Introduction
With improvements in living standards, colorectal
cancer has become one of the most common malignant tumors
worldwide. In China the morbidity of rectal cancer is 24
individuals per hundred thousand and it rose by 4% in the past
decade. It also ranks third amongst the other cancers in morbidity.
The incidence rate of rectal cancer, and particularly low rectal
cancer, has increased, accounting for 60–75% (1). Abdominoperineal resection (APR) has
remained the standard surgical procedure for the treatment of low
rectal cancer (2). However, certain
patients are unable to undergo APR treatment due to their inability
to tolerate the lower quality of life caused by the permanent anal
rechanneling following the procedure. Through carrying out
preoperative neoadjuvant chemotherapy and with the development of
surgical techniques, it is now possible to perform
sphincter-preserving resection (SPR) on cases of low and ultra-low
rectal cancer (3,4). SPR can improve postoperative quality of
life, and there is no difference compared with APR treatment in
terms of the degree of radical surgery required (5). Therefore, to a certain extent, SPR has
replaced APR in becoming the first choice treatment for cases of
low rectal cancer (6). However, the
decision with regard to the selection of APR or SPR surgery for
treatment remains controversial. Thus, the aim of the present study
was to investigate the associated factors of selecting SPR surgery
for the treatment of low rectal cancer. As it is known, the radical
resection treatment of high and midrectal cancers has been
standardized. Both of these methods are performed by receiving
sphincter preservation surgery, in which the former ones can be
treated by partial mesorectal excision and the later by total
mesorectal resection (7,8). However, for surgical therapy the low
rectal cancer remains controversial. In the past, some patients
receive APR as the standard surgical procedure (9) whereas others benefit from sphincter
preservation (2).
Materials and methods
Patients and clinicopathological
parameters
Between June 2006 and December 2009, a total of 330
patients, admitted to the Affiliated Tumor Hospital of Xinjiang
Medical University (Ürümqi, China) with
histopathologically-confirmed low rectal cancer, were enrolled in
the study. Rectal cancer with a distance of the tumor from the anal
verge (DTAV) of 3–7 cm was defined as low rectal cancer (10). All patients received radical surgery.
Clinicopathological features, including age, gender, ethnicity,
body mass index (BMI), history of diabetes, family medical history,
level of preoperative carcinoembryonic antigen (CEA), total
infiltrated circumference, DTAV, depth of invasion, tumor grade,
venous tumor embolism, growth type, tumor length and lymphatic
metastasis were fully reviewed. The tumor-node-metastasis (TNM)
stage was determined according to the American Joint Committee on
Cancer/International Union Against Cancer TNM staging system of
colorectal cancer (11). The
following criteria were used to exclude patients: Preoperative
adjuvant chemotherapy (18 cases), preoperative radiotherapy (8
cases), preoperative chemoradiotherapy (59 cases), confirmed
metastasis (63 cases) and rejection of APR treatment (13 cases).
The study was approved by the Medical Ethics Committee of the
Affiliated Tumor Hospital of Xinjiang Medical University (no.
W201324). Informed consent was obtained from all the patients prior
to their participation in the study.
Surgical pattern
Open surgery using the total mesorectal excision
(TME) (12) technique was
successfully performed on all 330 patients. Of these, 192 cases
(58.18%) received SPR and 138 cases (41.82%) underwent APR. All
postoperative incisal margins were pathologically confirmed as
negative.
