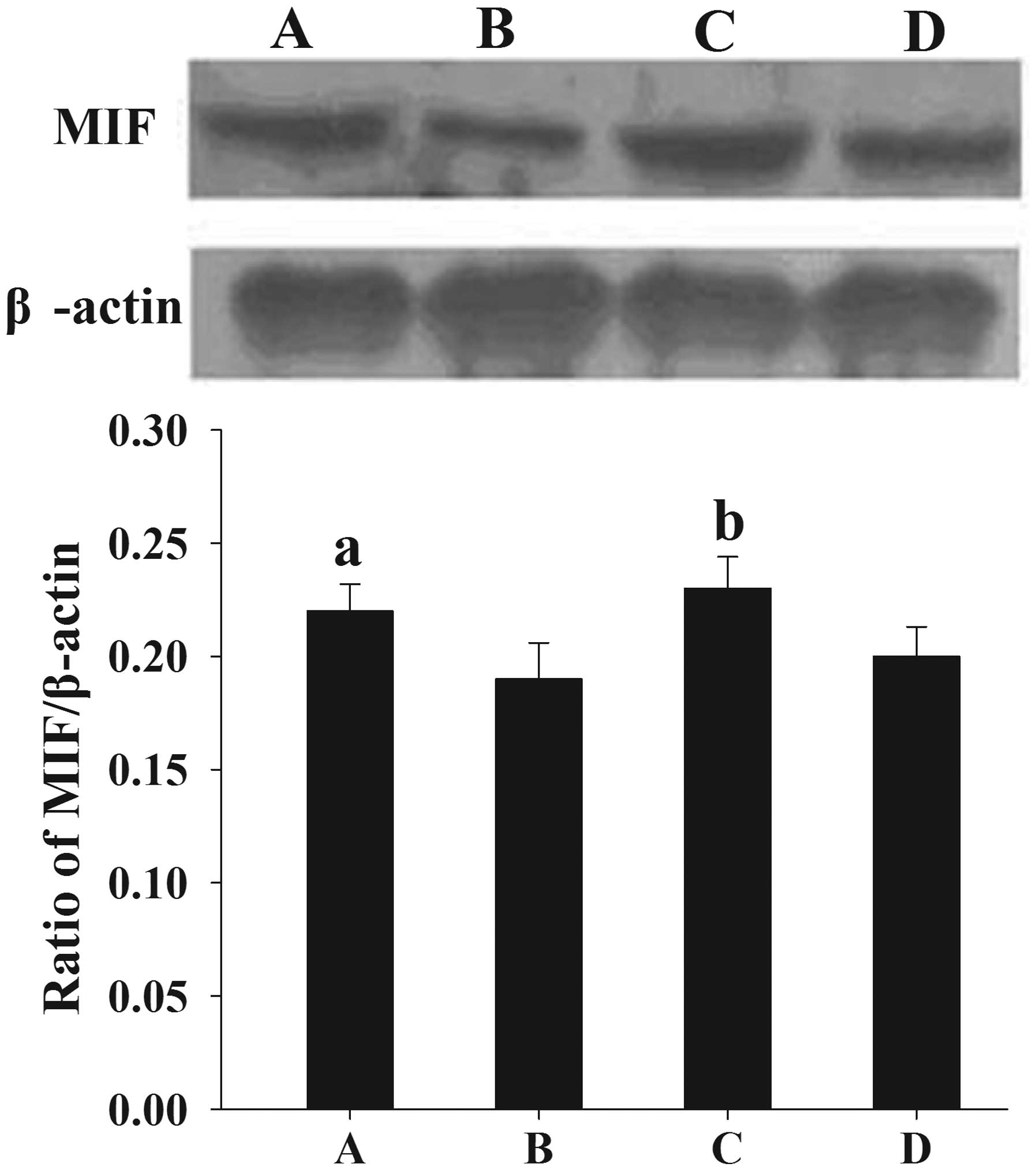
Following the normalization of each band of MIF from
different samples to β-actin, it was found that the mean levels of
MIF protein expression in women with endometriosis at the
proliferative and secretory phases (0.22±0.012 and 0.23±0.014,
respectively) were significantly higher than those in the control
women (0.19±0.016 and 0.20±0.013, respectively) (P<0.05)
(Fig. 1).
