Introduction
Atrial fibrillation (AF) is the most prevalent form
of arrhythmia observed in clinical practice, with a high population
prevalence in industrial and developing countries (1–3).
Furthermore, the prevalence of AF is increasing markedly in elderly
populations (4). AF is associated
with a three-fold risk of heart failure and a five-fold risk of
stroke (5,6). In addition to the significant rate of
morbidity, AF is associated with a 1.5–1.9-fold risk of mortality
(7). Therefore, AF has become a
substantial health burden for patients and societies worldwide.
According to the present guidelines for the
management of AF, antiarrhythmic drugs (AADs) are the primary
strategy for treating AF (5,6). However, the application of AADs has
encountered challenges due to their limited efficacy and potential
adverse effects. Thus, catheter ablation therapy has become a
generally adopted alternative technique for the treatment of AF,
particularly in cases of paroxysmal or/and persistent AF. However,
the management recommendations and guidelines have not yet reached
a consensus with regard to the use of catheter ablation for the
treatment of AF, primarily due to the differences in ablation
strategy and technique employed in different centers, in addition
to relevant complications (5,6,8). In previous years, a number of small to
moderately sized randomized controlled trials (RCTs) have been
published that directly compare the efficacy of catheter ablation
and AADs for the treatment of AF (9–19).
However, the number of patients enrolled in each study was limited.
Therefore, a meta-analysis was conducted in the present study to
comprehensively evaluate whether catheter ablation is superior to
AADs for the treatment of AF. In addition, the quality of the
results published by the previous studies was evaluated, as
recommended by the Cochrane Collaboration.
Materials and methods
Search strategy and inclusion
criteria
MEDLINE, Embase and the Cochrane Central Register of
Controlled Trials databases were searched for RCTs that compared
catheter ablation with AADs for the treatment of AF, without
language restrictions (last search update, May 1, 2014). In
addition, reference lists from initially identified articles were
retrieved in order to avoid the exclusion of any relevant studies.
The following medical subject heading terms were used: ‘Atrial
fibrillation’, ‘catheter ablation’ and ‘randomized controlled
trials’.
Studies were included if they satisfied the
following criteria: i) Study design was a RCT; ii) study population
consisted of human participants with paroxysmal, persistent or
long-standing persistent AF; iii) interventions included pulmonary
vein isolation, no matter which technique was used; and iv)
follow-up was ≥12 months.
Quality assessment
The methodological quality of each eligible study
(risk of bias) was evaluated as recommended in the Cochrane
Handbook for Systematic Reviews of Interventions (version 5.1.0)
(20). The risk of bias for each
trial was assessed on the basis of the prime endpoint of recurrence
of AF. The following criteria were evaluated and assigned a value
of ‘high’, ‘low’ or ‘unclear’ by two authors. With regard to
selection bias, the authors aimed to determine whether the method
of randomization was adequate and whether the treatment allocation
was concealed. In addition, performance and detection biases were
assessed by determining whether the participants and personnel were
blinded to the intervention, and whether the outcome assessor was
blinded to the intervention. With regard to attrition bias, the
authors determined whether any incomplete outcome data were
sufficiently assessed and handled, while reporting bias was
assessed by determining whether selective outcome reporting had
been identified. Finally, the existence of any additional sources
of bias was analyzed.
Data extraction
Two authors independently extracted relevant data
from the included trials, and disagreements were resolved by
discussion and consensus. The following data were extracted from
each RCT: Name of the first author, year of publication, number of
patients (intervention vs. control), age of populations,
composition of gender, time of follow-up, definition of primary
outcome and other important clinical information. In the case of a
trial being reported in multiple publications, the most complete
study or the article with the longest follow-up time was selected.
The primary endpoint was the recurrence of AF. Secondary endpoints
included all-cause mortality, stroke/transient ischemic attack
(TIA) and quality of life (QoL).
Statistical analysis
Data analysis was performed based on an
intention-to-treat analysis. Relative risks (RRs) with 95%
confidence intervals (CIs) were selected to compare the differences
for dichotomous outcomes, while weighted mean differences (WMDs)
with 95% CIs were used to compare continuous outcomes.
Heterogeneity among studies was analyzed using a
χ2-based Q test and I2 statistic. If
significant heterogeneity was identified (P<0.05 and
I2 >50%), a random-effects model was selected, whilst
a fixed-effects model was selected in all other cases.
Begg's funnel plot and Egger's test were used to
evaluate the significance of the publication bias. The
meta-analysis was performed using Stata software, version 11.0
(StataCorp, College Station, TX, USA) and Revman 5.1 software (The
Cochrane Collaboration). A two-tailed P-value of <0.05 was
considered to indicate a statistically significant difference.
Finally, the quality of the evidence was evaluated using the Grades
of Recommendation, Assessment, Development and Evaluation (GRADE)
system (21).
