Introduction
Acute renal failure (ARF) has a high incidence, and
acute tubular necrosis (ATN) is the main pathological manifestation
of this condition, which is also one of the leading causes of
mortality in critically ill patients. Numerous studies have shown
that bone marrow stem cells (BMSCs) possess the properties of
migrating and homing towards the injured tissues, and these stem
cells may be distinguished into renal intrinsic parenchymal cells,
including mesangial cells, tubular epithelial cells and podocytes
(1,2). A previous study applied BMSCs to the
repair treatment of acute tubular damage (1); however, recent experiments
investigating the promotive effects of BMSCs on kidney damage
repair are primarily based on in vitro cellular
transplantation, which has a long preparation period and multiple
involvements (3,4). Therefore, these studies lack clinical
feasibility in the treatment of ARF. A number of studies have found
that BMSC mobilisation agents, such as granulocyte
colony-stimulating factor (G-CSF) and stem cell factor (SCF), are
able to mobilise autologous BMSCs to promote the healing of
diseases, including kidney damage, myocardial necrosis, nerve
damage, liver damage and haematological malignancies (3–7).
Numerous scholars have hypothesised that when ATN
occurs, the renal tubular regeneration process is also involved in
various endogenous regulatory factors (8–10). Tögel
et al (11) hypothesised that
the period in which BMSCs produce a renal protective effect may be
achieved through a pathway similar to paracrine or autocrine
secretion. For instance, when hepatocyte growth factor (HGF),
epidermal growth factor (EGF) and insulin-like growth factor-1
(IGF-1) are administered exogenously, the cell cycle is
accelerated, thereby promoting cell proliferation and accelerating
the recovery from ATN. In the current study, ATN was simulated
experimentally in a laboratory, and SCF and G-CSF were
subcutaneously injected to mobilise the autologous BMSCs, with the
aim to observe the distribution of BMSCs in the renal tissues, as
well as the changes in HGF and EGF expression during the repair
process of ATN tubular epithelial cells. Thus, the aim of the
present study was to investigate the effects of mobilisation agents
on the level of BMSCs, as well as the possible mechanisms
underlying ATN repair.
Materials and methods
Establishment of models and
grouping
A total of 128 healthy male Sprague-Dawley rats
(age, 8–10 weeks; weight, 250–280 g) were provided by the
Experimental Animal Centre of Zhengzhou University (Zhengzhou,
China). The rats were housed separately and provided with food and
water ad libitum. Following urine screening to identify
non-healthy rats, the healthy rats were randomly divided into four
groups, which included the control, model, treatment and treatment
control groups. The study was conducted in strict accordance with
the recommendations from the ‘Guide for the Care and Use of
Laboratory Animals’ of the National Institutes of Health (Bethesda,
MD, USA). The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Xinxiang Medical
University (Xinxiang, China). The model and treatment groups were
established using the unilateral renal ischaemia-reperfusion injury
model, based on the method outlined by Supavekin et al
(12). After 24 h of modelling, the
treatment group was subcutaneously injected once a day with 200
µg/kg SCF (Chengdu Di'ao Jiuhong Pharmaceutical Co., Chengdu,
China) and 50 µg/kg G-CSF (North China Pharmaceutical Jintan
Biotechnology Co. Ltd, Shijiazhuang, China) for five consecutive
days (13). In the treatment control
group, the normal rats were injected with SCF and G-CSF at the same
time points and with the same doses. As BMSC mobilising agents
typically cause the number of peripheral stem cells to peak between
days three and five following treatment (14), postoperative day five was selected as
the starting time point for detection.
Specimen collection
Upon specimen collection on day 5, 10, 17 and 24,
eight rats from each group were randomly selected for specimen
reservation. For anaesthesia, 10% chloral hydrate solution (3.5
ml/kg) was intraperitoneally injected into the rats. Subsequently,
2-ml venous blood samples were collected and preserved in an
EDTA-coated Eppendorf tube. The left kidney was removed, and after
washing with saline, the kidney was cut into tissue sections
measuring 1.0×1.0×0.2 cm. A number of pieces were quickly placed
into liquid nitrogen, whereas the remaining sections were fixed in
10% neutral formalin buffer.