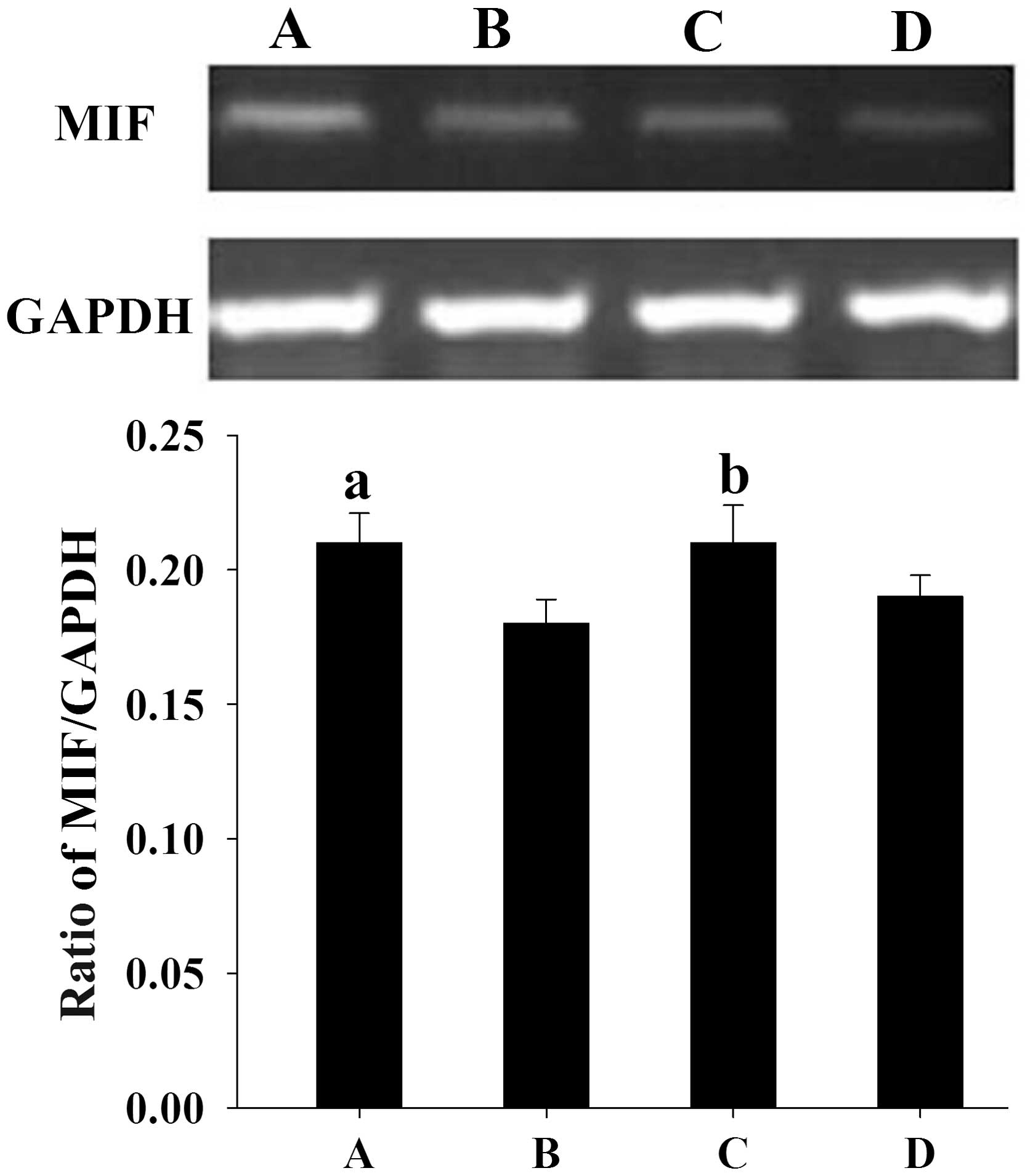
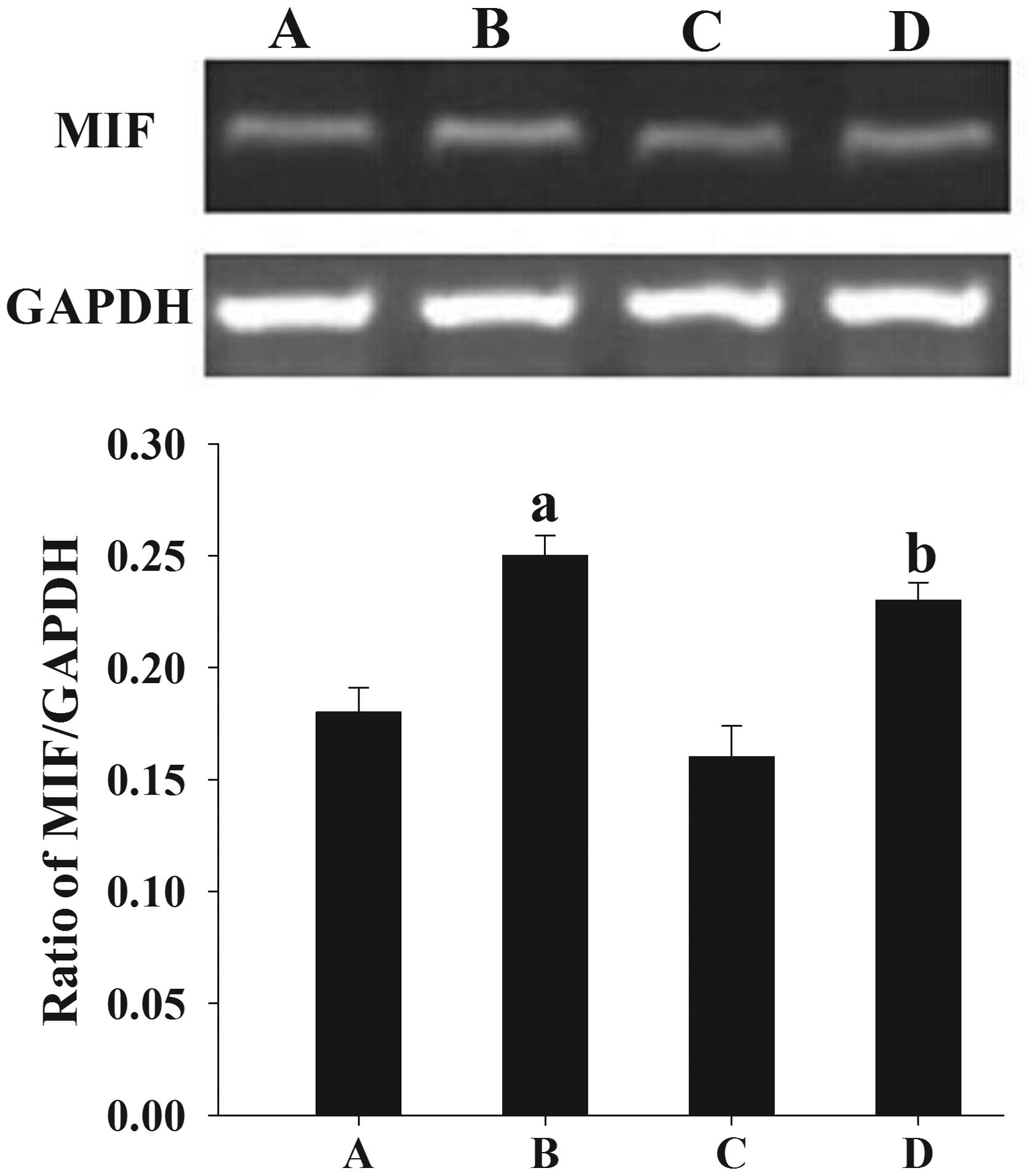
Similar to the results from the western blotting,
semi-quantitative PCR analysis showed that the mean levels of MIF
mRNA expression in women with endometriosis at the proliferative
and secretory phases (0.21±0.011 and 0.21±0.014, respectively) were
significantly higher than those in the control women (0.18±0.009
and 0.19±0.008, respectively) (P<0.05) (Fig. 2).