Results
Literature search and selection
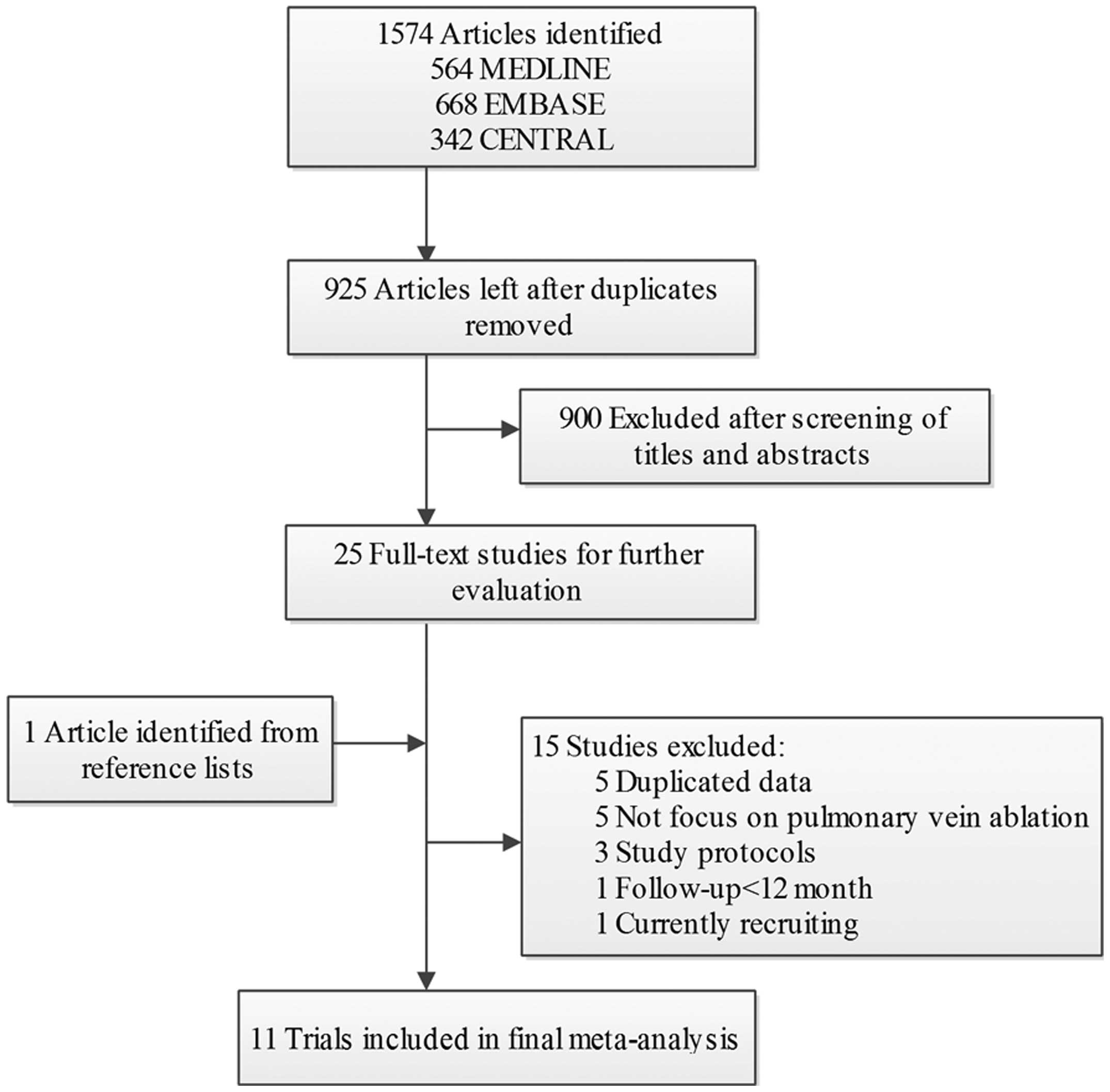
A flow diagram of the literature search is presented
in Fig. 1. Briefly, 1,917
potentially relevant articles were identified following electronic
and additional manual searches. Among the 1,917 publications
yielded, 900 articles were excluded following screening of the
titles and abstracts. Subsequently, 26 full-text articles were
assessed for eligibility. Among these, 15 articles were further
excluded for a variety of reasons, as described in Fig. 1. Finally, 11 RCTs involving 1,763
patients with AF were included in the meta-analysis (9–19).
Study characteristics and risk of bias
assessment
Primary characteristics of the 11 RCTs included in
the meta-analysis are shown in Table
I. The 11 trials were published between 2003 and 2013. Among
them, four trials enrolled only patients with paroxysmal AF
(13,15,16,18), two
trials enrolled patients with only persistent AF (11,19), and
the remaining five trials enrolled patients with paroxysmal and
persistent AF (9,10,12,14,17).
Three trials enrolled patients to receive pulmonary vein ablation
as the first-line therapy (10,17,18),
whereas the remaining studies included patients that had failed at
least one AAD treatment protocol or were intolerant of AADs. The
majority of the trials compared catheter ablation with AADs, with
the exception of one trial that compared catheter ablation plus
AADs with single AAD administration (12). All the AF patients assigned to
catheter ablation underwent a single ablation procedure in four of
the studies (9,10,12,14). In
the other trials, patients that received catheter ablation therapy
underwent two or more ablation procedures in the blanking period
when required.
 | Table I.Characteristics of the studies
included in meta-analysis. |
Table I.
Characteristics of the studies
included in meta-analysis.