Postoperative therapy
Radiotherapy was applied to all the patients with
rectal cancer of pT3N0M0 or
pT1–3N1–2Mx. The chemotherapy
scheme of FOLFOX6 was administered intravenously injected with
intravenously infused 130 mg/m2 oxaliplatin (L-OHP) that
lasted 3 h on the first day, an intravenously infused 300
mg/m2 calcium folinate (CF) injection, an intravenously
injected 400 mg/m2 5-FU injection and a 2,400
mg/m2 5-FU continuous intravenous infusion by a
micropump for 48 h, for 14 days per cycle and for 12 cycles in
total. For the recurrence therapy, the scheme of FOLFIRI was
performed at 2 weeks per therapeutic circle until the dose became
intolerable or invalid. This was performed on the first day with an
intravenously infused 350 mg/m2 Irinotecan (CPT-11)
injection, an intravenously infused 300 mg/m2 CF
injection, a 400 mg/m2 5-FU injection that was
intravenously injected and a 2,400 mg/m2 5-FU continuous
intravenous infusion by a micropump for 48 h. For the patients who
exhibited drug resistance, the chemotherapy scheme of XELOX was
used. This therapy consisted of the following on the first day: an
intravenously infused 130 mg/m2 L-OHP injection for 3 h,
1,000 mg/m2 capecitabine tablets, po., twice a day, from
the first to the fourth day, 3 weeks per cycle and for nine cycles
in total. These treatment criteria were according to the Ministry
of Health of China (13).
Follow-up assessments
All the patients enrolled in the study were
registered at the hospital and complete personal follow-up files of
the patients with explicit pathological diagnoses were established.
Following surgery, the patients were followed-up once every 3 weeks
within the first 6 months, once every 3 months for the subsequent
two years and once every 6 months thereafter until they succumbed
to the disease or their contact information was lost. Two follow-up
procedures were performed, namely outpatient or inpatient review
and a telephone follow-up, which included information regarding
postoperative chemotherapy, postoperative radiotherapy,
chemotherapy regimens, therapeutic course count, side effects,
recurrence and survival time. A digital examination was performed
each time. Other inspection methods carried out regularly included
computerized tomography scans (GE Discovery CT750 HD, GE Healthcare
Biosciences, Pittsburgh, PA, USA), magnetic resonance imaging
