Introduction
Hypertrophy of cardiomyocytes pathologically
contributes to left ventricular hypertrophy (LVH), which is induced
by hypertension (1). Thus,
elucidation of the mechanisms underlying the development and
regulation of cardiomyocyte hypertrophy is of great importance in
the prevention of cardiovascular diseases. Statins, or
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, have
been reported to exert multiple protective effects on the
cardiovascular system, independent of their classical functions on
lipoproteins. There is accumulating evidence that statins, in
addition to their classical functions on lipoproteins, also reduce
the level of cellular isoprenoid intermediates and inhibit the
isoprenylation of small molecular weight G-proteins of the Ras
superfamily (Ras/Rho), to subsequently exert protective effects on
the cardiovascular system (2).
Statins have been demonstrated to exert their effects on cell
proliferation and hypertrophy through mediating the membrane
translocation of Ras protein. The mitogen-activated protein kinase
(MAPK) signaling pathway and the phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (PKB) signaling pathway are known to be
involved in the biological effects of Ras protein (3). In addition, the PI3K/PKB pathway has
been demonstrated to be closely involved in the proliferation,
hypertrophy, apoptosis and survival process of cells (4).
The cellular kinase, PKB, is a serine/threonine
protein kinase that is activated via the PI3K signaling pathway. As
a common hub in a number of antiapoptotic pathways, PKB functions
through the phosphorylation of a plethora of downstream targets,
which in turn affect protective homeostatic, apoptotic and necrotic
pathways, as well as a number of other pathways, including those
associated with growth and development, and glucose metabolism
(5). PKB is an important mediator of
PI3K signaling, and numerous studies have implicated PKB-dependent
signaling pathways in the regulation of cardiac growth, contractile
function and coronary angiogenesis (6,7).
Phosphatase and tensin homolog (PTEN) is a lipid
phosphatase that predominantly dephosphorylates
phosphatidylinositol 3,4,5-trisphosphate [PtdIns-(3,4,5)-P3] to generate PtdIns-(4,5)-P2, and
therefore functions as a PI3K/PKB antagonist (8). In Drosophila, the loss of PTEN
has been shown to result in enhanced growth, whereas PTEN
overexpression has been demonstrated to decrease the cell number
and cell size (9). Furthermore, in
mice, the deletion of PTEN in cardiac muscle cells has been shown
to result in cardiac hypertrophy, which is associated with an
increase in individual myocyte size and PKB activity (10). These results are consistent with the
hypothesis that the loss of PTEN results in increased PI3K/PKB
activity, leading to cardiac hypertrophy. However, whether statins
exert an effect on the hypertrophy of cardiomyocytes remains
unknown, and the specific biological mechanism remains poorly
understood.
In the present study, the effects of simvastatin
were investigated on spontaneously hypertensive rats (SHRs) in
vivo and cardiomyocytes with serum-induced hypertrophy in
vitro. Subsequently, the effects of simvastatin on PKB and PTEN
expression in vivo and in vitro were investigated. In
addition, the effects of applying PTEN antisense
oligodeoxynucleotides on the biological effects of simvastatin in
cardiac myocytes were assessed in order to elucidate the function
of PTEN relative to the PKB pathway. Therefore, the aim of the
present study was to provide insight into the molecular mechanisms
modulating the hypertrophy of cardiomyocytes, and identify
potential therapeutic targets for the prevention of LVH induced by
hypertension.
Materials and methods
Animals
Thirty male SHRs and 15 Wistar-Kyoto (WKY) rats
(age, 8 weeks) were obtained from the Animal Center of Fuwai
Hospital (Beijing, China). The rats had free access to water and a
regular diet, and were housed at 22±1°C under a standard 12-h
light/dark cycle to acclimate for one week prior to the
experiments. All animals in the study were cared for, and the
experiments were performed according to, the established Guide for
the Care and Use of Laboratory Animals published by the National
Institutes of Health (NIH Publication No. 85-23, revised 1996). The
study was approved by the Institutional Review Board and the Animal
Care and Use Committee of the General Hospital of Lanzhou Military
Area Command (Lanzhou, China).
Determination of the rat tail artery
systolic pressure
The systolic pressure (mmHg) of the rat tail artery
was measured using a rat sphygmomanometer (RBP-1B-type, China Japan
Friendship Institute of Clinical Medicine, Beijing, China). All
measurements for the rat tail artery systolic pressure were
performed by the same investigator. The blood pressure of each
animal was measured prior to the initiation of the experiment, and
once a week until the end of the experiment.
Simvastatin administration for in vivo
study
Simvastatin (Wuhan C-bons Group, Wuhan, China) has
been widely administered clinically to prevent cardiovascular and
cerebrovascular diseases (11). Each
animal received simvastatin (10 mg/kg) once a day over a 10-week
study period. Control animals received the same volume of saline
during the same schedule.
Calculation of the left ventricular
mass (LVM) to body mass (BM) ratio
Following weighing, the animals were anesthetized
with an intraperitoneal injection of 3% pentobarbital sodium (40
mg/kg). The rat was sacrificed by exsanguination whilst still under
anaesthesia. The heart was removed immediately following euthanasia
and rinsed with 0.9% saline (4°C). Subsequently, the vessels,
cardiac atrium and right ventricular were quickly removed. After
blotting the saline with filter paper, the left ventricular and
interventricular septum were weighed, from which the LVM/BM ratio
was calculated. Finally, the tissue samples were cut into sections
at 6-µm thickness with an interval of 300 µm between each section
and stored in liquid nitrogen until required.