| Study | Ablation/AAD
patients, n | Gender (M/F), n | Age, years | Type of AF | Ablation
strategy | Follow-up, months
(success rate, %) | Primary outcome |
|---|
| Krittayaphong et
al (2003) | 15/15 | T:11/4 | T: 55.3±10.5 | Paroxysmal (70%) and
persistent AF | CPVA | 12 (100%) | AF |
|
|
| C: 8/7 | C: 48.6±15.4 |
|
|
|
| Wazni et al
(2005) | 33/37 | NA | T: 53±8 | Paroxysmal (96%) and
persistent AF | PVI | 12 | AF |
|
|
|
| C: 54±8 |
| (95.6) |
|
| Oral et al
(2006) | 77/69 | T: 67/10 | T: 55±9 | Chronic AF | CPVA | 12 | AT |
|
|
| C: 62/7 | C: 58±8 |
|
| (100) |
|
| Stabile et al
(2006) | 68/69 | T: 37/31 | T: 62.2±9 | Paroxysmal (67%)
and persistent AF | CPVA | 12 | AT |
|
|
| C: 44/25 | C: 62.3±10.7 |
|
| (97.1) |
|
| Jais et al
(2008) | 53/59 | T: 45/8 | T: 49.7±10.7 | Paroxysmal AF | PVI | 12 | AF |
|
|
| C: 49/10 | C: 52.4±11.4 |
|
| (96.4) |
|
| Forleo et al
(2009) | 35/35 | T: 20/15 | T: 63.2±8.6 | Paroxysmal (41%)
and persistent AF | PVI | 12 | AF |
|
|
| C: 23/12 | C: 64.8±6.5 |
|
| (100) |
|
| Wilber et al
(2010) | 106/61 | T: 73/33 | T: 55.5
(53.7–57.3) | Paroxysmal AF | PVI | 12 | Protocol-defined
treatment failure AT |
|
|
| C: 38/23 | C: 56.1
(52.9–59.4) |
|
| (95.2) |
|
| Pappone et
al (2011) | 99/99 | T: 69/30 | T: 55±10 | Paroxysmal AF | CPVA | 48 | AT |
|
|
| C: 64/35 | C: 57±10 |
|
| (100) |
|
| Nielsen et
al (2012) | 146/148 | T: 100/46 | T: 56±9 | Paroxysmal AF | CPVA | 24 | Burden of AF
AT |
|
|
| C: 106/42 | C: 54±10 |
|
| (95.9) |
|
| Morillo et
al (2012) | 66/61 | T: 51/15 | 55.3±10.5 | Paroxysmal (87.5%)
and persistent AF | PVI | 24 |
|
|
|
| C: 45/16 |
|
|
| (82.7) |
|
| Blandino et
al (2013) | 153/258 | T: 109/44 | T: 75±5 | Persistent AF | PVI | 60 | AF/AT |
|
|
| C: 186/72 | C: 76±5 |
|
| (100) |
|
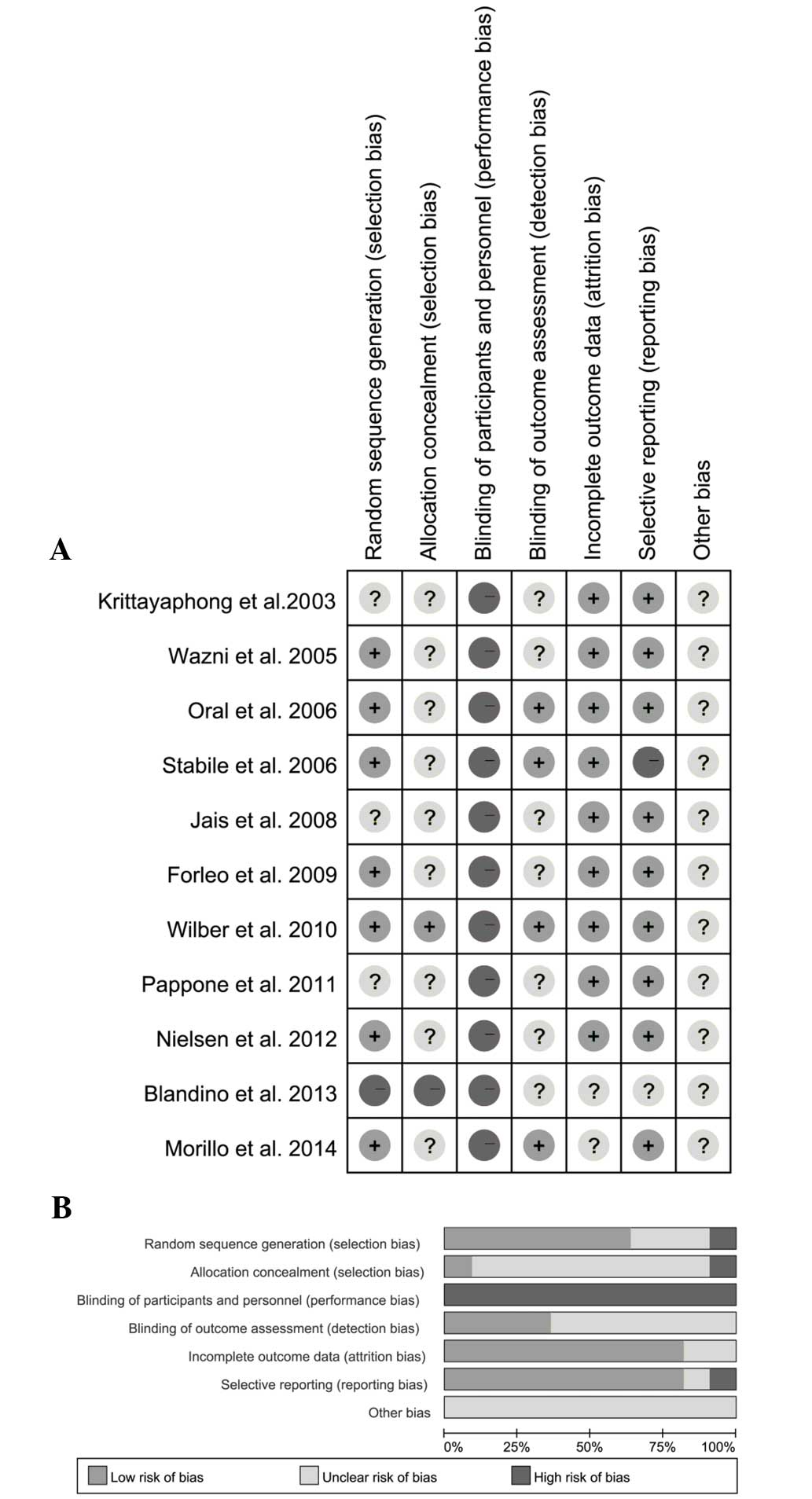
A risk of bias assessment was performed for each
trial, and the results are presented in Fig. 2A. Of the 11 trials, six trials
adopted appropriate methods to generate the random sequence
(10–12,14,15,18). One
study reported allocation concealment using sealed envelopes
(15); however, the methods of
concealment in other trials were not mentioned. Blinding of the
outcome assessors was reported in three trials (11,12,18). The
overall risk of bias is presented in Fig. 2B.