(1.5-T General Electric Medical Systems, Signa®, Milwaukee, USA),
electronic colonoscopy (PCF-200, Olympus®, Tokyo, Japan) and CEA
level measurements.
Statistical analysis
Using the SPR procedure as the dependent variable,
univariate analysis was performed with the χ2 test and
Fisher's exact test. Multivariate correlation analysis was carried
out with the logistic regression test. In addition, survival
analysis was performed using the log-rank test. All statistical
tests were conducted using SPSS 19.0 software (IBM SPSS, Armonk,
NY, USA). P≤0.05 was considered to indicate a statistically
significant difference.
Results
SPR procedure
Of the 192 patients who received SPR surgery, 16
cases underwent a unilateral inguinal lymphadenectomy due to
visible lymphadenectasis and nine cases experienced a bilateral
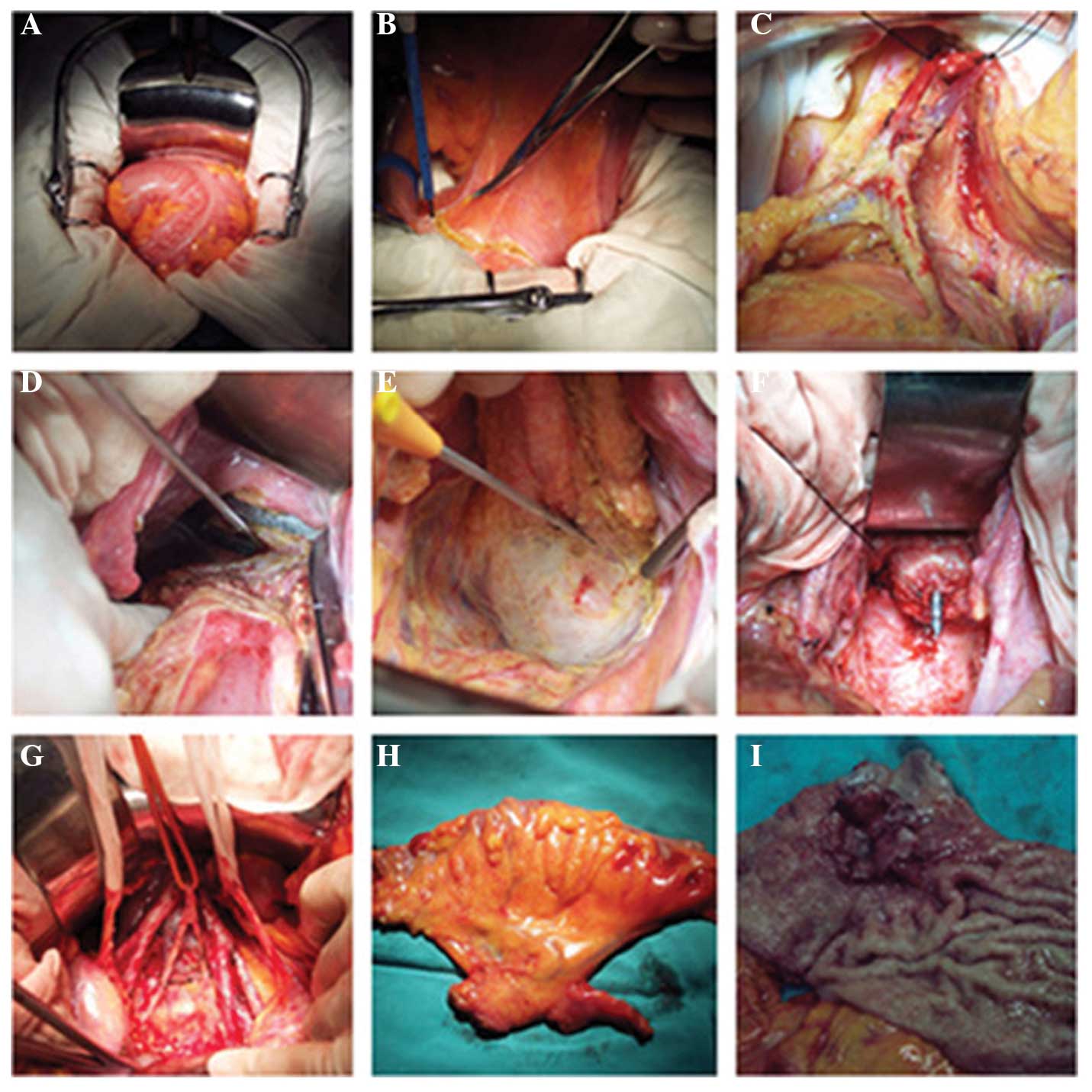
inguinal lymphadenectomy. A number of the key steps involved in the
SPR surgery are shown in Fig. 1.
Local recurrence results
Of the 330 patients, 192 cases (58.18%) received an
SPR and 138 cases (41.82%) underwent APR surgery. In the three-year
follow-up period, the total local recurrence rate in the pelvic
cavity was 4.24%. The local recurrence rate of the APR group was
3.62% (5/138), while the rate was 4.69% (9/192) in the SP group,
with no statistically significant difference (P>0.05; Table I).
 | Table I.Three-year local recurrence rate in
the APR and SP groups. |
Table I.
Three-year local recurrence rate in
the APR and SP groups.
| Group | Cases, n | Local recurrence, n
(%) | χ2 | P-value |
|---|
| SP | 192 | 9 (4.69) | 0.224 | 0.636 |
| APR | 138 | 5 (3.62) | – | – |
Univariate correlation analysis for
SPR surgery with associated clinicopathological features
For patients with low rectal cancer, the univariate
analysis results revealed that the sphincter-preserving factor was
associated with age, gender, ethnicity, BMI, total infiltrated
circumference, DTAV, depth of invasion and tumor grade, with
significant statistical difference (P<0.05). However, no
statistically significant associations were observed with the
family medical history, diabetes history, venous tumor embolism,
growth type, tumor length, lymphatic metastasis and preoperative
CEA level (P>0.05; Table
II).
 | Table II.Univariate analysis results of SP with
associated clinicopathological features for patients with low
rectal cancer. |
Table II.