Culture and identification of the
cardiomyocytes
Cardiomyocytes were cultured and identified
according to a previously reported method with slight modifications
(12). Briefly, the ventricles of
the Sprague Dawley rats (age, 1–3 days; either gender; Research
Center of Experimental Animals in the Fourth Military Medical
University, Xi'an, China) were harvested aseptically and cut into
1-mm3 pieces. The tissue samples were digested with 0.1%
type I collagenase (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for
5–10 min with the assistance of magnetic stirring. The digestion
procedures were repeated 5–7 times. Following centrifugation at 550
× g for 5 min at 37°C, the cell pellets were collected and
suspended in Dulbecco's modified Eagle's medium (DMEM; Gibco Life
Technologies, Grand Island, NY, USA) containing 15% neonatal bovine
serum (Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd., Hangzhou, China). The cells were seeded into culture flasks
and allowed to stand for 2 h at 37°C in 5% CO2
(CO2 incubator; NuAire, Hong Kong, China). After 2 h,
the difference in the adhesion ability of the cells enabled the
isolation of the cardiomyocytes. The isolated cardiomyocytes were
seeded into culture flasks or plates at a density of
4×105 cells/ml. After 48 h, 0.1 mmol/l bromodeoxyuridine
(Sigma-Aldrich) was added to inhibit the growth of the cells other
than the cardiomyocytes. The medium was changed every 2 days. The
cells were identified as cardiomyocytes by spontaneous beating
after 24 h and positive staining for α-smooth muscle actin. The
staining procedure is shown as previously described (13). The purity of the cells reached 95%,
which was satisfactory for the further experiments.
Intervention protocol
Stably cultured cells were divided into three
groups. In the control group, the cells were cultured with serum
free DMEM, while in the serum group, the cells were cultured with
DMEM containing 15% neonatal bovine serum. In the simvastatin
intervention group, the cells were cultured with DMEM containing
15% neonatal bovine serum, in addition to various concentrations of
simvastatin (10−5, 10−6, 10−7 and
10−8 mol/l). After 24 h, cell cultivation was terminated
for further evaluation of the indices.
Determination of the cardiomyocyte
surface area
Cardiomyocytes were seeded at a density of
4×105 cells/ml into six-well plates, with a cover slip
placed previously. The cells were subsequently cultured with medium
containing a variety of intervention factors. After 24 h of
culture, the cultivation was terminated and the cells on the cover
slip were fixed with 95% alcohol for 15 min in preparation for
hematoxylin and eosin staining. A Leica-Q500 image analysis system
(Leica Camera AG, Wetzlar, Germany) was used to determine the
cardiomyocyte surface area. Five observation fields were selected
randomly on each cover slip, and 5–8 cells within each observation
field were selected for the determination of the mean cardiomyocyte
surface area according to the image analysis system. The average of
the measurements was calculated.
Determination of the protein synthesis
rate using a 3H-leucine incorporation assay
Cardiomyocytes were seeded into 24-well plates at a
density of 4×105 cells/ml, with 1 ml in each well. Next,
3H-leucine, with a final concentration of 3.7×104 Bq/ml
(China Institute of Atomic Energy, Beijing, China) that had been
prewarmed to 37°C, was added to each well together with the
intervention factors. Following termination of the cultivation, the
cells were washed twice with phosphate-buffered saline (PBS) and
10% trichloroacetic acid for 5 min, which had both been precooled
to 4°C. Subsequently, 0.5 ml NaOH-1% SDS (0.3 mol/l) was added, and
the mixture was allowed to stand for 30 min at room temperature.
The cell lysates were harvested, transferred to glass-fiber filter
paper, and subjected to drying at 42°C. The radioactivity
(cpm/cell) of the cells was evaluated using a LS6500 liquid
scintillation counter (Beckman Coulter, Brea, CA, USA).
Western blot analysis
After 48 h cultivation, the cells were washed with
cold PBS, and lysed with 100 µl 1X SDS lysis buffer, which had been
prewarmed to 85°C. The lysates were collected in a microtube,
heated for 10 min, and then centrifuged at 12,000 × g for 10 min at
room temperature. Following collection of the supernatant, the
protein concentration was determined according to the Bradford
method (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Subsequently, 20 µg protein from each sample was boiled at 100°C
for 5 min, after which the proteins were separated through 5–8%
SDS-PAGE. The protein band was transferred to a nitrocellulose
membrane, and the membranes were blocked with 3% bovine serum
albumin solution overnight at 4°C. The detection of the different
proteins was conducted by overnight incubation of the membrane at
4°C with the required dilution of a specific primary antibody
(rabbit anti-mouse PKB monoclonal antibody; cat. no. SC-7686;
dilution, 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA; rabbit anti-mouse PTEN monoclonal antibody; dilution, 1:500;
Sigma-Aldrich). The membrane was further incubated with a
horseradish peroxidase-coupled goat anti-rabbit IgG (cat. no.
sc-2004; Santa Cruz Biotechnology, Inc.) for 1 h. After thoroughly
washing with 0.05% Tween 20, the protein bands were visualized
using 3,3′-diaminobenzidine (Sigma-Aldrich). LabWorks 3.0 UVP
software (UVP, Upland, CA, USA) was used to analyze the optical
density, which represented the relative protein concentration.
