Introduction
From the first orthotopic liver transplantation
(OLT) by Thomas Starzl (1), liver
transplantation has become an effective therapeutic option for the
treatment of end-stage liver disease. However, postoperative immune
rejection is the main cause for early stage liver dysfunction
following liver transplantation. Immune rejection is also
associated with various other complications, such as pulmonary
disease. In a previous study by Bozbas et al (2), pulmonary complications were detected in
42.1% of liver recipients; pneumonia, in 21.1%; and pleural
effusion on early postoperative chest radiographs, in 32.5%. In
clinical practice, an immune rejection response can be partly
controlled by a number of novel immunosuppressants, which are
required by almost all patients for prolonging the survival time.
However, these drugs are associated with a number of issues,
including high costs and numerous side effects. In addition,
chronic immune rejection is inevitable (3–5). A
number of in vitro studies (6–9) have
reported that mesenchymal stem cells (MSCs) produce an
immunosuppressive effect. MSCs have also been demonstrated, by Wood
et al (10), to have
important roles in the induction of immune tolerance following
organ transplantation in vivo. MSCs suppress allogeneic T
cell responses by secreting soluble factors, such as prostaglandin
E2, interleukin (IL)-10 and IL-6 (11). Kordelas et al suggested that
MSC-derived exosomes, which are released by exocytosis from the
plasma membrane, may benefit therapy-refractory patients with graft
versus-host disease (12).
Therefore, the aim of the present study was to clarify the
immunological effect induced by bone marrow MSCs in rats that had
undergone an OLT, in order to establish a theoretical base for
improving the survival times of patients undergoing a liver
transplantation.
Materials and methods
Animals and reagents
In total, 42 female Lewis rats and 42 female Brown
Norway rats were used as donors and recipients. Two male Lewis rats
were randomly chosen for the extraction of MSCs. All the rats
(weight, 180–220 g; age, three months) were provided by the
Experimental Animal Center of Xinjiang Medical University (Ürümqi,
China), and were housed with free access to water. Low glucose
Dubecco's modified Eagle's medium (LG-DMEM) was purchased from
Gibco Life Technologies (Carlsbad, CA, USA) and good grade fetal
bovine serum (FBS) was obtained from Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd. (Hangzhou, China). Trypsin (1:250)
was purchased from Blue quarter of Shanghai Science and Technology
Development Co. (Shanghai, China). Both L-glutamine and
poly-L-lysine were obtained from Sigma-Aldrich (St. Louis, MO,
USA). An inverted phase contrast microscope (CKX41-A32PH) was
obtained from Olympus Corporation (Tokyo, Japan). Rabbit anti-rat
antibodies targeted against interleukin (IL)-10 (cat. no.
bs-6761R), IL-12 (cat. no. bs-10641R) and transforming growth
factor (TGF)-α1 (cat. no. bs-0086R) were purchased from
Beijing Boao Sen Biotechnology Co., Ltd. (Beijing, China). A rabbit
two-step kit (PV6001) was obtained from Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd. (Beijing, China) and a SRY in
situ hybridization detection kit was purchased from Wuhan Bo
Shide Biological Engineering Co., Ltd. (MK1034; Wuhan, China).
Isolation, culture and identification
of MSCs
Following abdominal administration of 4% chloral
hydrate anesthesia (1 ml/100 g; Qingdao Yulong HAIZAO Co., Ltd.,
Qingdao, China), male Lewis rats were sacrificed and the femur was
obtained. The bone samples were immersed in 0.2%
penicillin-streptomycin solution (XB-100X; Guangzhou Xiang Bo
Biological Technology Co., Ltd., Guangzhou, China) and then flushed
by PBA-A. The femoral epiphysis was harvested and the cavitas
medullaris were repeatedly washed with LG-DMEM solution. Next,
~50 μl mixed bone marrow cell suspension was collected, dyed with
trypan blue and counted on a cell count board under a LEICA ortho
microscope (Leica Microsystems GmbH, Wetzlar, Germany). The cell
density was adjusted to 6–8×106/ml, after which 10% FBS
was added and the cells were inoculated in the culture flask for
culture. After 48 h, the liquid was changed for the first time and
the non-adherent cells were removed. When the cultured MSCs reached
~90% cell fusion degree, they were subcultured into new culture
bottles at a cell density of 2–5×105/ml. 0.125%
trypsinization was used for serial subcultivation. A cell
suspension of the third generational passage was collected with a
density of 2×104/ml and centrifuged at 250 × g for 5 min
at 4°C. Subsequently, the cells were passed through a 200 μm filter
and the density was adjusted to 2×106/ml. Finally, the
cells were stored in a refrigerator at 4°C (13,14).