Univariate analysis results of SP with
associated clinicopathological features for patients with low
rectal cancer.
| Clinicopathological
features | Cases, n | SP group, n
(%) | APR group, n
(%) | χ2 | P-value |
|---|
| Gender |
|
|
|
| 0.003 |
|
Male | 168 | 88
(52.38) | 80 (47.62) | 4.733 |
|
|
Female | 162 | 104 (64.20) | 58 (35.80) |
|
|
| Age, years |
|
|
|
| 0.002 |
|
≤40 | 34 | 10
(29.41) | 24 (70.59) | 13.004 |
|
|
41–60 | 134 | 81
(60.45) | 53 (39.55) |
|
|
|
≥61 | 162 | 101 (62.34) | 61 (37.66) |
|
|
| Tumor length,
cm |
|
|
|
| 0.317 |
|
<4 | 146 | 84
(57.53) | 62 (42.47) | 2.298 |
|
|
4.0–5.0 | 132 | 82
(62.12) | 50 (37.88) |
|
|
|
>5.0 | 52 | 26
(50.00) | 26 (50.00) |
|
|
| Ethnicity |
|
|
|
| 0.011 |
|
Han | 278 | 170 (61.15) | 108 (38.85) | 6.393 |
|
|
Uyghur | 52 | 22
(43.31) | 30 (46.69) |
|
|
| Growth type |
|
|
|
| 0.290 |
|
Ulcerative | 191 | 108 (56.54) | 83 (43.46) | 2.476 |
|
|
Mass | 125 | 78
(62.40) | 47 (37.60) |
|
|
|
Infiltrating | 14 | 6
(42.86) | 8 (57.14) |
|
|
| Tumor grade |
|
|
|
| <0.001 |
|
Well | 38 | 25
(65.79) | 13 (34.21) | 16.198 |
|
|
Moderate | 182 | 120 (65.93) | 62 (34.07) |
|
|
|
Poorly/anaplastic | 110 | 47
(42.73) | 63 (57.27) |
|
|
| Lymphatic
metastasis |
|
|
|
| 0.458 |
| N0 | 204 | 124 (60.78) | 80 (39.22) | 1.560 |
|
| N1 | 81 | 43
(55.56) | 38 (44.44) |
|
|
| N2 | 45 | 25
(51.85) | 20 (48.15) |
|
|
| Depth of
invasion |
|
|
|
| 0.001 |
|
T1/T2 | 84 | 59
(70.24) | 25 (29.76) | 14.645 |
|
| T3 | 126 | 79
(62.70) | 47 (37.30) |
|
|
| T4 | 120 | 54
(45.00) | 66 (55.00) |
|
|
| DTAV, cm |
|
|
|
| <0.001 |
|
3-<5 | 124 | 11 (8.87) | 113 (91.13) | 198.518 |
|
|
5–7 | 206 | 181 (87.86) | 25 (12.14) |
|
|
| Total infiltrated
circumference (cycle) |
|
|
|
| <0.001 |
|
<1/2 | 115 | 80
(69.57) | 35 (30.43) | 17.878 |
|
|
1/2-<3/4 | 124 | 75
(60.48) | 49 (39.52) |
|
|
|
≥3/4 | 91 | 37
(40.66) | 54 (59.34) |
|
|
| Preoperative CEA,
µg |
|
|
|
| 0.891 |
|
<5 | 219 | 128 (58.45) | 91 (41.55) | 0.019 |
|
| ≥5 | 111 | 64
(57.66) | 47 (42.34) |
|
|
| Venous tumor
embolus |
|
|
|
| 0.746 |
| No | 317 | 185 (58.35) | 132 (41.65) | 0.105 |
|
|
Yes | 13 | 7
(53.84) | 6 (46.16) |
|
|
| Diabetes
history |
|
|
|
|
|
| No | 286 | 167 (58.39) | 119 (41.61) | 0.039 | 0.844 |
|
Yes | 44 | 25
(56.82) | 19 (43.18) |
|
|
| BMI |
|
|
|
|
|
|
<25 | 181 | 115 (63.54) | 66 (36.46) | 4.723 | 0.030 |
|
≥25 | 149 | 77
(51.68) | 72 (48.32) |
|
|
| Tumor family
history |
|
|
|
| 0.727 |
| No | 266 | 156 (58.25) | 110 (41.75) | 0.122 |
|
|
Yes | 64 | 36
(56.25) | 28 (43.75) |
|
|
Multivariate correlation analysis for
SPR surgery with associated clinicopathological features
Multivariate correlation analysis indicated that the
sphincter-preserving factor was closely associated with DTAV and
the depth of invasion, with significant statistical difference
(P<0.05). Consequently, DTAV and the depth of invasion were
determined to be independent risk factors for SPR (Table III).
 | Table III.Multivariate correlation analysis of
SPR surgery with associated clinicopathological features for
patients with low rectal cancer. |
Table III.