Statistical analysis
Results are expressed as the mean ± standard
deviation, and differences among the groups were analyzed using
univariate analysis of variance and the least significant
difference test. SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for all the statistical analyses, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Simvastatin administration prevents
cardiomyocyte hypertrophy in SHRs
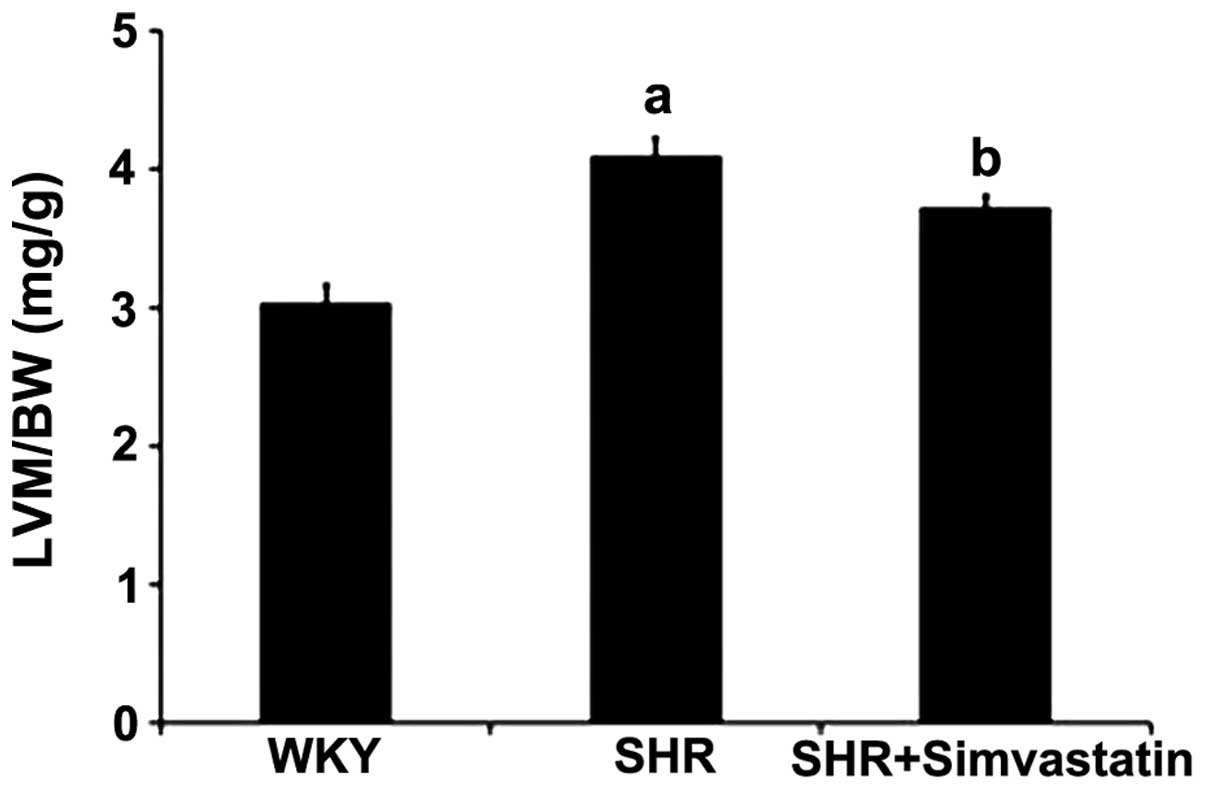
The LVM/BW ratio has been used to indicate
cardiomyocyte hypertrophy in certain in vivo experiments. In
the control (WKY) group, the LVM/BW ratio was 3.04±0.12 mg/g, while
in SHRs, the ratio was determined to be 4.10±0.13 mg/g. Treatment
with simvastatin was shown to reduce the extent of cardiomyocyte
hypertrophy in the SHRs, with simvastatin treatment decreasing the
LVM/BW ratio to 3.73±0.08 mg/g (Fig.
1).
