Introduction
Patau syndrome, also known as trisomy 13, is the
third most frequently occurring autosomal trisomy among newborns,
with a prevalence of 1 in 4,000 live births and 1 in 29,000 total
births (1). The condition was first
described by Smith et al (2)
in 1960. The conventional method utilized to establish a prenatal
diagnosis of Patau syndrome involves cytogenetic analysis through
amniocentesis or chorionic villous sampling; however, this is
time-consuming (14–21 days), labor-intensive, requires skilled
analysis and is not suitable for the large-scale screening of all
pregnant females (3,4).
Short tandem repeat (STR) loci, also known as
microsatellites or simple sequence repeats, are extensively used in
mapping studies, forensics and disease diagnosis due to their small
dimension and low mutation and high polymorphism rates (5). The use of quantitative fluorescence
polymerase chain reaction (QF-PCR) for the amplification of STR
markers is, to date, the most developed method for the rapid and
high sensitivity and specificity screening of chromosomal
aneuploidies involving chromosomes 21, 18, 13, X and Y (3,4,6,7).
Trisomies are identified by detecting three doses of an STR. The
clinical utility of the QF-PCR assay in several prenatal centers of
Europe has been confirmed by its high sensitivity and specificity
within 24–48 h (3,4,6,7). One of the most evident advantages of
QF-PCR is the automation of the procedure, allowing a high
throughput of samples at an extremely low cost; however, the allele
frequency and heterozygosity of STR markers vary among different
populations. It is important to select STR markers and analyze
their genetic polymorphisms prior to using them in prenatal
diagnosis in order to minimize the percentage of uninformative
QF-PCR results and increase the diagnostic accuracy (8–10).
Although several studies have reported the
application of QF-PCR in the prenatal diagnosis of common
chromosomal aneuploidies, the majority of the markers used were
based on a Caucasian population and were not suitable for a Chinese
population (3,4,6–10). Furthermore, few studies have
investigated the polymorphisms of STR markers on chromosome 13
(4,7,10). The
present investigation was carried out to study the genetic
polymorphisms of three STR markers, D13S305, D13S631 and D13S634,
on chromosome 13 in the Han population of Tianjin, China. To the
best of our knowledge, no data for these STR markers are currently
available for this population.
Materials and methods
Samples
Samples from 350 unrelated individual (200 males and
150 females) from the Han population of Tianjin, China were
collected by the Laboratory of Medical Genetics of the Tianjin
Medical University General Hospital (Tianjin, China) between
September 2010 and August 2012. The parents and grandparents of the
subjects were also from the same population. Routine karyotyping
with standard G-binding was performed on all samples to exclude
chromosomal abnormalities and the results showed that the karyotype
of all samples was normal.
This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Tianjin Medical University General Hospital. Written informed
consent was obtained from all participants.
DNA extraction and PCR
amplification
Genomic DNA was extracted from the peripheral blood
samples using a Whole Blood Genomic DNA Isolation kit (Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China)
according to the manufacturer's instructions. The characteristics
of the STR markers D13S305, D13S631 and D13S634 are listed in
Table I (http://www.ncbi.nlm.nih.gov/unists). Each STR marker
was amplified individually. PCR was carried out in a total reaction
volume of 25 µl, containing 100 ng genomic DNA, 0.1 µmol/l each
primer, 1X PCR buffer (containing MgCl2), 0.8 µmol/l
deoxynucleotide triphosphate and 1 unit Taq polymerase (Beijing
Dingguo Changsheng Biotechnology Co., Ltd.). The amplification was
performed in a GeneAmp® PCR System 9600 (Perkin Elmer, Waltham, MA,
USA). The protocol for the PCR was as follows: Initial denaturation
at 95°C for 5 min and 35 cycles consisting of denaturation at 95°C
for 30 sec, annealing at 55°C (D13S305 and D13S634) or 56°C
(D13S631) for 30 sec and extension at 72°C for 30 sec. The final
extension step was 72°C for 10 min.
 | Table I.Characteristics of the short tandem
repeat markers D13S305, D13S631 and D13S634. |
Table I.