Extraction of peripheral blood
CD34+ cells
Ficoll-Hypaque density gradient centrifugation was
performed to isolate the mononuclear cells from the peripheral
blood (15). Briefly, 2 ml venous
blood was fold diluted with Hank's solution (Sigma-Aldrich, St
Louis, MO, USA), then mixed and added to the Ficoll-Hypaque
lymphocyte separation medium (MP Biomedicals, LLC, Santa Ana, CA,
USA) along the tube wall. The mixture was centrifuged at 700 × g
for 20 min. The mononuclear cells aggregated and formed a white
film following centrifugation, which was pipetted with a capillary
tube and moved into an additional sterile tube. The cells were
washed with 2 ml Hank's solution, and centrifuged twice at 400 × g
for 10 min, after which the supernatant was discarded.
Subsequently, 10% foetal calf serum-containing RPMI 1640 (GE
Healthcare Life Sciences, Logan, UT, USA) was added for the
resuspension of the cells. A drop of cell suspension and a drop of
0.2% trypan blue dye (Sigma-Aldrich) were mixed, and the cell
numbers were counted within the four squares. The concentration of
the mononuclear cells was calculated as follows: (Cell number/1 ml
cell suspension) = [Cell number (in the four squares)/4] ×
104 × 2 (dilution factor).
Detection of peripheral blood
CD34+ cells
For CD34+ cell detection, 100 µl
fluorescein isothiocyanate (FITC)-CD34+ monoclonal
antibody (bs-0646R-FITC; Beijing Bioss Biotechnology Co., Ltd.,
Beijing, China) was added, and mixed at room temperature in the
dark for 25 min. The primary antibodies were incubated with
mononuclear cells extracted from peripheral blood. The mixture was
centrifuged twice in 1 ml phosphate-buffered saline (PBS) at 200 ×
g for 5 min. Next, 0.5 ml paraformaldehyde (1%) was added to
resuspend the cells, and a flow cytometric method (Epics-XL;
Beckman Coulter, Inc., Brea, CA, USA) was used to detect the
fluorescence value. From each tube, 105 cells were
counted using flow cytometry to detect the number of
CD34+ cells and analyse the percentage in the peripheral
blood. A negative control was also performed during the experiment,
using 100 µl PBS instead of the primary antibody.
Polymerase chain reaction (PCR)
Primers were designed according to the sequences in
GenBank and were as follows: HGF upstream, 5′-CTTCTGCCGGTCCTGTTG-3′
and downstream, 5′-CCACTTGACATACTATTG-3′; EGF upstream,
5′-AGACCAGGAACTGTCAG-3′ and downstream, 5′-AACTCAGAAGAACACGG-3′;
and β-actin upstream, 5′-CCTCGCCTTTGCCGATCC-3′ and downstream,
5′-TGATGGAGTACTTCTAGG-3′. All the primers were synthesised by
Shanghai Biological Engineering Co., Ltd. (Shanghai, China). TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA) was used to
extract the total mRNA from the kidneys of the rats, and cDNA was
synthesised using a reverse transcription kit (Takara Biotechnology
Co., Ltd., Dalian, China). The thermocycling conditions for the
PCR-amplified products were as follows. For HGF, initial
denaturation was performed at 94°C for 5 min, followed by 35 cycles
of denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec
and elongation at 72°C for 45 sec. A final elongation was conducted
at 72°C for 10 min. For EGF, the initial denaturation was performed
at 94°C for 5 min. Subsequently, 32 cycles of denaturation at 94°C
for 30 sec, annealing at 51°C for 30 sec and elongation at 72°C for
45 sec was performed, followed by a final elongation at 72°C for 10
min. Finally, the β-actin reaction underwent initial denaturation
at 94°C for 5 min, and then 30 cycles of denaturation at 94°C for
30 sec, annealing at 55°C for 30 sec and elongation at 72°C for 45,
followed by a final elongation at 72°C for 10 min. The final
storage temperature was 8°C. The reaction products were
photographed under ultraviolet light following electrophoresis. The
electrophoresis strips were analysed using image analysis software
(Olympus Stream Version 1.9; Olympus Corporation, Tokyo, Japan) and
the intensity of the strips was calculated by multiplying the area
and grey scale. To determine the HGF and EGF gene expression
levels, the ratio of the signal intensity of HGF and EGF was
calculated against that of β-actin.