Animal model establishment
Rat OLT models were established according to the
modified classic ‘two-cuff technique’ (15). A successful model was defined by the
recipient living for >48 h. In general, the rats were able to
drink water containing 10% glucose immediately following
consciousness, with free access to food one day after the OLT. All
the rats were divided equally at random into three groups. Group A
rats underwent the OLT only, group B were intramuscularly injected
with tacrolimus (FK506; 2 mg/kg/day) for one month following the
OLT, while group C rats were administered not only FK506, but also
a suspension of MSCs (1×106/200 g) infused through the
portal vein immediately following bile duct anastomosis. Ethical
approval was obtained from the Medical Ethics Committee of the
Affiliated Tumor Hospital of Xinjiang Medical University (no.
W-201309).
Specimen collection and
measurement
On day 7, six rats that had undergone an OLT were
randomly sacrificed in each group for hepatic function and
pathological analysis. Alanine aminotransferase (ALT), aspartate
aminotransferase (AST) and total bilirubin (TBIL) levels were
analyzed. Pathological examination was performed on the grafts
using hematoxylin and eosin staining, while the levels of various
cytokines, including TGF-α1, IL-10 and IL-12, were
measured by immunohistochemistry using a rabbit two-step kit
according to the manufacturer's instructions. SRY in situ
hybridization was applied to locate the MSCs containing a Y
chromosome (from the male donor). The remaining eight rats from
each group that had undergone an OLT were observed for survival
rates. The procedures were conducted according to the methods of
Wei et al (16).
Statistical analysis
SPSS 15.0 (SPSS, Inc., Chicago, IL, USA) software
was used for statistical analysis. Analysis of variance was used to
analyze the levels of AST, ALT and TBIL in the three groups. Least
significance difference analysis was performed to assess the
differences among the groups. Statistical analysis of the
expression of TGF-β1, IL-10 and IL-12 in the three
groups was carried out using a Kruskal-Wallis test. Comparisons
between the levels of these three cytokines in two groups were
conducted using a Mann-Whitney U test (adjusted α′=0.05/3) In
addition, Kaplan-Meier survival curves and the log-rank test were
performed to analyze the survival rates. Experimental data are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphological changes to the MSCs
At 72 h after the primary culture of MSCs, there
were a number of oval-shaped adherent cells and a small number of
cells undergoing stretching and deformation, presenting as a
polygon or fusiform-shaped (Fig.
1-A1). These cells proliferated rapidly and formed clones after
three to five days. A small number of cells were confluent (cells
in the first generational passage; Fig.
1–A2), and up to 90% confluency was achieved after seven or
eight days. The third generational passage cells were almost all