Characteristics of the short tandem
repeat markers D13S305, D13S631 and D13S634.
| Locus | Accession number | Primer sequence
(5′-3′) | Chromosome
location |
|---|
| D13S305 | L31091 |
f-FAM-TTGAGGACCTGTCGTTACG | 13q13.3 |
|
|
|
r-TTATAGAGCAGTTAAGGCACA |
|
| D13S631 | L30438 |
f-FAM-GGCAACAAGAGCAAAACTCT | 13q32.1 |
|
|
|
r-TAGCCCTCACCATGATTGG |
|
| D13S634 | L30363 |
f-FAM-TCCAGATAGGCAGATTCAAT | 13q14.3–13q22 |
|
|
|
r-CCTTCTTCTTCCCATTGATA |
|
Genotyping
A total of 1.5 µl PCR products, 8.5 µl deionized
formamide and a GeneScan™-500 ROX size standard (Applied
Biosystems, Foster City, CA, USA) were mixed and denatured at 94°C
for 2 min for capillary electrophoresis using an ABI 3730 sequencer
(Applied Biosystems). The results were analyzed using ABI Prism®
GeneMapper version 3.0 software (Applied Biosystems), and the
relative peak areas were automatically calculated by the software.
Two to three alleles of each locus was sequenced for confirmation
of repeat number using the ABI 3730 sequencer (Applied Biosystems).
The allele nomenclature followed the recommendations of the
International Society for Forensic Hemogenetics (11).
Statistical analysis
Allelic frequencies were calculated by direct
counting for males and females collectively. Each of the STR
markers was checked using the χ2 test to verify the
Hardy-Weinberg equilibrium (HWE). Genetic polymorphism data,
including expected heterozygosity, observed heterozygosity,
polymorphic information content (PIC), power of discrimination
(PD), power of exclusion (PE) and matching probability, were
calculated using the PowerStat V12 software (Promega Corp.,
Madison, WI, USA).
Results