Correlation between human endometrial
MIF expression and the serum levels of E2
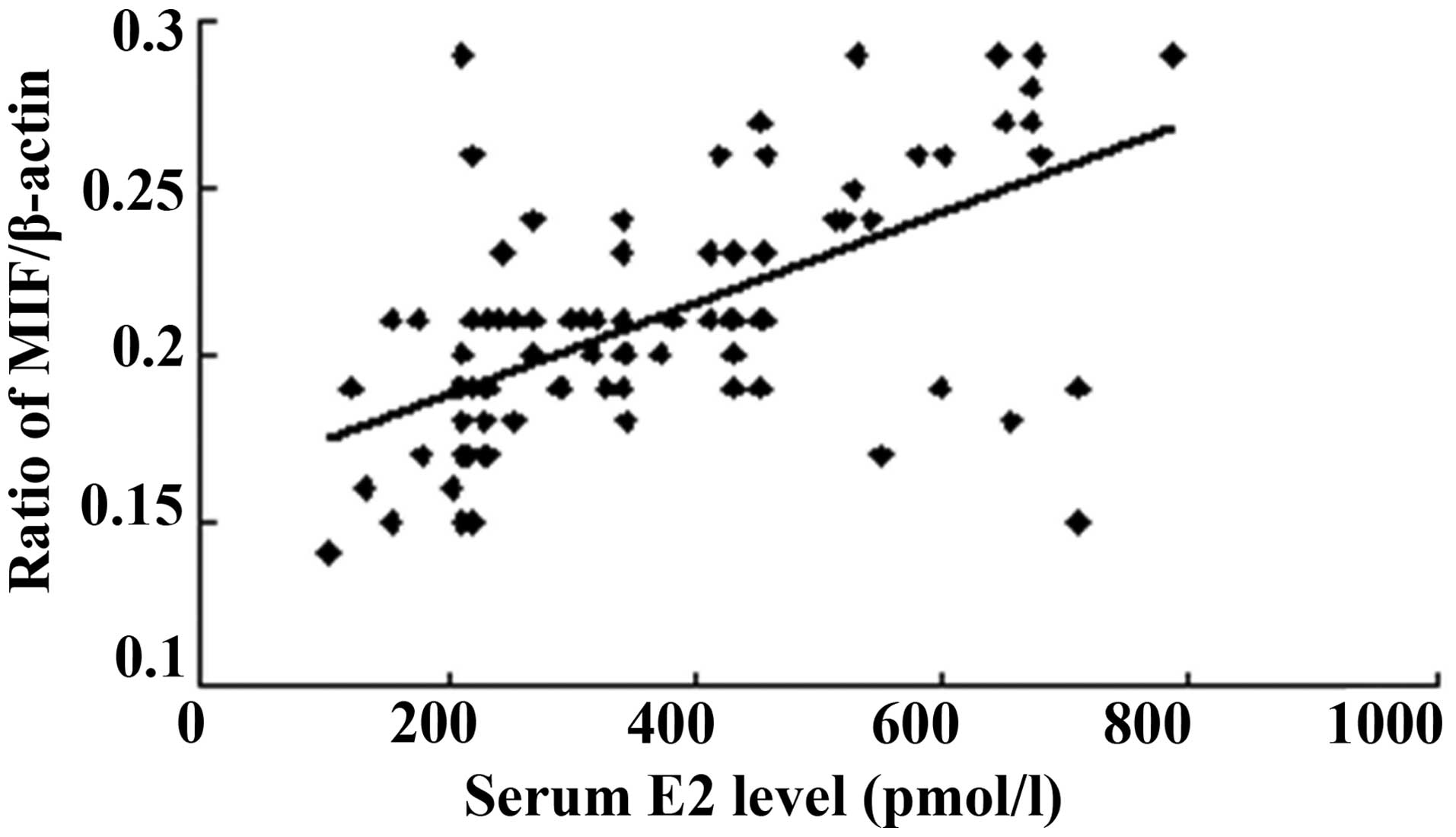
The serum E2 concentration was measured
at the proliferative and secretory phases of the menstrual cycle,
and the serum P concentration was determined at the secretory
phase. All patients in the present study showed a normal ovarian
steroid hormone profile. The serum E2 concentrations
ranged from 102.64 to 785.29 pmol/l, and a positive correlation was
found between the serum E2 level and MIF protein
expression (Fig. 3). As shown in
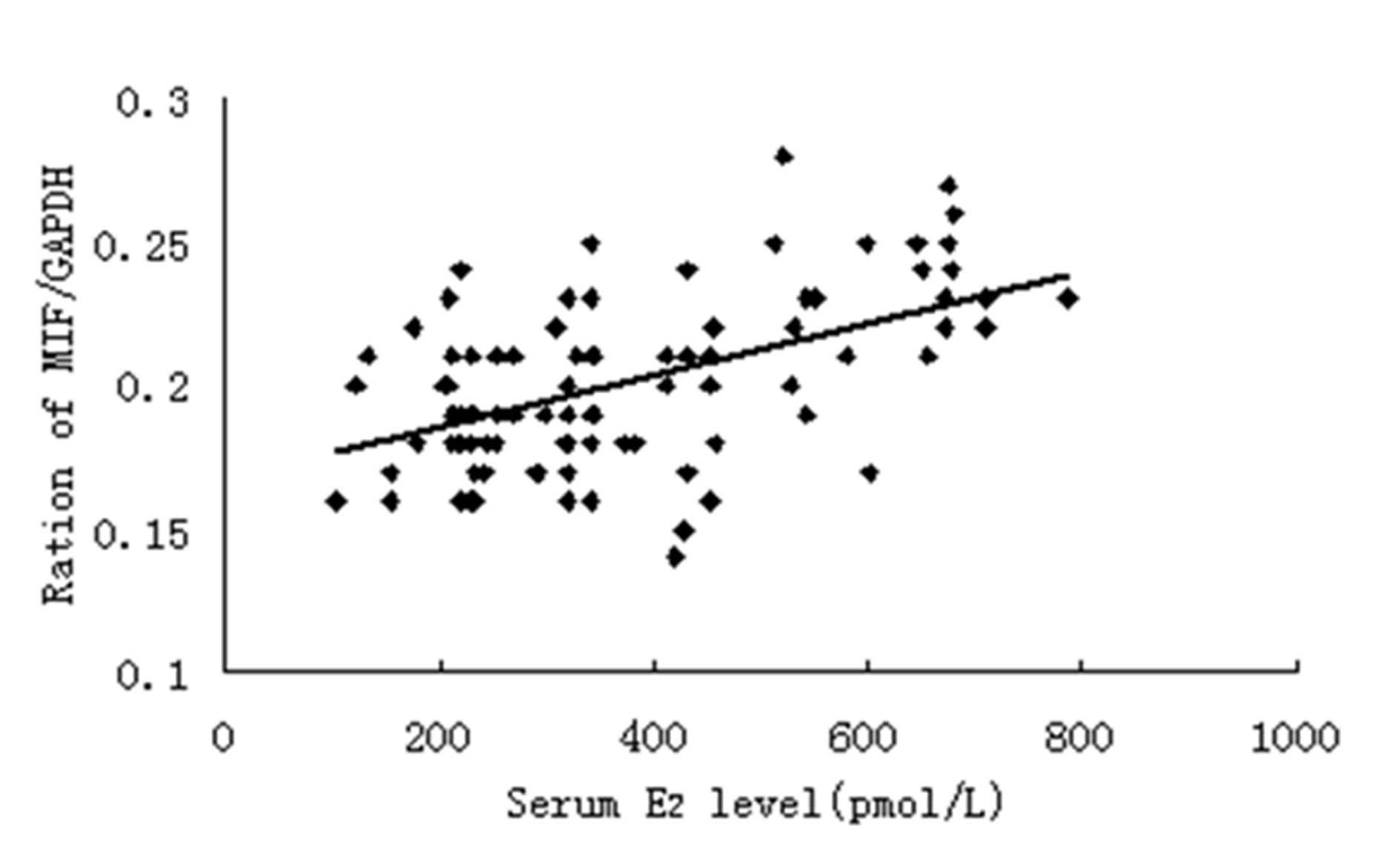
Fig. 4, a positive correlation was
also noted between the serum E2 level and MIF mRNA
expression.
Regulation of MIF expression by
E2 in human endometrial cells
Following treatment with E2, an elevated
expression of MIF protein (Fig. 5)
and mRNA (Fig. 6) was observed in
the cultured endometrial cells compared with the untreated
endometrial cells (P<0.05).
Discussion
In the present study, MIF protein and mRNA levels
were found to be increased in the endometrial tissues from women
with endometriosis. In a study by Arcuri et al (11), no significant differences in MIF
levels were found across the menstrual cycle. These results are
consistent with those of previous studies (11–13).