adhered to the wall and evenly spread, with a typical uniform long
spindle morphology. These cells also proliferated rapidly, and
>90% confluency was achieved after four or five days, presenting
as whirlpool and pectiniform shapes (Fig. 1-A3).
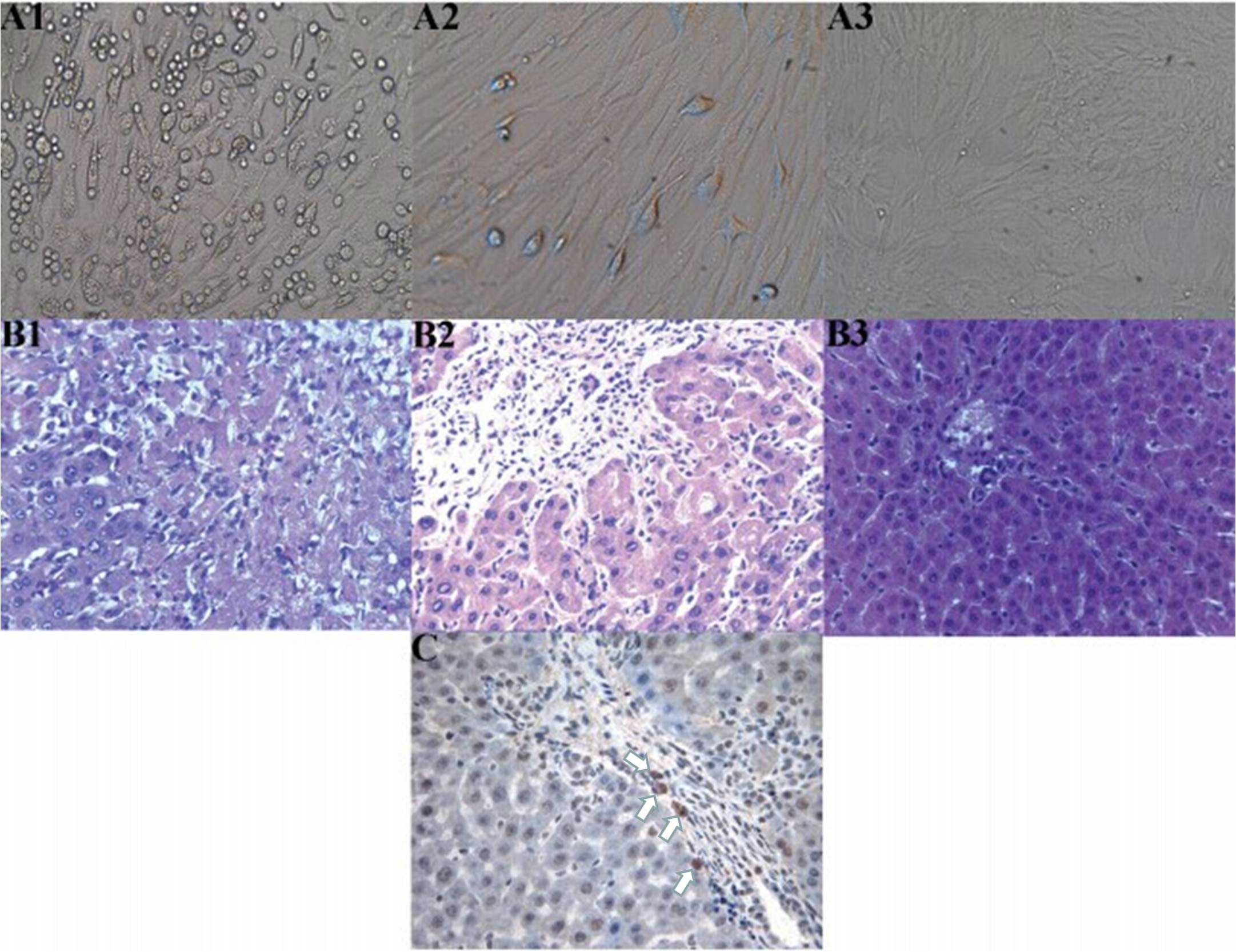 | Figure 1.(A) Cell culture, (B) hepatic
histopathological changes and (C) immunohistochemistry showing the
location of the mesenchymal stem cells (MSCs). (A) Morphological
changes of the MSCs; (A1) Primary culture for three days
(magnification, x100) revealed a number of oval-shaped adherent
cells and the partial stretching and deformation of cells,
presenting as a polygon or clostridium; (A2) First generational
passage cell culture (magnification, x200) showed a small number of
confluent cells; (A3) Third generational passage cell culture for
seven days (magnification, x50) exhibited whirlpool and pectiniform
shapes. (B) Hepatic histopathological changes in each group on day
7 after orthotopic liver transplantation (OLT; hematoxylin and
eosin stain; magnification, x200); (B1) Group A exhibited evident
necrosis of hepatic cells; (B2) Group B exhibited evident
inflammatory infiltration, predominantly concentrating in the
portal area, without cellular necrosis; (B3) Group C manifested
almost no evident inflammatory infiltration. (C) Location of MSCs
in the grafts of patients in group C on day 7 following OLT was
determined using SRY in situ hybridization (magnification,
x400). A number of positive cells (white arrows) can be observed,
with the cytoplasm and nucleus stained brown. |
General situation following the
OLT
Between days 2 and 3 after the OLT, the recipients
in group A appeared very lethargic and were not eating. The rats
responded slowly to an outside stimulus. After five to seven days,
the rats began to show progressive jaundice, loss of awareness,
apparent appetite reduction and gradual weight loss. The rats
continued to appear lethargic and have a poor response to outside
stimuli. Consequently, the rats succumbed naturally between days 9
and 13 after surgery. In the autopsy examination, no
intra-abdominal ascites or distortion of the biliary stent casing
were observed, while the edema of the liver and spleen was serious.
In groups B and C, within seven days of the OLT, the recipients
exhibited a passive mood, their diet was poor and their response to
outside stimuli appeared a little clumsy. However, in groups B and
C recovery was observed ~14 days after the OLT, with the rats
becoming active, improving their diet and gradually increasing in
weight at one month after surgery. After five weeks the recipients
in group B began to die, due to chronic immune rejection.
Hepatic functional measurements
On day 7 after the OLT, hepatic function in group C
was significantly improved compared with groups A and B (Table I).
 | Table I.Comparison of hepatic function among
the three groups on day 7 following OLT. |
Table I.