Sample amplification and repeat
sequences
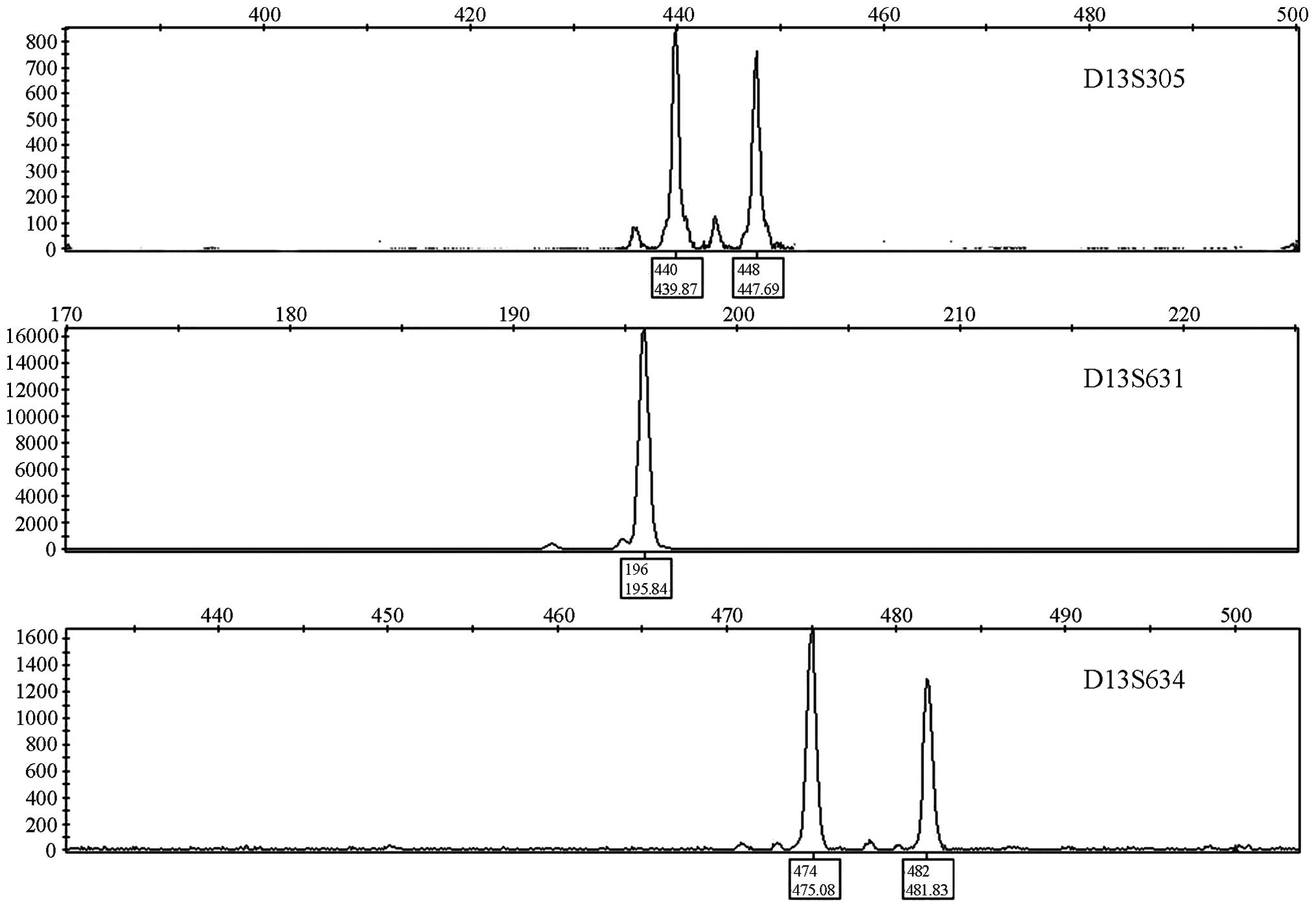
All samples were successfully amplified.
Heterozygotes showed two peaks with a ratio of 1:1 while
homozygotes showed only a single peak (Fig. 1). The D13S305, D13S631 and D13S634
STR markers were all tetranucleotide repeats, and the allele
nomenclature was based on the number of repeats. The repeat
sequences are shown in Table II.
Since no significant differences were found in the allele
frequencies between the males and females in the study, the data
were computed collectively.
 | Table II.Repeat sequences of the short tandem
repeat markers D13S305, D13S631 and D13S634. |
Table II.
Repeat sequences of the short tandem
repeat markers D13S305, D13S631 and D13S634.
| Locus | Repeat sequence |
|---|
| D13S305 | CTTT |
| D13S631 | ATCT |
| D13S634 | AAGA |
STR alleles
Eleven, seven and 11 alleles of the D13S305, D13S631
and D13S634 markers were observed, respectively. In total, 29
alleles were observed. Allele 8 was not found among the D13S634
markers analyzed. D13S305 was the most polymorphic marker with 11
different alleles, referred to as alleles 10–20, and higher genetic
polymorphism data compared to D13S634. D13S631 was the least
polymorphic marker with only seven alleles (referred to as alleles
9–15). Allele nomenclature, frequencies and fragment size of the
D13S305, D13S631 and D13S634 STR markers are shown in Table III. No significant deviations from
the HWE were observed in these STR markers (P>0.05) (Table IV).
 | Table III.Allele nomenclature, allele
frequencies and the fragment size of the D13S305, D13S631 and
D13S634 short tandem repeat markers. |
Table III.