Multivariate correlation analysis of
SPR surgery with associated clinicopathological features for
patients with low rectal cancer.
| Variable | β-value | SE | Wald value | P-value | OR | 95% CI |
|---|
| DTAV | 4.714 | 0.473 | 99.526 | <0.001 | 111.539 | 44.176–281.625 |
| Depth of
invasiona |
|
|
|
|
|
|
| T2
X13 |
|
| 11.234 | 0.004 |
|
|
| T3 X13
(1) | 0.892 | 0.432 |
4.271 | 0.039 | 2.441 | 1.047–5.689 |
| T4 X13
(2) | 1.900 | 0.582 | 10.669 | 0.001 | 6.686 | 2.138–20.910 |
Discussion
The TME technique proposed by Heald in 1982
(14) has been regarded as the gold
standard for the treatment of rectal cancer. The TME technique
significantly decreases the local postoperative recurrence rate of
rectal cancer (15). In certain
cases, selecting the surgical method is difficult due to the
specific tumor location of low rectal cancer. Retaining anal
function following radical surgery, local recurrence rate control
and improving postoperative quality of life have caused the
selection of the appropriate surgical method for the treatment of
low rectal cancer to be increasingly studied (16). The local recurrence rate is an
important index for evaluating the efficacy of the SPR outcome for
low rectal cancer, and this has been extensively studied. In
previous studies, Peeters et al reported a local recurrence
rate of ~10% (17), while You et
al reported a local recurrence rate of 6.9% (18), and in the study by Sun and Wang, a
local recurrence rate of 6.71% was determined (19). In the present study, the local
recurrence rate in the pelvic cavity of patients with low rectal
cancer was 4.24%, which was 3.62 and 4.69% in the APR and SP
groups, respectively, with no statistically significant difference
(P>0.05). Thus, the SPR surgical method did not increase the
local recurrence rate of low rectal cancer. This conclusion is
similar to the majority of previous studies (20,21).
Consequently, the selection of SPR surgery for the treatment of low
rectal cancer primarily depends on the accurate preoperative
assessment of the clinicopathological features of the patient,
which are beneficial to design a more reasonable surgical
scheme.
DTAV is regarded as the most important factor for
the determination of anal preserving surgery methods by the
majority of researchers (22,23).
Colorectal cancer has the particular biological characteristic of
upwards growth along the intestinal wall (24). However, the downward growth is
generally within 2.0 cm and growth of >2.0 cm presents in only
3% of cases (25,26). A consensus was recently reached that
a distal normal bowel resection of >2.0 cm was sufficient for
treatment (27). According to the US
National Comprehensive Cancer Network guidelines (revised in 2005),
resecting a 1–2-cm section of the distal rectal cancer in cases
where the DTAV is <5 cm is feasible. Based on the
characteristics of colorectal cancer anatomy, disconnecting the
ligament can extend the intestinal canal by 3–5 cm (28), which greatly increases the success
rate of low anastomosis procedures. At present, according to
clinical experience and literature reports (22,29,30), a
DTAV of 5 cm is the boundary for the selection of SPR surgery
treatment. Rectal cancer with a DTAV of 5–7 cm has a relatively
high rate of sphincter preservation since the intestinal tube is
sufficient in length for convenient anastomosis. In the present
study, a DTAV of 3–7 cm was selected, since a DTAV of <3 cm can
markedly decrease the effect of radical surgery. Rectal cancer with
a DTAV of >5 cm was shown to have a higher rate of sphincter
preservation (87.86%) compared with cases where the DTAV was 3–5 cm
(8.87%), and the difference was statistically significant
(P<0.05). Furthermore, multivariate analysis revealed that the
DTAV is an independent risk factor for treatment with SPR surgery
in patients with rectal cancer.