Comparison of hepatic function among
the three groups on day 7 following OLT.
| Groups | Cases (n) | ALT (U/l) | AST (U/l) | TBIL (μmol/l) |
|---|
| Group A | 6 | 733.00±69.45 | 645.00±94.16 | 98.50±5.58 |
| Group B | 6 |
274.50±50.47a |
344.50±60.03a |
38.17±2.86a |
| Group C | 6 |
114.67±21.96a,b |
151.33±16.68a,b |
17.00±1.41a,b |
Histopathological examination
On day 7 after the OLT, group A rats exhibited
strong acute rejection, and substantial necrosis of the hepatic
cells was observed. Group B rats presented with mild acute
rejection and there was evident inflammatory infiltration, mainly
concentrating in the portal area, without cellular necrosis. Group
C rats manifested the mildest rejection and there was almost no
observations of inflammatory infiltration, with the structures
clearly visible. In the three groups, pathological changes caused
by acute immune rejection were classified according to the standard
recommended by Williams et al (17) (Table
II and Fig. 1-B).
 | Table II.Comparison of graft pathological
classification among the three groups on day 7 following OLT. |
Table II.
Comparison of graft pathological
classification among the three groups on day 7 following OLT.
| Groups | Class zero | Class one | Class two | Class three |
|---|
| Group A | 0 | 0 | 0 | 6 |
| Group B | 0 | 3 | 3 | 0 |
| Group C | 3 | 3 | 0 | 0 |
Immunohistochemical results
TGF-α1 was mainly secreted by MSCs and
their differentiated cells, including T helper 1 (Th1) cells. IL-10
was primarily secreted by T helper 2 cells, mononuclear cells, MSCs
and their differentiated cells. IL-12 was predominantly secreted by
antigen-presenting-cells (APCs), such as dendritic cells, and
mononuclear cells and Th1 cells. The majority of these cells were
found in the portal area. When the nucleus and part of the
cytoplasm were stained with brown particles, the cells were
regarded to exhibit positive expression. A total of five
high-density positive cell areas in the portal area were randomly
selected under a microscope (magnification, x400) to observe the
proportion of positive cells. If the percentage of positive cells
was <10%, 10–25%, 26–50% and >50%, the cells were regarded as
negative (–), weak positive (+), medium positive (++) and strong
positive (+++), respectively. Weak positive and medium positive
samples were considered to exhibit low expression, while strong
positive samples were considered to have high expression (18) (Table
III).
 | Table III.Expression of TGF-β1,
IL-10 and IL-12 in the three groups after OLT. |
Table III.
Expression of TGF-β1,
IL-10 and IL-12 in the three groups after OLT.
| Groups | n |
TGF-β1 | IL-10 | IL-12 |
|---|
|
|
|
|
|---|
|
|
| – | + |
| ++ | +++ | – | + |
| ++ | +++ | – | + |
| ++ | +++ |
|---|
| A | 6 | 2 | 4 |
| 0 | 0 | 2 | 4 |
| 0 | 0 | 0 | 1 |
| 5 | 0 |
| B | 6 | 0a | 2 |
| 4 | 0 | 0 | 0 |
| 1 | 5 | 1 | 5 |
| 0 | 0 |
| C | 6 | 0 | 0 |
| 1 | 5 | 0b | 0 |
| 0 | 6 | 6 | 0 |
| 0 | 0 |
|
| H |
|
|
| 13.859 |
|
|
|
| 15.065 |
|
|
|
| 14.606 |
|
|
| P |
|
|
| 0.001 |
|
|
|
| 0.001 |
|
|
|
| 0.001 |
|
|
SRY in situ hybridization
On day 7 after the OLT, the results revealed a
number of positive cells, with part of the cytoplasm and the
nucleus stained brown. These cells were the MSCs obtained from the
male donor rats containing the Y chromosome, and were primarily
found in the portal area (Fig.
1-C).