Allele nomenclature, allele
frequencies and the fragment size of the D13S305, D13S631 and
D13S634 short tandem repeat markers.
|
| Locus |
|---|
|
|
|
|---|
| Allele | D13S305 (bp) | D13S631 (bp) | D13S634 (bp) |
|---|
| 5 |
|
| 0.0029 (454) |
| 6 |
|
| 0.0043 (458) |
| 7 |
|
| 0.0014 (462) |
| 8 |
|
|
|
| 9 |
| 0.0043 (188) | 0.0200 (470) |
| 10 | 0.0029 (416) | 0.0586 (192) | 0.2171 (474) |
| 11 | 0.0071 (420) | 0.3471 (196) | 0.0657 (478) |
| 12 | 0.0100 (424) | 0.2857 (200) | 0.2900 (482) |
| 13 | 0.0543 (428) | 0.1386 (204) | 0.2329 (486) |
| 14 | 0.1071 (432) | 0.1471 (208) | 0.1143 (490) |
| 15 | 0.1929 (436) | 0.0186 (212) | 0.0457 (494) |
| 16 | 0.2429 (440) |
| 0.0057 (498) |
| 17 | 0.1914 (444) |
|
|
| 18 | 0.1357 (448) |
|
|
| 19 | 0.0457 (452) |
|
|
| 20 | 0.0100 (456) |
|
|
 | Table IV.Genetic polymorphism data of the
D13S305, D13S631 and D13S634 short tandem repeat markers. |
Table IV.
Genetic polymorphism data of the
D13S305, D13S631 and D13S634 short tandem repeat markers.
|
| Locus |
|---|
|
|
|
|---|
| Statistic | D13S305 | D13S631 | D13S634 |
|---|
| He | 0.833 | 0.754 | 0.796 |
| Ho | 0.809 | 0.714 | 0.757 |
| PIC | 0.810 | 0.714 | 0.765 |
| PD | 0.948 | 0.893 | 0.927 |
| PE | 0.615 | 0.451 | 0.522 |
| MP | 0.052 | 0.107 | 0.073 |
| HWE (P-value) | 0.878 | 0.647 | 0.397 |
Genetic polymorphism data
Genetic polymorphism data for the D13S305, D13S631
and D13S634 STR markers are shown in Table IV. Heterozygosity for the three
markers ranged between 0.714 and 0.809 in this study. The results
showed that D13S305 was the most informative marker, with
heterozygosity at 0.809, while D13S631 was the least informative
marker, with heterozygosity at 0.714. The PIC values ranged between
0.714 (D13S631) and 0.810 (D13S305), the PD values ranged between
0.893 (D13S631) and 0.948 (D13S305) and the PE ranged between 0.451
(D13S631) and 0.615 (D13S305). The study showed that D13S305 was
the most polymorphic marker among these STR markers.
Discussion
The use of QF-PCR to amplify STR markers in order to
diagnose chromosomal aneuploidies was first applied in 1993
(12). As this approach has
developed, it has been widely used in numerous countries for >10
years and has proved to be an accurate, less labor-intensive and
robust prenatal test for chromosomal aneuploidies (3,4,6–10). One
of the most evident advantages of QF-PCR is the automation of the
procedure, allowing a high throughput of samples at a low cost. In
general, the use of three or four highly polymorphic STR markers
per chromosome allows a high diagnostic accuracy in the majority of
samples (13). Although the
incidence of Patau syndrome is less than that of trisomy 21 (Down
syndrome) and trisomy 18 (Edwards syndrome), there have been a
number of patients with trisomy 13 who have survived past the first
decade, even reaching 146 months (14). Furthermore, few papers have reported
the application of the QF-PCR method in detecting trisomy 13 or
investigated the polymorphisms of STR markers on chromosome 13. The
aim of the present study was to select STR markers on chromosome 13
that were highly polymorphic for the Chinese population and to
optimize the PCR system in order to design a novel, multiplex PCR
assay to rapidly diagnose chromosomal abnormalities.