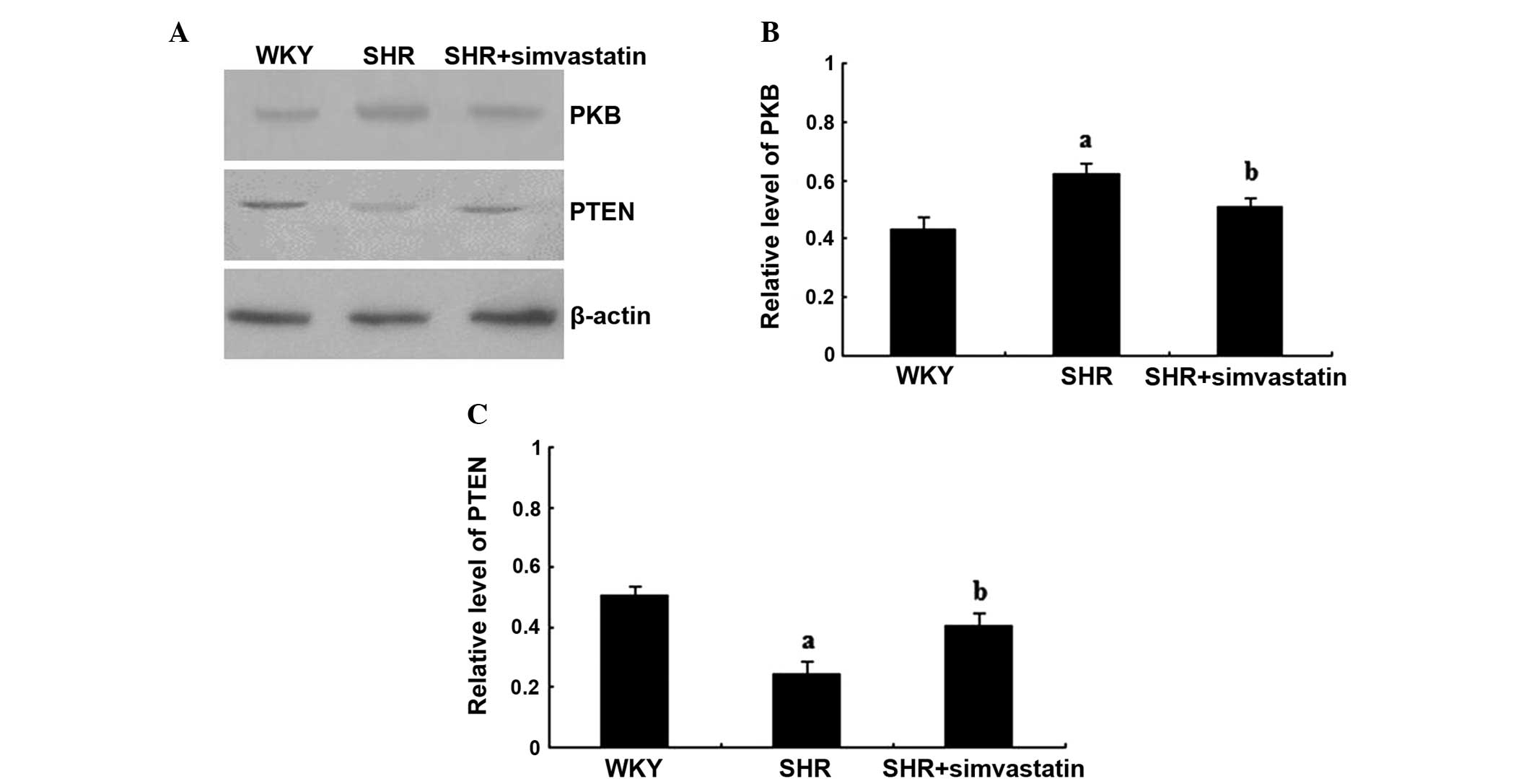
Simvastatin inhibits PKB expression,
but increases PTEN expression in SHRs
In order to identify the pathway affected by
simvastatin with regard to cardiomyocyte hypertrophy, the protein
expression levels of PKB and PTEN were investigated in the study.
In the SHR group, the PKB protein expression levels were
significantly greater when compared with that in the WKY group
(P<0.01), while the level in the simvastatin + SHR group was
significantly lower when compared with the SHR group (P<0.01).
By contrast, the protein expression level of PTEN in the SHR group
was significantly lower when compared with that in the WKY group
(P<0.01), while expression levels were significantly increased
in the simvastatin treatment group, as compared with that of the
SHR group (P<0.01; Fig.
2A–C).
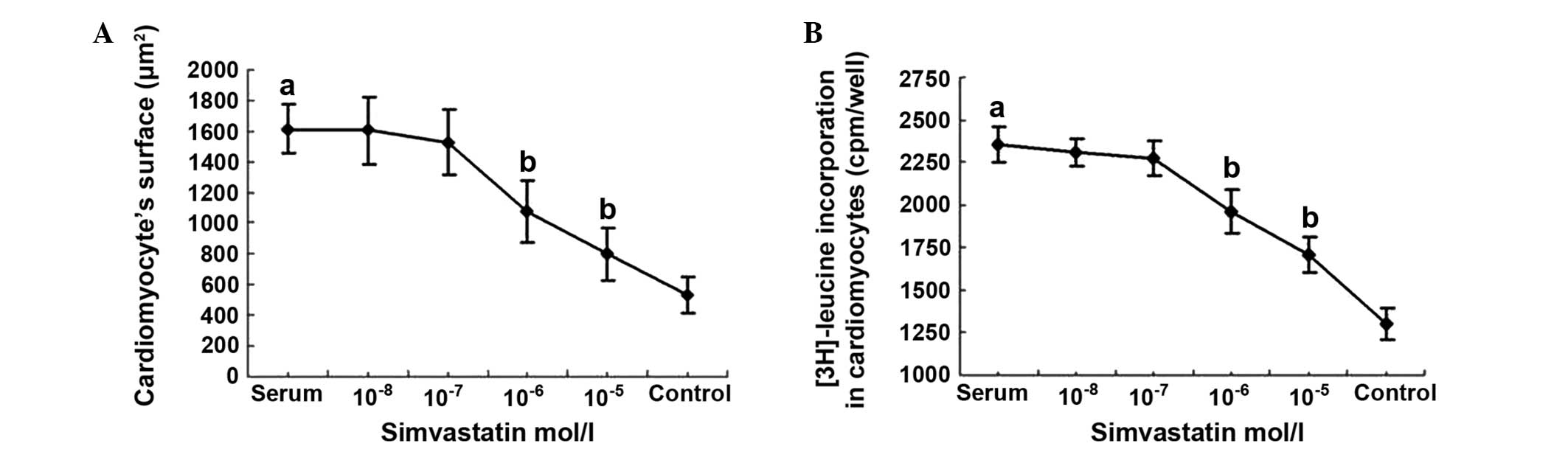
Effects of simvastatin on the surface
area and 3H-leucine incorporation in cultured cardiomyocytes
Following treatment with 15% neonatal bovine serum
for 24 h, the cardiomyocyte surface area was 1,611.16±160.75
µm2, which was significantly higher when compared with
that of the serum-free control group (538.04±118.60 µm2;
P<0.01). Following treatment with different concentrations of
simvastatin together with 15% fetal bovine serum, the cardiomyocyte
surface area was determined to be significantly lower in the
10−5 and 10−6 mol/l simvastatin intervention
groups (799.84±167.70 and 1,076.88±199.28 µm2,
respectively), as compared with the serum group (P<0.01).