A consensus has not been reached on whether the
depth of invasion is an independent risk factor for SPR surgery in
patients with low rectal cancer. However, the majority of studies
(22,29,31,32)
support the hypothesis that the depth of invasion is an independent
risk factor. The studies report that the rectum below the
peritoneum has no serosa layer covered. Rectal tumors are able to
infiltrate into the tissue outside the rectum and pelvis, which
increases the difficulty of SPR surgery and increases the local
recurrence rate (33,34). In the present study, univariate and
multivariate analyses indicated that the rate of sphincter
preservation was associated with the depth of invasion, and
statistically significant differences were observed (P<0.05).
Thus, the deeper the rectal tumor infiltrate, the lower the rate of
sphincter preservation. Furthermore, univariate analysis results
revealed that the sphincter-preserving factor was strongly
associated with the total infiltrated circumference, which was
similar to the results of a study by Cong (22). According to the growth
characteristics of the malignant tumor, the longer the growth cycle
and the wider the tumor infiltrates, the more difficult the surgery
becomes. When rectal cancer infiltration reaches close to a
complete cycle of the rectum, the depth of invasion is primarily in
the T3 or T4 stage, and the adjacent tissue of the intestinal tube
may be infiltrated. Consequently, SPR surgery becomes too difficult
to be implemented.
Xinjiang Uygur Autonomous Region in the northwest of
China comprises numerous ethnic groups that have different diets,
living habits and plateau environments. Therefore, ethnicity was an
important research parameter in the current study. However, the
results of the present study demonstrated that SPR surgery success
was not associated with ethnicity. Liu et al (35) reported that the rates of obesity in
the Kazak and Uygur ethnic populations were 40.1 and 28.9%,
respectively, which were markedly higher compared with the Han
ethnicity (18.4%). The increase in surgical difficulty as a result
of obesity may decrease the rate of sphincter preservation in
patients from the Uygur ethnicity (36,37).
Furthermore, on an economical and cognitive level, rectal cancer in
Uygur populations is generally diagnosed much later in the disease
stage, resulting in a larger tumor size and deeper invasion, which
greatly influences the rate of sphincter preservation (38–40). In
the present study, univariate analysis demonstrated that the
sphincter-preserving factor was significantly associated with
gender, BMI, age and tumor grade. The reasons for this may be as
follows: i) The male pelvis is narrower and smaller, which
increases the difficulty of the surgery for patients with rectal
cancer; ii) young patients tend to be diagnosed at a later stage of
the disease; thus, there is more extensive invasion of adjacent
tissue; iii) obesity increases the surgical difficulty,
particularly with regard to sphincter preservation; and iv) a low
differentiated rectal tumor may lead to deeper tumor invasion
(41,42).
In conclusion, there are numerous risk factors with
regard to sphincter preservation for patients with low rectal
cancer. The sphincter-preserving factor was demonstrated to be
associated with certain clinicopathological features, including
DTAV and the depth of invasion. Therefore, careful preoperative
evaluation of the associated risk factors may be beneficial for
selecting the precise surgical pattern (SPR or APR) and ensuring an
accurate surgical procedure, which may subsequently enhance the
rate of sphincter preservation and improve the quality of life of
patients with low rectal cancer.
Acknowledgements
The authors thank the patients for their
participation in the study. The study was supported by grants from
the National 11th Five-Year Science & Technology
Support Program of China (no. 2006BAI02A06) and the Science &
Technology Innovation Fund of Xinjiang Medical University (no.
XJC201267).
References
|
1
|
Wu ZD and Wu JH: Surgery. 7th. People's
Medical Publishing House; Beijing, China: pp. 4922008, PubMed/NCBI
|
|
2
|
Mauvais F, Sabbagh C, Brehant O, et al:
The current abdominoperineal resection: oncological problems and
surgical modifications for low rectal cancer. J Visc Surg.