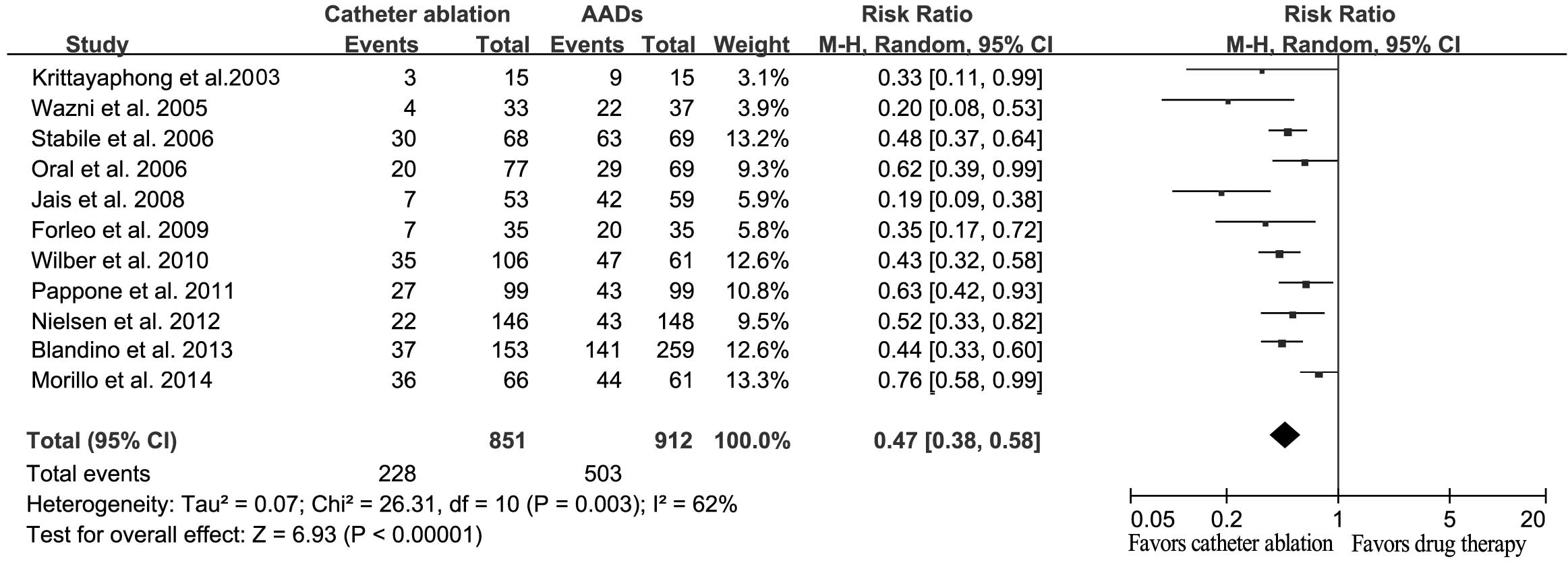
Analysis of the primary outcome,
recurrence of AF
The majority of the included trials considered the
recurrence of AF and/or atrial tachyarrhythmia as their primary end
point. Therefore, the overall effect of catheter ablation against
AADs for the recurrence of AF was assessed. The results indicated
that catheter ablation was able to significantly reduce the
recurrence of AF, as compared with AADs (RR, 0.47; 95% CI,
0.38–0.58; P<0.001; Fig. 3).
Significant heterogeneity was detected among the trials (Q=26.31;
I2=62%; P=0.003).
Three trials enrolled drug-naive patients and the
result was similar to the overall effect (RR, 0.50; 95% CI,
0.27–0.92; P=0.03). Sensitivity analysis indicated that no single
study significantly altered the combined effect, which ranged
between 0.45 (95% CI, 0.37–0.55) and 0.41 (95% CI, 0.43–0.62).