However, in the 10−7 and 10−8 mol/l
simvastatin intervention groups, the cardiomyocyte surface areas
(1,529.32±212.83 and 1,606.84±220.81 µm2, respectively)
were not significantly different when compared with that of the
serum group (P>0.05; Fig.
3A).
The 3H-leucine incorporation rate in the 15%
neonatal bovine serum group (2,360±106 cpm/well) was significantly
greater when compared with that in the serum-free group (1,305±92
cpm/well; P<0.01). The incorporation rates were 1,707±101 and
1,962±125 cpm/well in the 10−5 and 10−6 mol/l
simvastatin intervention groups, respectively, and these values
were significantly lower when compared with the serum group
(P<0.01). In the 10−7 and 10−8 mol/l
simvastatin intervention groups, the incorporation rates were
2,280±105 and 2,311±80 cpm/well, respectively; however, these
values were not significantly different when compared with that of
the serum group (P>0.05; Fig.
3B).
Effects of simvastatin on PKB and PTEN
expression in the cultured cardiomyocytes
PKB protein expression levels were significantly
higher in the serum group when compared with the control group
(P<0.01). Furthermore, the PKB protein expression levels were
significantly lower in the 10−5 and 10−6
mol/l simvastatin intervention groups when compared with the serum
group (P<0.01). However, there were no statistically significant
differences in the levels when comparing the 10−7 and
10−8 simvastatin intervention groups with the serum
group (P>0.05). By contrast, simvastatin was found to increase
the PTEN protein expression levels in a concentration-dependent
manner. The protein expression levels of PTEN in the
10−5, 10−6 and 10−7 mol/l
simvastatin + serum groups were significantly higher when compared
with the serum group (all P<0.05); however, the expression
levels were all lower when compared with the control group (all
P<0.01; Fig. 4A–C).
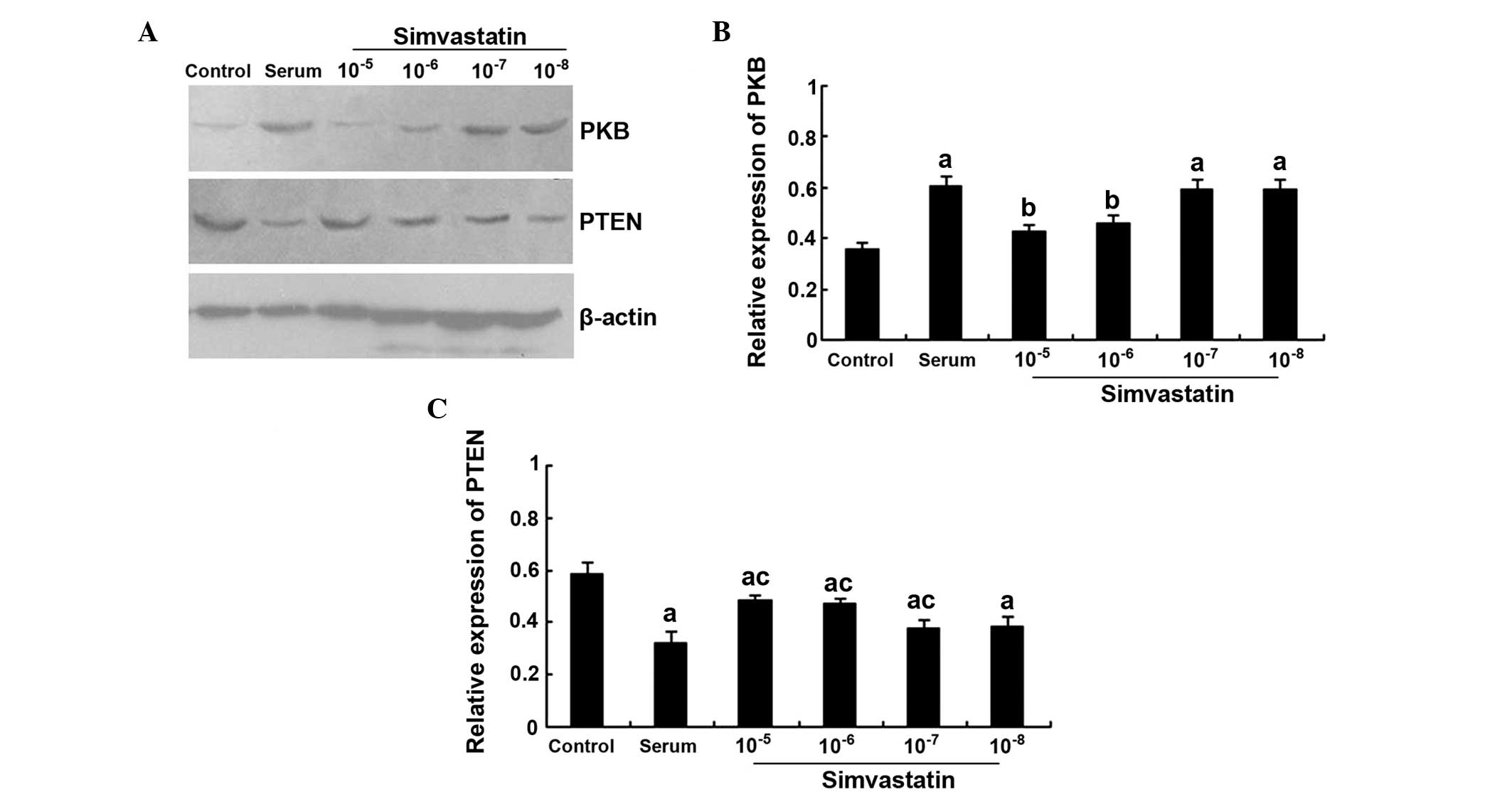 | Figure 4.Effects of simvastatin on PKB and PTEN
expression in cultured cardiomyocytes. (A) Representative western
blot, and quantitative determination of (B) PKB and (C) PTEN
protein expression levels in the control, serum, and
10−5, 10−6, 10−7 and