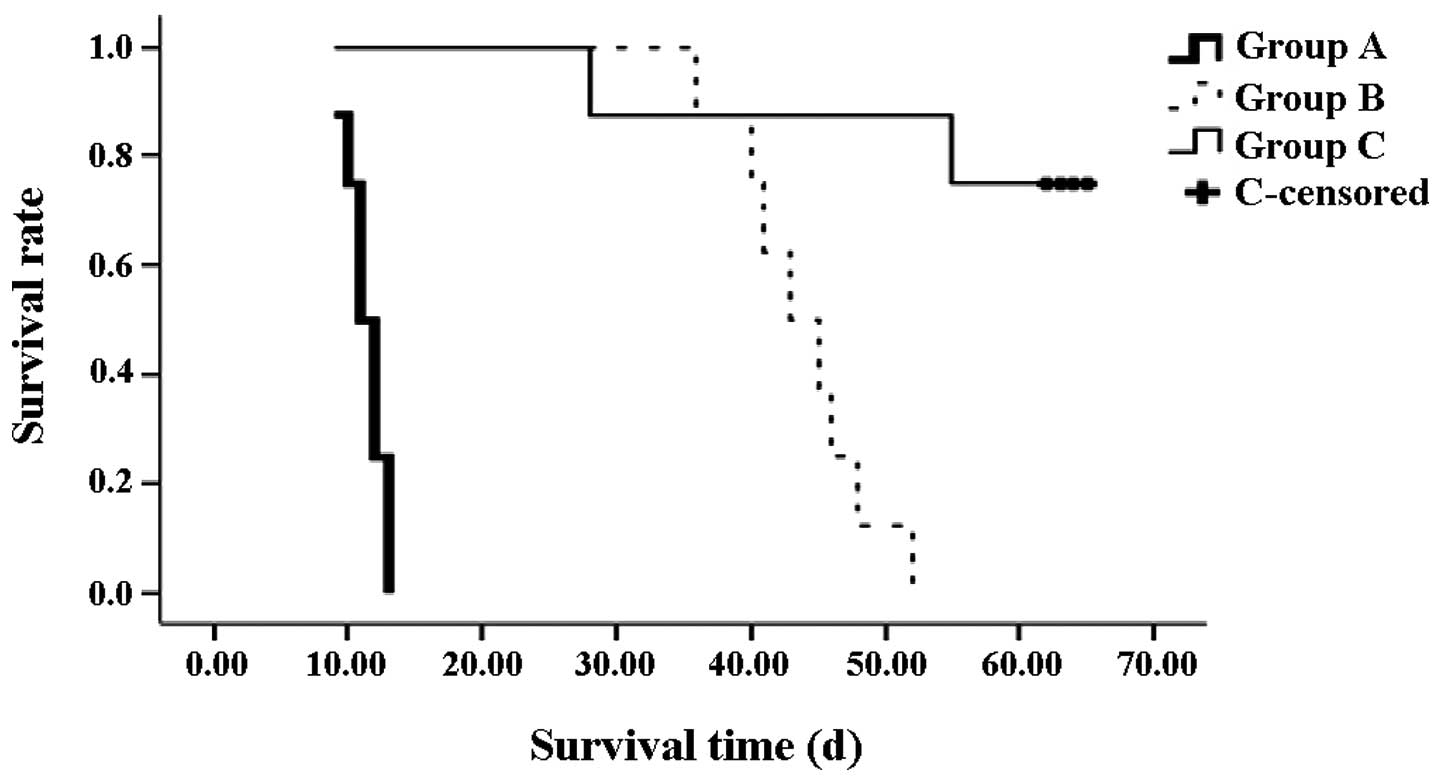
Survival analysis
From the Kaplan-Meier survival curve, the survival
times in groups B (median, 44 days) and C (median, 63 days) were
shown to be significantly longer compared with the rats in group A
(median, 11 days; both P<0.01). In addition, the survival rate
of the rats in group C was significantly higher compared with group
B (P<0.01). On day 28 after the OLT, one rat from group C
succumbed to biliary tract obstruction. An additional rat succumbed
on day 55; however, the cause was unknown (Fig. 2).
Discussion
MSCs are characterized by their ability to
self-renew and differentiate into various cell lineages. The cells
have a wide range of sources. Research into the clinical
application of MSCs is increasing, with in vitro and in
vivo studies (19–21) demonstrating the immunoregulatory
effect of MSCs. Bartholomew et al (22) found that MSCs were able to prolong
allogeneic skin graft survival in baboons, despite the cells being
collected from a ‘third party’ (not from the donors or recipients).
In addition, MSC infusion suppressed immune rejection, while the
recipients did not inflict an immune rejection against the MSCs.
Schatton et al (23) also
reported that MSCs had the same effect in a model of allogeneic
heart transplantation.
Clinical liver transplantation has become an
effective treatment for advanced chronic liver disease and certain
cases of acute liver failure. Postoperative acute immune rejection
seriously affects the outcome of liver transplantation. In clinical
practice, a number of novel immunosuppressants are used to maintain
liver function by controlling immune rejection following liver
transplantation. However, administration of these drugs is limited
due to the high-cost, severe toxicity and side effects. In
addition, these drugs are unable to prevent chronic immune
rejection. The ideal pathway of immunosuppressants for prolonging
graft survival is to induce specific immune tolerance of
recipients-to-grafts (24,25). Previous studies (26,27) have
indicated that the third generational passage cells of MSCs had a
strong proliferation capability and vital force, with the cell
survival rate reaching >90%. In the present study, these
conditions were also demonstrated by elaborative observation under
an inverted microscope during the whole process of MSC culture
(Fig. 1A). Therefore, the third
generational passage cells of donor MSCs were synchronously infused
into recipients during the OLT. Compared with the other groups,
synchronously infused MSCs combined with postoperative
immunosuppressant therapy were shown to reduce the dose of
postoperative immunosuppressant application, relieve acute immune
rejection and induce immune tolerance, through measuring hepatic
function, observing hepatic pathological changes, detecting immune
cytokines and observing the survival times of the recipients. These
effects may be due to a number of reasons. Firstly, MSCs may
immunoregulate the functions of host T cells (28–30) by
direct regulation (31,32), where the MSCs change the immune
function through direct contact between cells to regulate the ratio
changes of T cell subsets, or indirect regulation (33–35),
where MSCs may inhibit the growth and activation of T cells to
cause relative changes to the T cell subsets in order to induce a
lower T cell response, through the secretion of immune cytokines,
including TGF-β1, hepatocyte growth factor, and
metabolic products, such as prostaglandin E2 or
indole-2,3-dioxygenase. Beyth et al (9) indicated that MSCs affected the
maturation of normal APCs indirectly to cause T cells to become
unresponsive, through the secretion of IL-10 and the inhibition of
monocyte IL-12 secretion, ultimately inhibiting immune rejection.