MIF, which is a 12.5-kDa cytokine that inhibits the
migration and chemotaxis of macrophages (14,15), was
first identified as a T-cell-derived lymphokine (16,17). MIF
exerts a wide range of immunostimulatory and proinflammatory
effects (18) and is produced not
only by activated T cells and macrophages, but also by the
endothelial and epithelial cells of several organs (19–21). MIF
is important in tumor growth and angiogenesis (22–24), and
has been suggested to have a pivotal function in cell proliferation
and differentiation, angiogenesis and wound healing (18,25).
Endometriosis, although not neoplastic, shares features with
malignant cells and the metastasis in endometriosis may include
certain mechanisms proposed in cancer (26). The higher expression of MIF in
endometriosis could be associated with the fact that the
endometrial cells from women with endometriosis have a
predisposition to adhere and grow outside of the uterus (4).
In the present study it was found that the
expression of MIF in endometrial tissues was correlated with the
serum level of E2. This result suggests that estrogen
contributes to the regulation of MIF in endometrial tissue;
however, there are contradictory theories regarding the association
between estrogen and MIF. It has previously been demonstrated that
MIF expression is upregulated in the wound healing process of
estrogen-deficient mice, and that estrogen treatment can directly
inhibit MIF production by murine macrophages (27). Houdeau et al (28) found that estrogen could decrease MIF
production in the female rat colon, which may have affected the
susceptibility of the colon to inflammation. In addition, Hsieh
et al (29) revealed that the
protective effects of estrogen on lung injury following traumatic
hemorrhage were mediated via the downregulation of lung MIF
production. Regarding the endometrium, however, the expression of
MIF has been shown to be a dynamic process. At the start of the
proliferative phase of the menstrual cycle, the MIF protein levels
are low. MIF expression then increases during the mid-late
proliferative phase and reaches a maximum level around ovulation,
prior to undergoing a progressive reduction to moderate levels in
the mid-secretory phase and a final further reduction in the late
secretory phase (30). These changes
are synchronous with the fluctuation of estrogen and are consistent
with the observations in the present study. Any inconsistencies in
data concerning estrogen and MIF levels are likely due to
inter-tissue/-cellular differences.