10−8 mol/l simvastatin intervention groups.
aP<0.01, vs. control group; bP<0.01,
vs. serum group; cP<0.05, vs. serum group. PKB,
protein kinase B; PTEN, phosphatase and tensin homolog. |
PTEN antisense oligodeoxynucleotides
decrease the level of PTEN and inhibit the effect of simvastatin on
cardiomyocytes
Inhibition of the biological effects of simvastatin
on the cardiomyocytes was observed following treatment with PTEN
antisense oligodeoxynucleotides. The protein expression levels of
PTEN in the PTEN antisense oligodeoxynucleotides group were
significantly lower compared with those in the simvastatin group
(P<0.01); however, there were no statistically significant
differences when comparing the sense or mismatch groups with the
simvastatin group (P>0.05; Fig. 5A
and B). Accordingly, the cardiomyocyte surface area (Fig. 5C) and the 3H-leucine incorporation
rate (Fig. 5D) in the
10−6 mol/l simvastatin + 5×10−6 mol/l PTEN
antisense oligodeoxynucleotides group were significantly greater
compared with the values observed in the simvastatin single
treatment group (P<0.01). However, the values were lower
compared with those of the serum group (P<0.01). By contrast,
there were no statistically significant differences in the protein
expression levels when comparing the PTEN sense and mismatch
oligodeoxynucleotides group with the simvastatin group
(P>0.05).
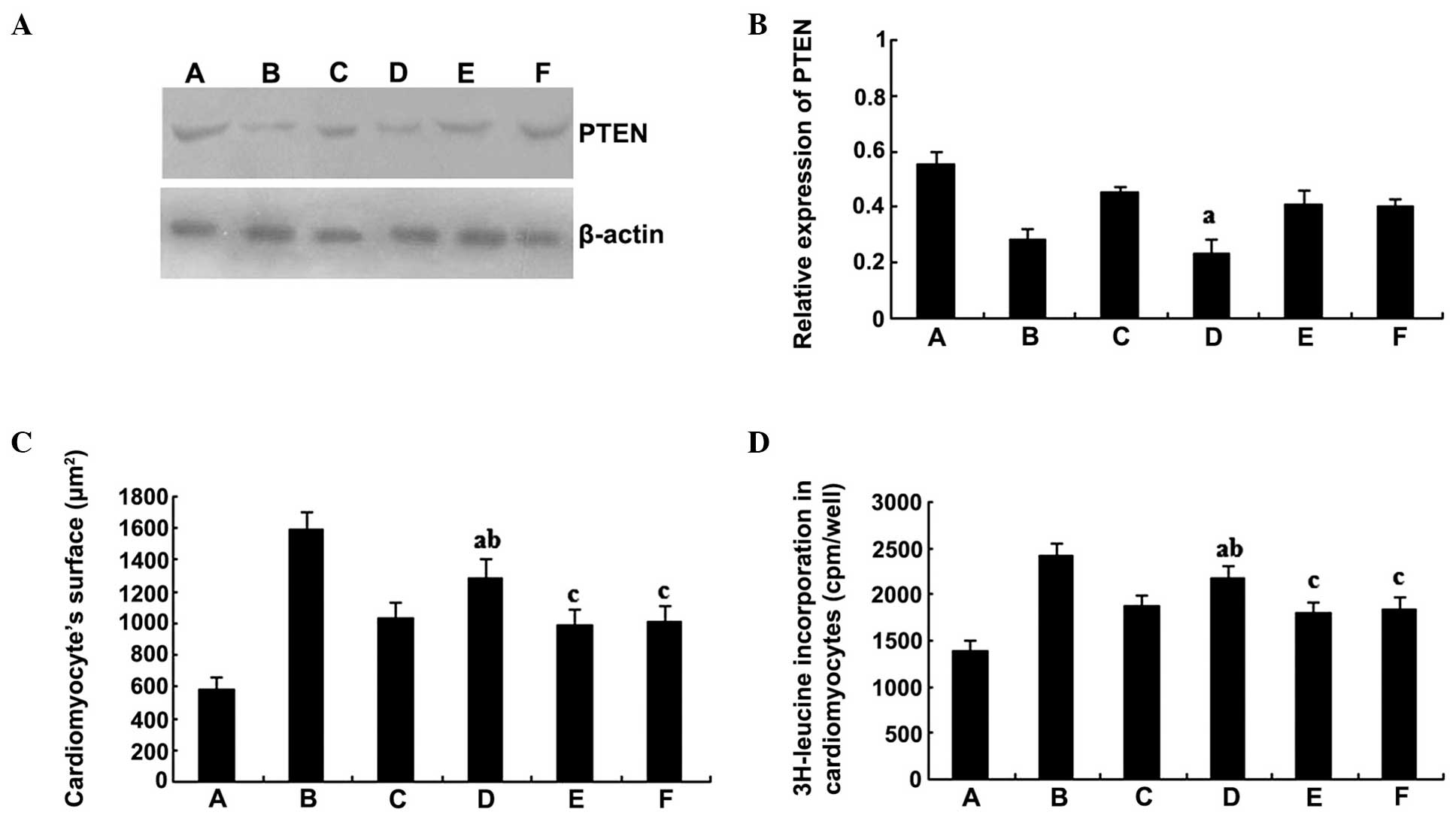 | Figure 5.PTEN antisense oligodeoxynucleotides
decrease the level of PTEN protein expression and inhibit the
effects of simvastatin on cardiomyocytes. (A) Representative
western blot and (B) quantitative determination of PTEN protein
expression levels. (C) Cardiomyocyte surface area and (D)
3H-leucine incorporation rate of the various groups.
aP<0.01, vs. 10−6 mol/l simvastatin group;
bP<0.01, vs. serum group; cP>0.05, vs.
10−6 mol/l simvastatin group. PTEN, phosphatase and
tensin homolog; A, control group; B, serum group; C,
10−6 mol/l simvastatin group; D, 10−6 mol/l
simvastatin + PTEN antisense oligodeoxynucleotides group; E,
10−6 mol/l simvastatin + PTEN sense
oligodeoxynucleotides group; F, 10−6 mol/l simvastatin +
PTEN mismatch oligodeoxynucleotides group. |