In the current study, strong positive expression of the immune
cytokines, TGF-β1 and IL-10, was observed in group C,
and expression was more evident compared with group B. Expression
in group A was weak positive. In addition, the expression of IL-12
in groups A, B and C was observed to be medium positive, weak
positive and negative, respectively. MSCs are known to increase the
secretion of TGF-β1 and IL-10 (36) and inhibit the secretion of IL-12
following OLT, which is in accordance with the aforementioned
conclusions (37). An additional
possible mechanism by which MSCs may exert their effects is that
MSCs may inhibit the formation of cytotoxic lymphocytes and reduce
the activation of NK cells (38).
Finally, MSCs may depress the differentiation and maturation of
dendritic cells and interfere with the function of endocytosis;
MSCs may also upregulate CD8+CD28−T cells to
inhibit immune rejection (39).
FK506 is an immunosuppressant and MSCs have an inhibitory immune
rejection effect. Consequently, in the present study, the survival
times of the recipients in group C were significantly longer
compared with group A (P<0.01) and group B (P<0.01), with a
median survival time of >63 days. On day 7 after the OLT, the
levels of AST, ALT and TBIL in group C were significantly lower
compared with group A (P<0.01) and group B (P<0.01; Table I). With regard to histopathological
examination, group A exhibited severe immune rejection, while the
reaction in group C appeared significantly milder when compared
with groups A and B. Thus, synchronous infusion of MSCs in a rat
model of OLT was shown to reduce the dose of postoperative
immunosuppressants applied, improving the long-term immune
tolerance.
MSCs are hypothesized to inhibit immune rejection
primarily through direct contact between cells and soluble immune
cytokines by paracrine regulation. The pathway is very significant
in alleviating transplant immune rejection (40–43).
Thereby, it was necessary to determine the homing ability of MSCs
to the graft liver in group C. Currently, there are a variety of
methods to determine the homing ability. In the present study, Y
chromosome location was selected (donors and recipients were
female, while donors for MSC extraction were male). MSCs have a
powerful homing ability and the liver is the main homing organ for
transplantation cells, with 29–45% of transplantation cells
locating in the liver (20,21). In the present study, MSCs were
infused (1×106/200 g) into the recipients via the portal
vein immediately after anastomosing the bile duct. Through SRY
in situ hybridization, positive cells with areas of the
cytoplasm and nucleus stained brown were observed on day 7 after
the OLT, primarily on the portal area (Fig. 1C). These results suggested that, to a
certain degree, the homing ability and immunosuppressive effects of
MSCs were associated with MSC application.
In conclusion, MSCs may contribute to reducing the
postoperative immunosuppressant dose by inhibiting immune rejection
and inducing immune tolerance. In addition, MSCs have a wide range
of sources and are easily isolated in culture; thus, MSC therapy
has good potential for reducing the severe toxicity and side
effects of immunosuppressant drugs, suppressing immune rejection
and inducing immune tolerance following clinical liver
transplantations in the near future. However, the detailed
mechanisms require further research.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (no. 30760239) and the Science
& Technology Innovation Fund of Xinjiang Medical University
(no. XJC201267).
References
|
1
|
Starzl TE, Marchioro TL, Faris TD, Carey
TA and Otte JB: The therapeutic potential of whole organ
transplantation. J Am Med Womens Assoc. 21:207–209. 1966.PubMed/NCBI
|
|
2
|
Bozbas SS, Eyuboglu FO, Ozturk Ergur F,
Gullu Arslan N, Sevmis S, Karakayali H, et al: Pulmonary
complications and mortality after liver transplant. Exp Clin
Transplant. 6:264–270. 2008.PubMed/NCBI
|
|
3
|
Tanaka T, Takatsuki M, Soyama A, Torashima
Y, Kinoshita A, Yamaguchi I, et al: Evaluation of immune function
under conversion from Prograf to Advagraf in living donor liver
transplantation. Ann Transplant. 18:293–298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bogdanos DP, Gao B and Gershwin ME: Liver
immunology. Compr Physiol. 3:567–598. 2013.PubMed/NCBI
|
|
5
|
Gerlach UA, Vogt K, Schlickeiser S, Meisel
C, Streitz M, Kunkel D, et al: Elevation of CD4+
differentiated memory T cells is associated with acute cellular and
antibody-mediated rejection after liver transplantation.