A previous study demonstrated that estrogen is able
to act through nonclassical membrane receptors, leading to rapid
intracellular responses (31). The
exact pathway underlying the regulation of MIF by estrogen requires
further study.
In the present study it was found that MIF
expression in the endometrial cells from women with endometriosis
was more sensitive to E2 than the MIF expression in
endometrial cells from women without endometriosis. During the
menstrual cycle, the human endometrium undergoes profound and
dynamic changes that are accordant with the changes in the levels
of steroid hormones, such as E2, which is a critical
hormone that favors the development and maintenance of
endometriosis (9). The oversensitive
upregulation of MIF expression may contribute to the pathogenesis
and progression of endometriosis.
In conclusion, the present study has shown for the
first time, to the best of our knowledge, that MIF expression in
the endometrial tissues of women with endometriosis is
significantly increased, that MIF expression is associated with the
level of E2, and that MIF expression in endometrial
cells from women with endometriosis is more sensitive to
E2 than that in cells from women without endometriosis.
These findings suggest that MIF contributes to the pathogenesis of
endometriosis and is regulated by steroid hormones. The mechanisms
by which increased endometrial MIF levels in endometriosis affect
the reproductive function of the patients require further
elucidation. In addition, further investigations into the use of
MIF as a clinical diagnostic marker and as a therapeutic target are
warranted.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81100407).
Abbreviations:
|
E2
|
17β-estradiol
|
|
MIF
|
macrophage migration inhibitory
factor
|
References
|
1
|
Sampson JA: Peritoneal endometriosis due
to the menstrual dissemination of endometrial tissue into the
peritoneal cavity. Am J Obstet Gynecol. 14:422–469. 1927.
|
|
2
|
Strathy JH, Molgaard CA, Coulam CB and
Melton JL III: Endometriosis and infertility: A laparoscopic study
of endometriosis among fertile and infertile women. Fertil Steril.
38:667–672. 1982.PubMed/NCBI
|
|
3
|
Kao L, Germeyer A, Tulac S, et al:
Expression profiling of endometrium from women with endometriosis
reveals candidate genes for disease-based implantation failure and
infertility. Endocrinology. 144:2870–2881. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ulukus M, Cakmak H and Arici A: The role
of endometrium in endometriosis. J Soc Gynecol Investig.
13:467–476. 2006.PubMed/NCBI
|
|
5
|
Larson DF and Horak K: Macrophage
migration inhibitory factor: Controller of systemic inflammation.
Crit Care. 10:1382006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bernhagen J, Calandra T, Mitchell RA, et
al: MIF is a pituitary-derived cytokine that potentiates lethal
endotoxaemia. Nature. 365:756–759. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berkkanoglu M and Arici A: Immunology and
endometriosis. Am J Reprod Immunol. 50:48–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akoum A, Metz CN, Al-Akoum M and Kats R:
Macrophage migration inhibitory factor expression in the
intrauterine endometrium of women with endometriosis varies with
disease stage, infertility status, and pelvic pain. Fertil Steril.
85:1379–1385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deroo BJ and Korach KS: Estrogen receptors
and human disease. J Clin Invest. 116:561–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brosens IA, Cornillie F, Koninckx P and
Vásquez G: Evolution of the Revised American Fertility Society
Classification of Endometriosis. Fertil Steril. 44:714–716.
1985.PubMed/NCBI
|
|
11
|
Arcuri F, Ricci C, Ietta F, et al:
Macrophage migration inhibitory factor in the human endometrium:
Expression and localization during the menstrual cycle and early
pregnancy. Biol Reprod. 64:1200–1205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin W, Chen S, Li M, Wang B, Qu X and
Zhang Y: Expression of macrophage migration inhibitory factor in
human endometriosis: relation to disease stage, menstrual cycle and
infertility. J Obstet Gynaecol Res. 36:344–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akoum A, Metz CN, Al-Akoum M and Kats R:
Macrophage migration inhibitory factor expression in the
intrauterine endometrium of women with endometriosis varies with
disease stage, infertility status, and pelvic pain. Fertil Steril.