Discussion
The salient finding of the present study was that
simvastatin reduces cardiomyocyte hypertrophy by increasing PTEN
expression. In the current study, simvastatin administration was
demonstrated to prevent rat cardiomyocyte hypertrophy and inhibit
PKB expression in vivo and in vitro. By contrast,
simvastatin was shown to significantly increase PTEN expression in
the SHRs and cultured cardiomyocytes. In addition, treatment with
PTEN antisense oligodeoxynucleotides significantly inhibited the
expression of PTEN and blocked the effect of simvastatin on
cardiomyocytes. These results demonstrate possible molecular
pathways underlying the effects of simvastatin on the hypertrophy
of cardiomyocytes, and indicate that simvastatin may be an
effective pharmacological compound against cardiomyocyte
hypertrophy.
Statins are well accepted to not only have
satisfactory lipid regulatory effects, but also inhibit the
proliferation of vascular smooth muscle cells and cardiac
fibroblasts (14,15). Statin administration is associated
with a decreased incidence of cardiovascular events and mortality
in patients with cardiovascular risk. However, the effects of
statins on cardiomyocytes and the specific molecular mechanisms
underlying their action remain uncertain.
A previous study demonstrated that fetal bovine
serum containing endothelin I, angiotensin II and fibroblast growth
factors is able to induce the hypertrophy of cardiomyocytes
(16). In the present study,
simvastatin was shown to inhibit the serum-induced increase in cell
surface area and the 3H-leucine incorporation rate in
cardiomyocytes, while significantly decreasing the PKB expression
levels in a concentration-dependent manner. Leucine is a precursor
of protein synthesis, and the 3H-leucine incorporation assay is
widely used for the detection of protein synthesis in cells. The
results of the present study indicated that simvastatin inhibited
the hypertrophy and protein synthesis of cardiomyocytes, which may
be significant in alleviating hypertension-induced LVH, improving
heart function and delaying the development of cardiac hypertrophy
to heart failure.