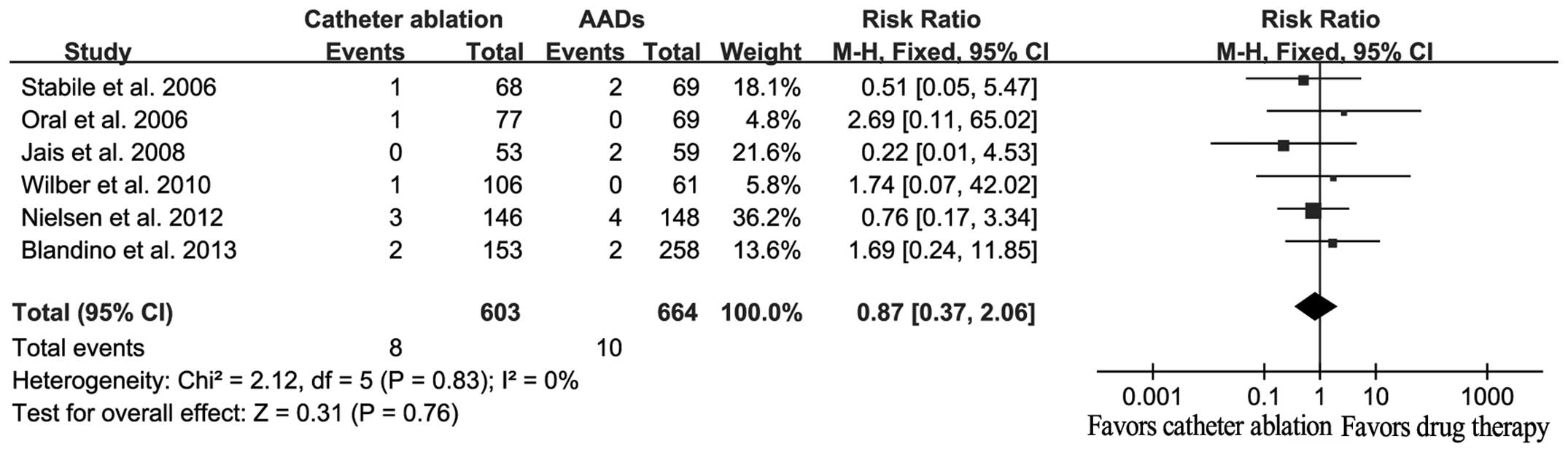
Analysis of the secondary outcomes,
all-cause mortality, stroke and/or TIA, and QoL
A total of 18 mortalities were reported in six
studies, among which eight cases had received catheter ablation and
10 cases had received AADs. No statistically significant difference
in the mortality rate was detected between the catheter ablation
and AAD treatment groups (RR, 0.87; 95% CI, 0.37–2.06; P=0.76;
Fig. 4), with no evidence for
significant heterogeneity (Q=2.12, I2=0%, P=0.83).
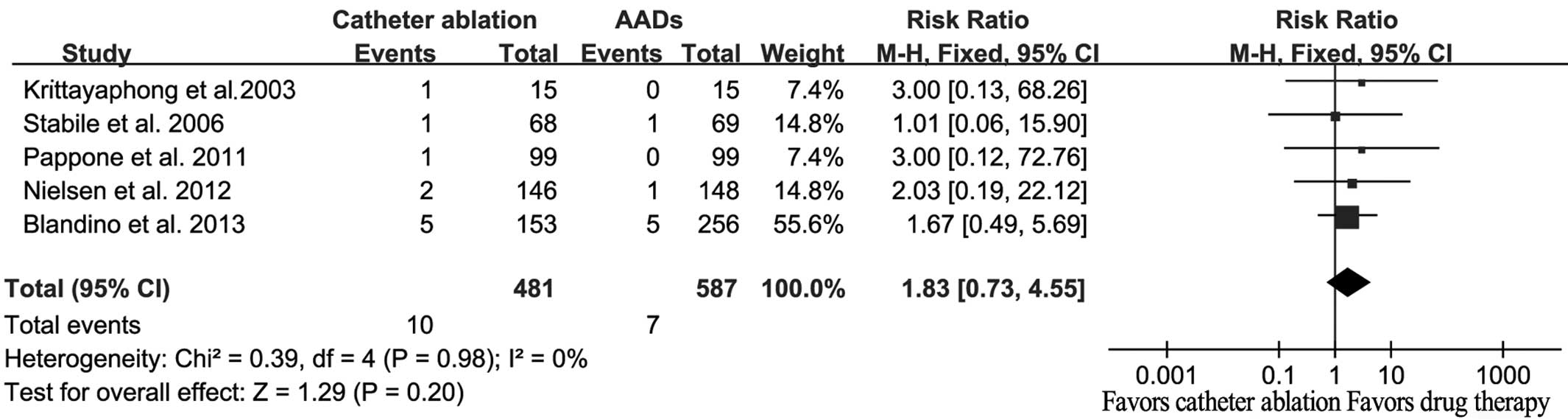
A total of 17 strokes/TIA were reported, among which
10 events occurred in catheter ablation patients and seven events
occurred in AAD patients. However, no statistically significant
difference was detected between the catheter ablation and AAD
therapies (RR, 1.83; 95% CI, 0.73–4.55; P=0.20; Fig. 5), and there was no evident
heterogeneity (Q=0.39; I2=0%; P=0.98).