85:1379–1385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calandra T and Roger T: Macrophage
migration inhibitory factor: A regulator of innate immunity. Nat
Rev Immunol. 3:791–800. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hermanowski-Vosatka A, Mundt SS, Ayala JM,
et al: Enzymatically inactive macrophage migration inhibitory
factor inhibits monocyte chemotaxis and random migration.
Biochemistry. 38:12841–12849. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
David JR: Delayed hypersensitivity in
vitro: Its mediation by cell-free substances formed by lymphoid
cell-antigen interaction. Proc Natl Acad Sci USA. 56:72–77. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bloom BR and Bennett B: Mechanism of a
reaction in vitro associated with delayed-type hypersensitivity.
Science. 153:80–82. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishihira J: Macrophage migration
inhibitory factor (MIF): Its essential role in the immune system
and cell growth. J Interferon Cytokine Res. 20:751–762. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Donnelly SC, Haslett C, Reid PT, et al:
Regulatory role for macrophage migration inhibitory factor in acute
respiratory distress syndrome. Nature Med. 3:320–323. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imamura K, Nishihira J, Suzuki M, et al:
Identification and immunohistochemical localization of macrophage
migration inhibitory factor in human kidney. IUBMB Life.
40:1233–1242. 1996. View Article : Google Scholar
|
|
21
|
Suzuki M, Sugimoto H, Nakagawa A, Tanaka
I, Nishihira J and Sakai M: Crystal structure of the macrophage
migration inhibitory factor from rat liver. Nat Struct Biol.
3:259–266. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chesney J, Metz C, Bacher M, Peng T,
Meinhardt A and Bucala R: An essential role for macrophage
migration inhibitory factor (MIF) in angiogenesis and the growth of
a murine lymphoma. Mol Med. 5:181–191. 1999.PubMed/NCBI
|
|
23
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour-associated macrophages in tumour progression:
Implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogawa H, Nishihira J, Sato Y, et al: An
antibody for macrophage migration inhibitory factor suppresses
tumour growth and inhibits tumour-associated angiogenesis.
Cytokine. 12:309–314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishihira J, Ishibashi T, Fukushima T, Sun
B, Sato Y and Todo S: Macrophage migration inhibitory factor (MIF):
Its potential role in tumor growth and tumor-associated
angiogenesis. Ann NY Acad Sci. 995:171–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeitvogel A, Baumann R and
Starzinski-Powitz A: Identification of an invasive,
N-cadherin-expressing epithelial cell type in endometriosis using a
new cell culture model. Am J Pathol. 159:1839–1852. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ashcroft GS, Mills SJ, Lei K, et al:
Estrogen modulates cutaneous wound healing by downregulating
macrophage migration inhibitory factor. J Clin Invest.
111:1309–1318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Houdeau E, Moriez R, Leveque M, et al: Sex
steroid regulation of macrophage migration inhibitory factor in
normal and inflamed colon in the female rat. Gastroenterology.
132:982–993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh YC, Frink M, Hsieh CH, et al:
Downregulation of migration inhibitory factor is critical for
estrogen-mediated attenuation of lung tissue damage following
trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol.
292:L1227–L1232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kats R, Al-Akoum M, Guay S, Metz C and
Akoum A: Cycle-dependent expression of macrophage migration
inhibitory factor in the human endometrium. Hum Reprod.
20:3518–3525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ropero AB, Soria B and Nadal A: A
nonclassical estrogen membrane receptor triggers rapid differential
actions in the endocrine pancreas. Mol Endocrinol. 16:497–505.
2002. View Article : Google Scholar : PubMed/NCBI
|