148:e85–e93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rullier E, Denost Q, Vendrely V, Rullier A
and Laurent C: Low rectal cancer: classification and
standardization of surgery. Dis Colon Rectum. 56:560–567. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Richardson DP, Porter GA and Johnson PM:
Population-based use of sphincter-preserving surgery in patients
with rectal cancer: is there room for improvement. Dis Colon
Rectum. 56:704–710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huh JW, Jung EJ, Park YA, et al:
Sphincter-preserving operations following preoperative
chemoradiation: an alternative to abdominoperineal resection for
lower rectal cancer. World J Surg. 32:1116–1123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mir SA, Chowdri NA, Parray FQ, et al:
Sphincter-saving surgeries for rectal cancer: A single center study
from Kashmir. South Asian J Cancer. 2:227–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen WQ, Zheng RS, Zhang SW, Zeng HM and
Zou XN: The incidences and mortalities of major cancers in China,
2010. Chin J Cancer. 33:402–405. 2014.PubMed/NCBI
|
|
8
|
Lopez-Kostner F, Lavery IC, Hool GR,
Rybicki LA and Fazio VW: Total mesorectal excision is not necessary
for cancers of the upper rectum. Surgery. 124:612–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heald RJ, Moran BJ, Ryall RD, Sexton R and
MacFarlane JK: Rectal cancer: the Basingstoke experience of total
mesorectal excision, 1978–1997. Arch Surg. 133:894–899. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siani LM, Ferranti F, Benedetti M, et al:
Laparoscopic versus open total mesorectal excision for stage I-III
mid and low rectal cancer: a retrospective 5 years analysis. G
Chir. 33:404–408. 2012.PubMed/NCBI
|
|
11
|
American Joint Committee on Cancer (AJCC),
. TNM staging of colorectal cancer. 7th. Springer; New York, USA:
pp. 173–206. 2010
|
|
12
|
Atallah S, Albert M, DeBeche-Adams T,
Nassif G, Polavarapu H and Larach S: Transanal minimally invasive
surgery for total mesorectal excision (TAMIS-TME): a stepwise
description of the surgical technique with video demonstration.
Tech Coloproctol. 17:321–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ministry of Health of the People's
Republic of China, . Chinese Standard for The Management of
Colorectal Cancer. 2010. Beijing, China: pp. 23–24, (In
Chinese).
|
|
14
|
Heald RJ, Husband EM and Ryall RD: The
mesorectum in rectal cancer surgery-the clue to pelvic recurrence.
Br J Surg. 69:613–616. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maurer CA, Renzulli P, Kull C, et al: The
impact of the introduction of total mesorectal excision on local
recurrence rate and survival in rectal cancer: long-term results.
Ann Surg Oncol. 18:1899–1906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anderin C, Granath F, Martling A, et al:
Local recurrence after prone vs supine abdominoperineal excision
for low rectal cancer. Colorectal Dis. 15:812–815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peeters KC, Tollenaar RA, Marijnen CA, et
al: Risk factors for anastomotic failure after total mesorectal
excision of rectal cancer. Br J Surg. 92:211–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
You YN, Baxter NN, Stewart A, et al: Is
the increasing rate of local excision for stage I rectal cancer in
the United States justified?: a nationwide cohort study from the
National Cancer Database. Ann Surg. 245:726–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun HY and Wang KK: Application experience
of total mesorectum resection. Chin J Gen Pract. 7:1075–1076.
2009.
|
|
20
|
Nash GM, Weiss A, Dasgupta R, Gonen M,
Guillem JG and Wong WD: Close distal margin and rectal cancer
recurrence after sphincter-preserving rectal resection. Dis Colon
Rectum. 53:1365–1373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JC, Yu CS, Lim SB, Kim CW, Kim JH and
Kim TW: Abdominoperineal resection and low anterior resection:
comparison of long-term oncologic outcome in matched patients with
lower rectal cancer. Int J Colorectal Dis. 28:493–501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cong ZJ, Hu LH, Xing JJ, Zhang W, Fu CG,
Yu ED and Zhong M: Risk factors associated with
sphincter-preserving resection in patients with low rectal cancer.
Int Surg. 99:330–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin ST, Heneghan HM and Winter DC:
Systematic review of outcomes after intersphincteric resection for
low rectal cancer. Br J Surg. 99:603–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keum MA, Lim SB, Kim SA, et al:
Clinicopathologic factors affecting recurrence after curative
surgery for stage I colorectal cancer. J Korean Soc Coloproctol.