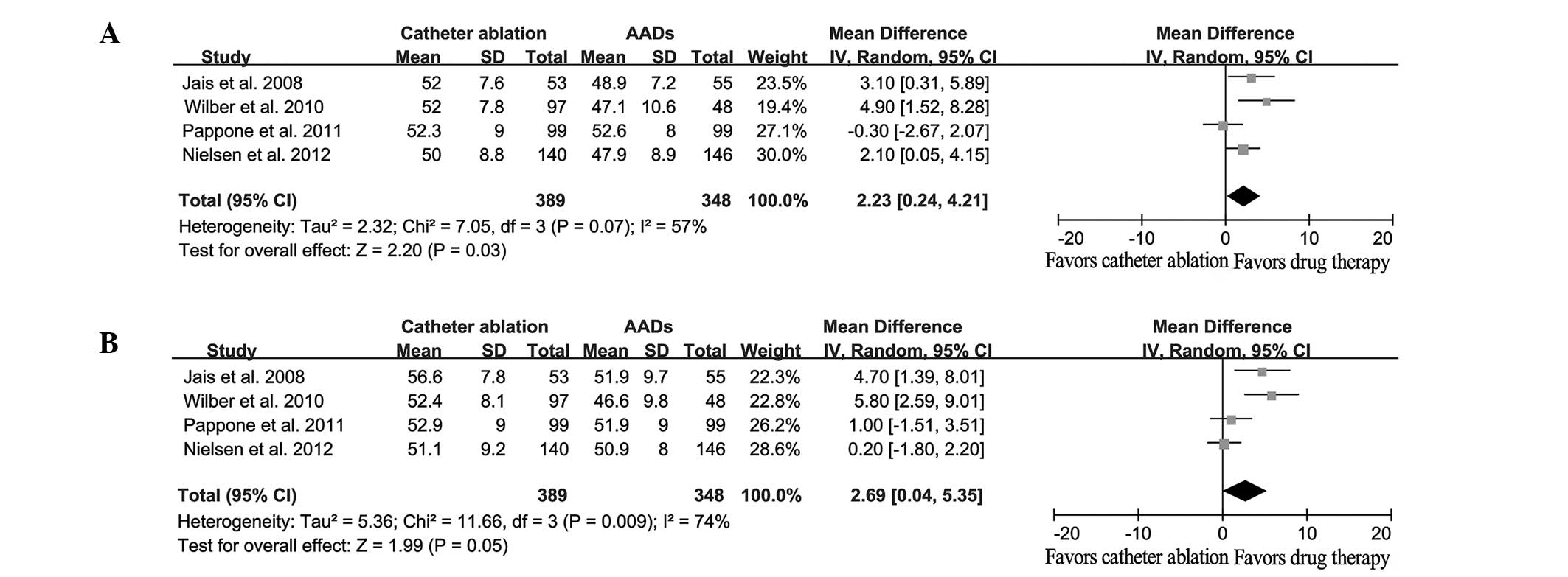
Four studies included results with regard to the
differences in the QoL outcome, including the physical component
summary (PCS) and the mental component summary (MCS). When compared
with the baseline observations, the catheter ablation and AAD
treatment groups exhibited a significantly improved QoL at the end
of the study. However, catheter ablation was shown to result in
improved QoL outcomes compared with AADs (PCS: WMD, 2.23; 95% CI,
0.24–4.21; P=0.03; MCS: WMD, 2.69; 95% CI, 0.04–5.35; P=0.05;
Fig. 6).
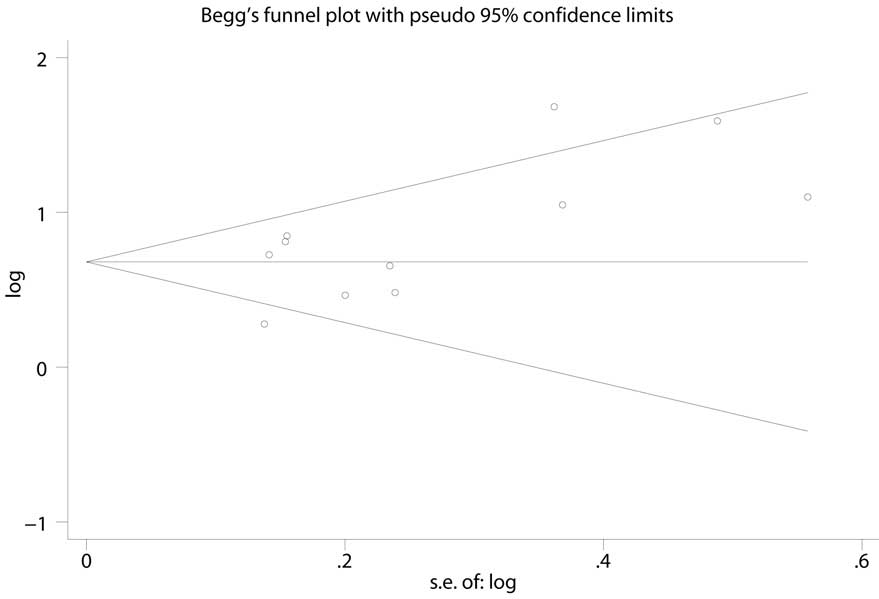
Quality assessment and publications
bias
The quality of the evidence was evaluated using the
GRADE system. As shown in Table II,
the evidence quality of the outcomes ranged between low and high.
Potential publication bias was assessed using Begg's funnel plot
and Egger's test, and the results indicated that there was no
potential publication bias (Fig. 7;
Egger's test, P=0.066).
 | Table II.Summary of findings with regard to
the quality of the evidence of outcomes. |
Table II.
Summary of findings with regard to
the quality of the evidence of outcomes.
|
| Illustrative
comparative risks (95% CI) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Outcomes | Assumed risk of
AADs | Corresponding risk
catheter ablation | RR (95% CI) | Participants
(studies), n | Quality of the
evidence (GRADE) |
|---|
| AF recurrence | 552 per 1,000 | 259 per 1,000
(210–320) | 0.47
(0.38–0.58) | 1,763 (11) |
++++High |
| All-causes
mortality | 15 per 1,000 | 13 per 1,000
(6–31) | 0.87
(0.37–2.06) | 1,267 (6) |
+++−Moderatea |
| Stroke/TIA | 12 per 1,000 | 22 per 1,000
(9–54) | 1.83
(0.73–4.55) | 1,068 (5) |
+++−Moderatea |
| QoL PCS | – | – | 2.23
(0.24–4.21) | 737 (4) |
++−−Lowb,c |
| QoL MCS | – | – | 2.69
(0.04–5.35) | 737 (4) |
++−−Lowb,c |
Discussion
The primary finding of the present meta-analysis was
that the recurrence of AF was notably reduced in patients that
received catheter ablation therapy, as compared with those that
received AADs. Furthermore, catheter ablation treatment was shown
to result in an improved QoL when compared with AADs. However, no
statistically significant difference was identified between two
groups with regard to the incidence of all-cause mortality and
stroke/TIA.
In the present study, a significant reduction in
recurrent AF was observed in the patients who underwent catheter
ablation therapy, as compared with the patients that received AADs.