Transplantation. 95:1512–1520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, et al: Human bone marrow
stromal cells suppress T-lymphocyte proliferation induced by
cellular or nonspecific mitogeni stimuli. Blood. 99:3838–3843.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rasmusson I, Ringdén O, Sundberg B and Le
Blanc K: Mesenchymal stem cells inhibit the formation of cytotoxic
T lymphocytes, but not activated cytotoxic T lymphocytes or natural
killer cells. Transplantation. 76:1208–1213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le Blanc K, Rasmusson I, Götherström C,
Seidel C, Sundberg B, Sundin M, et al: Mesenchymal stem cells
inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on
phytohaemagglutinin-activated lymphocytes. Scand J Immunol.
60:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beyth S, Borovsky Z, Mevorach D,
Liebergall M, Gazit Z, Aslan H, et al: Human mesenchymal stem cells
alter antigen-presenting cell maturation and induce T-cell
unresponsiveness. Blood. 105:2214–2219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wood KJ, Bushell A and Hester J:
Regulatory immune cells in transplantation. Nat Rev Immunol.
12:417–430. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
English K, Ryan JM, Tobin L, Murphy MJ,
Barry FP and Mahon BP: Cell contact, prostaglandin E(2) and
transforming growth factor beta 1 play non-redundant roles in human
mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+
regulatory T cells. Clin Exp Immunol. 156:149–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kordelas L, Rebmann V, Ludwig AK, Radtke
S, Ruesing J, Doeppner TR, et al: MSC-derived exosomes: a novel
tool to treat therapy-refractory graft-versus-host disease.
Leukemia. 28:970–973. 2014.PubMed/NCBI
|
|
13
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, et al: Multilineage potential of adult
human mesenchymal stem cells. Science. 284:143–147. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minguell JJ, Erices A and Conget P:
Mesenchymal stem cells. Exp Biol Med (Maywood). 226:507–520.
2001.PubMed/NCBI
|
|
15
|
Kamada N and Calne RY: A surgical
experience with five hundred thirty liver transplants in the rat.
Surgery. 93:64–69. 1983.PubMed/NCBI
|
|
16
|
Wei F, Wang TZ, Zhang J, Yuan ZY, Tian HY,
Ni YJ, et al: Mesenchymal stem cells neither fully acquire the
electrophysiological properties of mature cardiomyocytes nor
promote ventricular arrhythmias in infarcted rats. Basic Res
Cardiol. 107:2742012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Williams JW, Peters TG, Vera SR, Britt LG,
van Voorst SJ and Haggitt RC: Biopsy-directed immunosuppression
following hepatic transplantation in man. Transplantation.
39:589–596. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kojc N, Zidar N, Vodopivec B and Gale N:
Expression of CD34, alpha-smooth muscle actin, and transforming
growth factor beta1 in squamous intraepithelial lesions and
squamous cell carcinoma of the larynx and hypopharynx. Hum Pathol.
36:16–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi T and Song SU: Immunomodulatory
properties of mesenchymal stem cells and their therapeutic
applications. Arch Pharm Res. 35:213–221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim YH, Wee YM, Choi MY, Lim DG, Kim SC
and Han DJ: Interleukin (IL)-10 induced by CD11b(+) cells and
IL-10-activated regulatory T cells play a role in immune modulation
of mesenchymal stem cells in rat islet allografts. Mol Med.
17:697–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong ZF, Huang XJ and Yin ZY:
Immunocharacteristics of bone marrow mesenchymal stem cell.
Zhonghua Gan Zang Bing Za Zhi. 17:53–58. 2009.(In Chinese).
PubMed/NCBI
|
|
22
|
Bartholomew A, Sturgeon C, Siatskas M,
Ferrer K, McIntosh K, Patil S, et al: Mesenchymal stem cells
suppress lymphocyte proliferation in vitro and prolong skin graft
survival in vivo. Exp Hematol. 30:42–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schatton T, Yang J, Chandraker A, Sayegh
MH and Frank MH: In vivo immunomodulatory function of
ABCB5+ dermal mesenchymal stem cells. Transplantation.