Different laboratories applying QF-PCR for prenatal
diagnosis typically use individual markers or commercial rapid
aneuploidy detection kits (15);
however, the majority of these markers have been based on the
Caucasian population and are not suitable for the Chinese
population, such as D13S742 and D13S258. In our preliminary
experiment it was found that the marker D13S742 was not polymorphic
in the Han population of Tianjin, China, and that the
heterozygosity was only 0.475. Furthermore, the marker D13S258
failed to amplify. In addition to the level of heterozygosity, the
characteristics of the repeats were taken into consideration in the
selection of the STR markers. Tetranucleotide repeats were
preferred due to the occurrence of shutters and the mutation rate
being low (16). The location of the
STR markers on the target chromosome is also crucial. In the
present study, three STR markers distributed along the long arm of
chromosome 13 were selected; these exhibited no overlap, which
could have increased the possibility of detecting partial monosomy
or trisomy (17). These STR markers,
D13S305, D13S631 and D13S634, were selected from a total of eight
STR markers, D13S631, D13S634, D13S258, D13S303, D13S256, D13S628,
D13S742 and D13S305, based on their extensive use in the literature
(4,7,10,15,17–19),
data on their repeat number, heterozygosity and genetic
polymorphisms and our preliminary experiment, and were shown to be
highly polymorphic in the Han population of Tianjin, China.
Previous studies have also analyzed the polymorphism
of these three STR markers and used them in prenatal diagnosis.
Cirigliano et al (4) found
nine, eight and 12 alleles of the D13S305, D13S631 and D13S634
markers in a Spanish and Italian population. The heterozygosity was
0.75, 0.78 and 0.85, respectively. The authors then performed
QF-PCR on 43,000 clinical samples and found 127 cases of trisomy
13, for which the QF-PCR results were consistent with those of the
cytogenetic analysis (4). Putzova
et al (17) found 11 and
eight alleles of D13S305 and D13S631, respectively, in a Czech
population, and the heterozygosity of each marker was 0.809 and
0.827. With regard to D13S305 and D13S634, Cho et al
(18) found 11 and 10 alleles,
respectively, in a Korean population, while Nasiri et al
(10) observed nine and eight
alleles, respectively, in an Iranian population. The heterozygosity
values in the study by Cho et al (18) were 0.84 and 0.74, respectively, while
those in the study by Nasiri et al (10) were 0.7850 and 0.8807, respectively.
Onay et al (15) observed
seven, eight and 12 alleles of D13S305, D13S631 and D13S634
markers, respectively, in a Turkish population and found that the
heterozygosity was 0.75, 0.78 and 0.85. One case of trisomy 13 was
detected using QF-PCR. Baig et al (19) found nine and 11 alleles of D13S631
and D13S634 in a Singaporean population with heterozygosity at
0.768 and 0.839, respectively. In the same study, QF-PCR was
performed to detect aneuploidies; 47 autosomal trisomies and 16
gender chromosome aneuploidies were identified, including 30 cases
of trisomy 13.
In conclusion, the present study has shown that the
D13S305, D13S631 and D13S634 STR markers are highly polymorphic in
the Han population of Tianjin, China and has provided basic data
that can be used in human population genetic studies. These STR
markers can be applied in the prenatal diagnosis of Patau
syndrome.
References
|
1
|
Lin HY, Lin SP, Chen YJ, et al: Clinical
characteristics and survival of trisomy 13 in a medical center in
Taiwan, 1985–2004. Pediatr Int. 49:380–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith DW, Patau K, Therman E and Inhorn
SL: A new autosomal trisomy syndrome: multiple congenital anomalies
caused by an extra chromosome. J Pediatr. 57:338–345. 1960.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faas BH, Cirigliano V and Bui TH: Rapid
methods for targeted prenatal diagnosis of common chromosome
aneuploidies. Semin Fetal Neonatal Med. 16:81–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cirigliano V, Voglino G, Ordoñez E, et al:
Rapid prenatal diagnosis of common chromosome aneuploidies by
QF-PCR, results of 9 years of clinical experience. Prenat Diagn.