Restoration of a sinus rhythm is considered to improve the
long-term survival rates and reduce the incidence of stroke/TIA in
general AF patients (22,23). In addition, a previous observational
study indicated that catheter ablation is superior to AADs in
reducing the all-cause mortality rate (24). However, in the present meta-analysis,
no statistically significant difference was detected between the
catheter ablation and AAD therapy with regard to the rates of
mortality and stroke/TIA, for which there are a number of possible
explanations. Firstly, the current study design was different to
that of the aforementioned observational study, with only RCTs
included in the current meta-analysis. RCTs are considered to
provide the most robust evidence for clinical practice; however,
the number of participants enrolled may be insufficient to obtain
statistical differences. Secondly, the duration of the follow-up
period in the majority of the trials was only 12 months, which may
be too short to detect a representative quantity of adverse events,
particularly considering that patients in RCTs are typically
younger and exhibit a low prevalence of structural heart disease.
These factors limited the value of the studies for the evaluation
of the long-term efficacy of catheter ablation. However, the
ongoing multicenter, randomized Catheter Ablation versus
Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial
(no. NCT00911508), which plans to enroll >3,000 patients, may
resolve this problem.
QoL is a key factor to consider when selecting a
method of AF management. Theoretically, early catheter ablation is
able to avoid the requirement for long-term drug employment and the
subsequent side effects. The present study indicated that patients
who underwent catheter ablation therapy had an improved QoL
compared with patients that received AADs. However, the assessment
of QoL is subjective, and as catheter ablation is distinct from
drug therapy, blinding of the assessment is impossible.
Furthermore, according to the GRADE system, the quality of evidence
of QoL as a parameter is assessed as ‘low’. Therefore, further
studies may be required to elucidate this issue.
Cost-effectiveness is an additional key factor to
take into consideration when comparing treatments for AF. However,
was the ablation technique and duration differed between study
centers, it is difficult to precisely evaluate the
cost-effectiveness of catheter ablation compared with AADs for AF
therapy. The limited information available did not permit a
consensus on the cost-effectiveness of catheter ablation for AF
(25–28). Thus, the cost comparison of catheter
ablation and AADs requires further investigation in future
RCTs.
Prior to the present study, a number of previous
meta-analyses were published that compared catheter ablation with
AADs for the treatment of AF (29–31).
However, the present study possesses a number of advantages
compared with the previous studies. Firstly, the present
meta-analysis included more recently published trials, and the
number of AF patients included in this meta-analysis was twice the
number reported in previous studies. Furthermore, the present study
analyzed a number of outcome markers, in addition to the primary
endpoint (recurrent AF), including all-cause mortality, stroke/TIA
and the change of QoL. In addition, in the present study, the
quality of evidence was assessed using the GRADE system, as
proposed by the Cochrane Collaboration.
However, the current meta-analysis contains a number
of limitations, and the present results require cautious
interpretation. Firstly, the trials included in the meta-analysis
used different ablation techniques and different methods to monitor
the recurrence of AF. These variations are consistent with the
current status in clinical practice. Secondly, unlike real clinical
practice, AF patients included in the present analysis were
relatively young, with no serious structural heart diseases, and
only one study (11) enrolled
elderly patients (≥70 years). Thus, when appraising the results of
the present study in the real clinical practice setting, the
aforementioned limitations should be considered.
In conclusion, the results of the present study
demonstrated that catheter ablation therapy is superior to AADs in
reducing the recurrence of AF and improving the QoL. However, there
is insufficient evidence to suggest that catheter ablation is
superior to AADs in reducing the long-term severe adverse events,
including all-cause mortality and stroke/TIA. This issue may be
clarified by the future CABANA trial (no. NCT00911508).
References
|
1
|
Naccarelli GV, Varker H, Lin J and
Schulman KL: Increasing prevalence of atrial fibrillation and
flutter in the United States. Am J Cardiol. 104:1534–1539. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilke T, Groth A, Mueller S, et al:
Incidence and prevalence of atrial fibrillation: An analysis based
on 8.3 million patients. Europace. 15:486–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou ZQ and Hu DY: An epidemiological
study on the prevalence of atrial fibrillation in the Chinese
population of mainland China. J Epidemiol. 18:209–216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piccini JP, Hammill BG, Sinner MF, et al:
Incidence and prevalence of atrial fibrillation and associated
mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc
Qual Outcomes. 5:85–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camm AJ, Lip GY, De Caterina R, et al: ESC
Committee for Practice Guidelines (CPG): 2012 focused update of the
ESC Guidelines for the management of atrial fibrillation: An update
of the 2010 ESC Guidelines for the management of atrial
fibrillation. Developed with the special contribution of the
European Heart Rhythm Association. Eur Heart J. 33:2719–2747. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wann LS, Curtis AB, January CT, et al:
2006 Writing Committee Members; ACCF/AHA Task Force Members: 2011
ACCF/AHA/HRS focused update on the management of patients with
atrial fibrillation (updating the 2006 guideline): A report of the
American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines. Circulation.
123:104–123. 2011.PubMed/NCBI
|
|
7
|
Benjamin EJ, Wolf PA, D'Agostino RB,
Silbershatz H, Kannel WB and Levy D: Impact of atrial fibrillation
on the risk of death: The Framingham Heart Study. Circulation.