28:49–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du JY and Zhang Y: Clinical analysis of 45
cases receiving sphincter-preserving operation of low rectal
cancer. Chongqing Yi Yao. 39:589–590. 2010.(In Chinese).
|
|
26
|
Williams NS, Dixon MF and Johnston D:
Reappraisal of the 5 centimetre rule of distal excision for
carcinoma of the rectum: a study of distal intramural spread and of
patients' survival. Br J Surg. 70:150–154. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seo SI, Yu CS, Kim GS, et al:
Characteristics and risk factors associated with permanent stomas
after sphincter-saving resection for rectal cancer. World J Surg.
37:2490–2496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Açar Hİ and Kuzu MA: Perineal and pelvic
anatomy of extralevator abdominoperineal excision for rectal
cancer: cadaveric dissection. Dis Colon Rectum. 54:1179–1183. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Temple LK, Romanus D, Niland J, Veer AT,
Weiser MR, Skibber J, Wilson J, Raiput A, Benson A, Wong YN and
Schrag D: Factors associated with sphincter-preserving surgery for
rectal cancer at national comprehensive cancer network centers. Ann
Surg. 250:260–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bordeianou L, Maguire LH, Alavi K, et al:
Sphincter-sparing surgery in patients with low-lying rectal cancer:
techniques, oncologic outcomes, and functional results. J
Gastrointest Surg. 18:1358–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orsenigo E, Di Palo S, Vignali A and
Staudacher C: Laparoscopic intersphincteric resection for low
rectal cancer. Surg Oncol. 16 (Suppl 1):S117–S120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chuwa EW and Seow-Choen F: Outcomes for
abdominoperineal resections are not worse than those of anterior
resections. Dis Colon Rectum. 49:41–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bugg WG, Andreou AK, Biswas D, et al: The
prognostic significance of MRI-detected extramural venous invasion
in rectal carcinoma. Clin Radiol. 69:619–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhangu A, Fitzgerald JE, Slesser A, et al:
Prognostic significance of extramural vascular invasion in T4
rectal cancer. Colorectal Dis. 15:e665–e671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu C, Ma X, Ma YT, et al: Prevalence on
overweight and obesity in Han, Uygur and Hazakh in adults from
Xinjiang. Zhonghua Liu Xing Bing Xue Za Zhi. 31:1139–1143. 2010.(In
Chinese). PubMed/NCBI
|
|
36
|
Chern H, Chou J, Donkor C, et al: Effects
of obesity in rectal cancer surgery. J Am Coll Surg. 211:55–60.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aytac E, Lavery IC, Kalady MF and Kiran
RP: Impact of obesity on operation performed, complications and
long-term outcomes in terms of restoration of intestinal continuity
for patients with mid and low rectal cancer. Dis Colon Rectum.
56:689–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu HM, Li XJ, Wang L, et al: Analysis of
clinic pathological characteristics and prognosis between Uyghur
and Han people with rectal cancer. Chongqing Yi Xue. 44:478–481.
2015.
|
|
39
|
Sun ZQ, Wang HJ, Zhao ZL, et al:
Significance of HPV Infection and Genic Mutation of APC and K-ras
in Patients with Rectal Cancer. Asian Pacific J Cancer Prev.
14:121–126. 2013. View Article : Google Scholar
|
|
40
|
Yusup A, Wang HJ, Rahmutula A, et al:
Clinical features and prognosis in colorectal cancer patients with
different ethnicities in Northwest China. World J Gastroenterol.
19:7183–7188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen ZH, Song XM, Chen SC, et al: Risk
factors for adverse outcome in low rectal cancer. World J
Gastroenterol. 18:64–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li SY, Yu B, Liang ZJ, et al: Clinical
study of 102 cases of abdominal-anus resection with telescopic
anastomosis of colon rectal mucosa for lower segment of rectal
cancer. Zhonghua Wai Ke Za Zhi. 41:812–814. 2003.(In Chinese).
PubMed/NCBI
|