In addition to the classical function of statins on
lipoproteins, there is accumulating evidence that statins also
reduce the level of cellular isoprenoid intermediates and inhibit
the isoprenylation of small molecular weight G-proteins of Ras/Rho,
to subsequently exert protective effects on the cardiovascular
system (2). Statins play a role in
cell proliferation and hypertrophy through mediating the membrane
translocation of Ras protein, and the MAPK and PI3K/PKB signaling
pathways are involved in the biological functions of Ras protein
(3). In addition, the PI3K/PKB
pathway has been found to be closely involved in the proliferation,
hypertrophy, apoptosis and survival process of cells (4). Nevertheless, it is unclear how the
pathway is involved in the statin-modulated hypertrophy process of
cardiomyocytes. In the present study, simvastatin was demonstrated
to reverse the serum-induced hypertrophy of cardiomyocytes, as well
as decrease the protein expression levels of PKB. Therefore, the
results indicate that PKB is an important signal transmission
molecule involved in the hypertrophy of cardiomyocytes. Simvastatin
is able to downregulate PKB expression, modulate the activity of
downstream kinases and transcription factors, and decrease the
expression of protein synthesis-associated genes, in order to
inhibit the hypertrophy of cardiomyocytes.
PTEN expression levels may be involved in myocardial
hypertrophy, which can be inferred since simvastatin was shown to
elevate the PTEN expression level. External prohypertrophic factors
downregulate PTEN expression in cardiomyocytes, which subsequently
activates downstream kinases and transcription factors through the
PI3K/PKB pathway, thus motivating myocardium hypertrophy. This may
be one of the molecular mechanism through which simvastatin
inhibits the hypertrophy of cardiomyocytes and reverses LVH. The
biological effects of simvastatin on cardiomyocytes may be partly
inhibited by intervention with PTEN antisense
oligodeoxynucleotides. In the present study, application of PTEN
antisense oligodeoxynucleotides reduced the simvastatin-induced
increase in PTEN expression, and also decreased the cardiomyocyte
surface area and 3H-leucine incorporation rate, indicating that
PTEN is involved in the effect of simvastatin on the hypertrophy of
cardiomyocytes.
In conclusion, the results of the present study
indicate that simvastatin is able to reverse cardiomyocyte
hypertrophy induced by fetal bovine serum, possibly through
increasing PTEN expression, thereby inhibiting the PI3K/PKB
pathway. Therefore, the present study provides a novel theoretical
and experimental basis for the alleviation of LVH at the cellular
and molecular levels, and, furthermore, offers new insights for the
prevention of hypertension-induced LVH.
References
|
1
|
Zheng H and Lu GM: Reduction of prohibitin
expression contributes to left ventricular hypertrophy via
enhancement of mitochondrial reactive oxygen species formation in
spontaneous hypertensive rats. Free Radic Res. 49:164–174. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koh KK, Sakuma I and Quon MJ: Differential
metabolic effects of distinct statins. Atherosclerosis. 215:1–8.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugden PH: Ras, Akt, and
mechanotransduction in the cardiac myocyte. Circ Res. 93:1179–1192.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paez J and Sellers WR: PI3K/PTEN/AKT
pathway. A critical mediator of oncogenic signaling. Cancer Treat
Res. 115:145–167. 2003.PubMed/NCBI
|
|
6
|
Shiojima I and Walsh K: Regulation of
cardiac growth and coronary angiogenesis by the Akt/PKB signaling
pathway. Genes Dev. 20:3347–3365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Berlo JH, Maillet M and Molkentin JD:
Signaling effectors underlying pathologic growth and remodeling of
the heart. J Clin Invest. 123:37–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stuenaes JT, Bolling A, Ingvaldsen A, et
al: Beta-adrenoceptor stimulation potentiates insulin-stimulated
PKB phosphorylation in rat cardiomyocytes via cAMP and PKA. Br J
Pharmacol. 160:116–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maire CL, Ramkissoon S, Hayashi M, Haidar
S, Ramkissoon L, DiTomaso E and Ligon KL: Pten loss in Olig2
expressing neural progenitor cells and oligodendrocytes leads to
interneuron dysplasia and leukodystrophy. Stem Cells. 32:313–326.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crackower MA, Oudit GY, Kozieradzki I, et
al: Regulation of myocardial contractility and cell size by
distinct PI3K-PTEN signaling pathways. Cell. 110:737–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Cheng X, Liao YH, et al:
Simvastatin regulates myocardial cytokine expression and improves
ventricular remodeling in rats after acute myocardial infarction.
Cardiovasc Drugs Ther. 19:13–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simpson P, McGrath A and Savion S: Myocyte
hypertrophy in neonatal rat heart cultures and its regulation by
serum and by catecholamines. Circ Res. 51:787–801. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi YS, Dusting GJ, Stubbs S, et al:
Differentiation of human adipose-derived stem cells into beating
cardiomyocytes. J Cell Mol Med. 14:878–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clunn GF, Sever PS and Hughes AD: Calcium
channel regulation in vascular smooth muscle cells: Synergistic
effects of statins and calcium channel blockers. Int J Cardiol.
139:2–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Q, Cui W, Zhang HL, Hu HJ, Zhang YN,
Liu DM and Liu J: Atorvastatin suppresses aldosterone-induced
neonatal rat cardiac fibroblast proliferation by inhibiting ERK1/2
in the genomic pathway. J Cardiovasc Pharmacol. 61:520–527. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santalucía T, Christmann M, Yacoub MH and
Brand NJ: Hypertrophic agonists induce the binding of c-Fos to an
AP-1 site in cardiac myocytes: Implications for the expression of
GLUT1. Cardiovasc Res. 59:639–648. 2003. View Article : Google Scholar : PubMed/NCBI
|