98:946–952. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Verma A, Macle L, Cox J and Skanes ACCCS
Atrial Fibrillation Guidelines Committee: Canadian Cardiovascular
Society atrial fibrillation guidelines 2010: Catheter ablation for
atrial fibrillation/atrial flutter. Can J Cardiol. 27:60–66. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krittayaphong R, Raungrattanaamporn O,
Bhuripanyo K, et al: A randomized clinical trial of the efficacy of
radiofrequency catheter ablation and amiodarone in the treatment of
symptomatic atrial fibrillation. J Med Assoc Thai. 86 (Suppl
1):8–16. 2003.PubMed/NCBI
|
|
10
|
Wazni OM, Marrouche NF, Martin DO, et al:
Radiofrequency ablation vs. antiarrhythmic drugs as first-line
treatment of symptomatic atrial fibrillation: A randomized trial.
JAMA. 293:2634–2640. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oral H, Pappone C, Chugh A, et al:
Circumferential pulmonary-vein ablation for chronic atrial
fibrillation. N Engl J Med. 354:934–941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stabile G, Bertaglia E, Senatore G, et al:
Catheter ablation treatment in patients with drug-refractory atrial
fibrillation: A prospective, multi-centre, randomized, controlled
study (Catheter Ablation For The Cure Of Atrial Fibrillation
Study). Eur Heart J. 27:216–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jais P, Cauchemez B, Macle L, et al:
Catheter ablation versus antiarrhythmic drugs for atrial
fibrillation: Rhe A4 study. Circulation. 118:2498–2505. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Forleo GB, Mantica M, De Luca L, et al:
Catheter ablation of atrial fibrillation in patients with diabetes
mellitus type 2: Results from a randomized study comparing
pulmonary vein isolation versus antiarrhythmic drug therapy. J
Cardiovasc Electrophysiol. 20:22–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilber DJ, Pappone C, Neuzil P, et al:
Comparison of antiarrhythmic drug therapy and radiofrequency
catheter ablation in patients with paroxysmal atrial fibrillation:
A randomized controlled trial. JAMA. 303:333–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pappone C, Vicedomini G, Augello G, et al:
Radiofrequency catheter ablation and antiarrhythmic drug therapy: A
prospective, randomized, 4-year follow-up trial: The APAF study.
Circ Arrhythm Electrophysiol. 4:808–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morillo CA, Verma A, Connolly SJ, Kuck KH,
Nair GM, Champagne J, Sterns LD, Beresh H, Healey JS and Natale
ARAAFT-2 Investigators: Radiofrequency ablation vs antiarrhythmic
drugs as first-line treatment of symptomatic atrial fibrillation
(RAAFT 2): A randomized trial. JAMA. 311:692–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cosedis Nielsen J, Johannessen A,
Raatikainen P, et al: Radiofrequency ablation as initial therapy in
paroxysmal atrial fibrillation. N Engl J Med. 367:1587–1595. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blandino A, Toso E, Scaglione M, et al:
Long-term efficacy and safety of two different rhythm control
strategies in elderly patients with symptomatic persistent atrial
fibrillation. J Cardiovasc Electrophysiol. 24:731–738. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0. updated.
March. 2011The Cochrane Collaboration. 2011, Available from.
https://www.cochrane-handbook.org
|
|
21
|
Guyatt GH, Oxman AD, Vist GE, et al GRADE
Working Group: GRADE: An emerging consensus on rating quality of
evidence and strength of recommendations. BMJ. 336:924–926. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ionescu-Ittu R, Abrahamowicz M,
Jackevicius CA, et al: Comparative effectiveness of rhythm control
vs rate control drug treatment effect on mortality in patients with
atrial fibrillation. Arch Intern Med. 172:997–1004. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsadok MA, Jackevicius CA, Essebag V, et
al: Rhythm versus rate control therapy and subsequent stroke or
transient ischemic attack in patients with atrial fibrillation.
Circulation. 126:2680–2687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pappone C, Rosanio S, Augello G, et al:
Mortality, morbidity and quality of life after circumferential
pulmonary vein ablation for atrial fibrillation: Outcomes from a
controlled nonrandomized long-term study. J Am Coll Cardiol.
42:185–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khaykin Y, Wang X, Natale A, et al: Cost
comparison of ablation versus antiarrhythmic drugs as first-line
therapy for atrial fibrillation: An economic evaluation of the
RAAFT pilot study. J Cardiovasc Electrophysiol. 20:7–12. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McKenna C, Palmer S, Rodgers M, et al:
Cost-effectiveness of radiofrequency catheter ablation for the
treatment of atrial fibrillation in the United Kingdom. Heart.
95:542–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reynolds MR, Zimetbaum P, Josephson ME,
Ellis E, Danilov T and Cohen DJ: Cost-effectiveness of
radiofrequency catheter ablation compared with antiarrhythmic drug
therapy for paroxysmal atrial fibrillation. Circ Arrhythm
Electrophysiol. 2:362–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noro M, Kujime S, Ito N, et al: Cost
effectiveness of radiofrequency catheter ablation vs. medical
treatment for atrial fibrillation in Japan. Cost performance for
atrial fibrillation. Circ J. 75:1860–1866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Noheria A, Kumar A, Wylie JV Jr and
Josephson ME: Catheter ablation vs. antiarrhythmic drug therapy for
atrial fibrillation: A systematic review. Arch Intern Med.
168:581–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Piccini JP, Lopes RD, Kong MH, Hasselblad
V, Jackson K and Al-Khatib SM: Pulmonary vein isolation for the
maintenance of sinus rhythm in patients with atrial fibrillation: A
meta-analysis of randomized, controlled trials. Circ Arrhythm
Electrophysiol. 2:626–633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonanno C, Paccanaro M, La Vecchia L,
Ometto R and Fontanelli A: Efficacy and safety of catheter ablation
versus antiarrhythmic drugs for atrial fibrillation: A
meta-analysis of randomized trials. J Cardiovasc Med (Hagerstown).
11:408–418. 2010. View Article : Google Scholar : PubMed/NCBI
|