82:185–186. 2006. View Article : Google Scholar
|
|
24
|
Pan MX, Hou WL, Zhang QJ, Gong DH, Cheng
Y, Jian GD and Gao Y: Infusion of autologous mesenchymal stem cells
prolongs the survival of dogs receiving living donor liver
transplantation. Nan Fang Yi Ke Da Xue Xue Bao. 29:1783–1786.
2009.(In Chinese). PubMed/NCBI
|
|
25
|
Zhang LS, Liu QF, Huang K, Zhang Y, Fan ZP
and Huang SL: Mesenchymal stem cells for treatment of
steroid-resistant chronic graft-versus-host disease. Zhonghua Nei
Ke Za Zhi. 48:542–546. 2009.(In Chinese). PubMed/NCBI
|
|
26
|
Colter DC, Class R, DiGirolamo CM and
Prockop DJ: Rapid expansion of recycling stem cells in cultures of
plastic-adherent cells from human bone marrow. Proc Natl Acad Sci
USA. 97:3213–3218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang Y, Jahagirda BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al: Pluripotency of
mesenchymal stem cells derived from adult marrow. Nature.
418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ye Z, Wang Y, Xie HY and Zheng SS:
Immunosuppressive effects of rat mesenchymal stem cells:
involvement of CD4+CD25+ regulatory T cells.
Hepatobiliary Pancreat Dis Int. 7:608–614. 2008.PubMed/NCBI
|
|
29
|
Sekiya I, Larson BL, Smith JR, Pochampally
R, Cui JG and Prockop DJ: Expansion of human adult stem cells from
bone marrow stroma: conditions that maximize the yields of early
progenitors and evaluate their quality. Stem Cells. 20:530–541.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia X, Chen W, Ma T, Xu G, Liu H, Liang C,
et al: Mesenchymal stem cells administered after liver
transplantation prevent acute graft-versus-host disease in rats.
Liver Transpl. 18:696–706. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krampera M, Glennie S, Dyson J, Scott D,
Laylor R, Simpson E, et al: Bone marrow mesenchymal stem cells
inhibit the response of naive and memory antigen-specific T cells
to their cognate peptide. Blood. 101:3722–3729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Popp FC, Renner P, Eggenhofer E, Slowik P,
Geissler EK, Piso P, et al: Mesenchymal stem cells as
immunomodulators after liver transplantation. Liver Transpl.
15:1192–1198. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, et al: Human bone marrow
stromal cells suppress T-lymphocyte proliferation induced by
cellular or nonspecific mitogenic stimuli. Blood. 99:3838–3843.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tobin LM, Healy ME, English K and Mahon
BP: Human mesenchymal stem cells suppress donor CD4(+) T cell
proliferation and reduce pathology in a humanized mouse model of
acute graft-versus-host disease. Clin Exp Immunol. 172:333–348.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meisel R, Zibert A, Laryea M, Göbel U,
Däubener W and Dilloo D: Human bone marrow stromal cells inhibit
allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated
tryptophan degradation. Blood. 103:4619–4621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Niu J, Yue W, Song Y, Zhang Y, Qi X, Wang
Z, et al: Prevention of acute liver allograft rejection by
IL-10-engineered mesenchymal stem cells. Clin Exp Immunol.
176:473–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han J, Zhao J, Xu J and Wen Y: Mesenchymal
stem cells genetically modified by lentivirus-mediated
interleukin-12 inhibit malignant ascites in mice. Exp Ther Med.
8:1330–1334. 2014.PubMed/NCBI
|
|
38
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y,
Yu XD and Mao N: Human mesenchymal stem cells inhibit
differentiation and function of monocyte-derived dendritic cells.
Blood. 105:4120–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pan GZ, Yang Y, Zhang J, Liu W, Wang GY,
Zhang YC, et al: Bone marrow mesenchymal stem cells ameliorate
hepatic ischemia/reperfusion injuries via inactivation of the
MEK/ERK signaling pathway in rats. J Surg Res. 178:935–948. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuroda Y, Kitada M, Wakao S and Dezawa M:
Bone marrow mesenchymal cells: how do they contribute to tissue
repair and are they really stem cells. Arch Immunol Ther Exp
(Warsz). 59:369–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pournasr B, Mohamadnejad M, Bagheri M,
Aghdami N, Shahsavani M, Malekzadeh R and Baharvand H: In vitro
differentiation of human bone marrow mesenchymal stem cells into
hepatocyte-like cells. Arch Iran Med. 14:244–249. 2011.PubMed/NCBI
|
|
43
|
Secchiero P, Corallini F, Zavan B, Tripodo
C, Vindigni V and Zauli G: Mesenchymal stem cells display
hepato-protective activity in lymphoma bearing xenografts. Invest
New Drugs. 30:803–807. 2012. View Article : Google Scholar : PubMed/NCBI
|