29:40–49. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tariq MA, Ullah O, Riazuddin SA and
Riazuddin S: Allele frequency distribution of 13 X-chromosomal STR
loci in Pakistani population. Int J Legal Med. 122:525–528. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hills A, Donaghue C, Waters J, et al:
QF-PCR as a stand-alone test for prenatal samples: the first 2
years' experience in the London region. Prenat Diagn. 30:509–517.
2010.PubMed/NCBI
|
|
7
|
Papoulidis I, Siomou E, Sotiriadis A, et
al: Dual testing with QF-PCR and karyotype analysis for prenatal
diagnosis of chromosomal abnormalities. Evaluation of 13,500 cases
with consideration of using QF-PCR as a stand-alone test according
to referral indications. Prenat Diagn. 32:680–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quaife R, Wong LF, Tan SY, Chua WY, Lim
SS, Hammersley CJ and Yeo HL: QF-PCR-based prenatal detection of
aneuploidy in a southeast Asian population. Prenat Diagn.
24:407–413. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andonova S, Vazharova R, Dimitrova V,
Mazneikova V, Toncheva D and Kremensky I: Introduction of the
QF-PCR analysis for the purposes of prenatal diagnosis in Bulgaria
- estimation of applicability of 6 STR markers on chromosomes 21
and 18. Prenat Diagn. 24:202–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nasiri H, Noori-Dalooi MR, Dastan J and
Ghaffari SR: Investigation of QF-PCR application for rapid prenatal
diagnosis of chromosomal aneuploidies in Iranian population. Iran J
Pediatr. 21:15–20. 2011.PubMed/NCBI
|
|
11
|
Bär W, Brinkmann B, Budowle B, et al: DNA
recommendations. Further report of the DNA Commission of the ISFH
regarding the use of short tandem repeat systems. International
Society for Forensic Haemogenetics. Int J Legal Med. 110:175–176.
1997.PubMed/NCBI
|
|
12
|
Mansfield ES: Diagnosis of Down syndrome
and other aneuploidies using quantitative polymerase chain reaction
and small tandem repeat polymorphisms. Hum Mol Genet. 2:43–50.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jain S, Panigrahi I, Sheth J and Agarwal
S: STR markers for detecting heterogeneity in Indian population.
Mol Biol Rep. 39:461–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iliopoulos D, Sekerli E, Vassiliou G,
Sidiropoulou V, Topalidis A, Dimopoulou D and Voyiatzis N: Patau
syndrome with a long survival (146 months): a clinical report and
review of literature. Am J Med Genet A. 140:92–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Onay H, Ugurlu T, Aykut A, et al: Rapid
prenatal diagnosis of common aneuploidies in amniotic fluid using
quantitative fluorescent polymerase chain reaction. Gynecol Obstet
Invest. 66:104–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nadeem A, Babar ME, Hussain M and Tahir
MA: Development of pentaplex PCR and genetic analysis of X
chromosomal STRs in Punjabi population of Pakistan. Mol Biol Rep.
36:1671–1675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Putzova M, Pecnova L, Dvorakova L,
Soldatova I, Goetz P and Stejskal D: OmniPlex - a new QF-PCR assay
for prenatal diagnosis of common aneuploidies based on evaluation
of the heterozygosity of short tandem repeat loci in the Czech
population. Prenat Diagn. 28:1214–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho EH, Park BY, Kang YS and Lee EH:
Validation of QF-PCR in a Korean population. Prenat Diagn.
29:213–216. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baig S, Ho SS, Ng BL, et al: Development
of quantitative-fluorescence polymerase chain reaction for the
rapid prenatal diagnosis of common chromosomal aneuploidies in
1,000 samples in Singapore. Singapore Med J. 51:343–348.
2010.PubMed/NCBI
|