Immunohistochemistry
For immunohistochemistry, the
streptomycin-avidin-biotin-peroxidase complex method (no. SP-0023;
Beijing Bioss Biotechnology Co., Ltd.) was used. Paraffin sections
of 4 µm were conventionally dewaxed and hydrated, and the citrate
buffer was microwave repaired. The sections were placed under high
fire for 5 min and low fire for 5 min, followed by closure with 5%
bovine serum albumin (Sigma-Aldrich). Subsequently, rabbit anti-rat
CD34+ (1:200; no. bs-0646R; Beijing Bioss Biotechnology
Co., Ltd.), rabbit anti-rat HGF (1:200; no. bs-1025R; Beijing Bioss
Biotechnology Co., Ltd.) and rabbit anti-rat EGF polyclonal
antibodies (1:100; no. bs-2008R; Beijing Bioss Biotechnology Co.,
Ltd., China) were used as the primary antibodies for overnight
incubation at 4°C. The sections were washed with PBS, and a
secondary horseradish peroxidase-conjugated goat anti-rabbit IgG
antibody (1:500; Zhongshan Goldenbridge Biotechnology Co, Ltd.,
Beijing, China) was added and incubated at 37°C for 30 min. After
washing with PBS, the sections were stained using diaminobenzidine.
PBS was used instead of the primary antibody in the control
group.
Evaluation of the immunohistochemical
results
The staining results for CD34, HGF and EGF were
evaluated from ten non-overlapped view fields of each slice that
were randomly selected under x200 magnification (Bx51/Bx52;
Olympus). An IDA-2000 computer image automatic analysis system
(Olympus) was used to investigate the positive immunohistochemical
signals in the aforementioned selected view fields. The percentages
of cells positive for CD34, HGF or EGF relative to the total number
of renal tubular cells in the selected fields were calculated, with
the mean values shown as the immunohistochemical results.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis, and all the data are expressed
as the mean ± standard deviation. Intergroup comparisons were
performed using one-way analysis of variance, whereas pairwise
intergroup comparisons were conducted using the least significant
difference method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Percentage changes in the
CD34+ cell count
On postoperative day five, the percentages of
CD34+ mononuclear cells in the model, treatment and
treatment control groups reached a peak. Compared with the control
group, the differences were statistically significant (P<0.05),
and the ratio for the treatment group was significantly higher
compared with that for the model and treatment control groups.
Furthermore, the ratio for the treatment control group was
significantly higher compared with that for the model group
(P<0.05). The percentage of CD34+ cells gradually
decreased over time. On postoperative days 10 and 17, the
percentages of CD34+ cells in the treatment group were
significantly higher compared with the percentages for the other
groups (Table I).
 | Table I.Percentage of CD34+
mononuclear cells in the peripheral blood. |
Table I.
Percentage of CD34+
mononuclear cells in the peripheral blood.
| Time (days) | Cases (n) | Control group
(%) | Model group (%) | Treatment group
(%) | Treatment control
group (%) |
|---|
| 5 | 8 |
0.13±0.01 |
0.90±0.06a,c |
2.07±0.08a,b,c |
1.41±0.04a |
| 10 | 8 |
0.13±0.02 |
0.56±0.44 |
1.61±0.07a,b,c |
1.03±0.04a |
| 17 | 8 |
0.13±0.02 |
0.42±0.49 |
0.88±0.05a,b,c |
0.54±0.18 |
| 24 | 8 |
0.13±0.02 |
0.22±0.03 |
0.31±0.77 |
0.24±0.02 |
The percentages of CD34+ cells in the
renal tissues of the model and treatment groups increased
significantly on postoperative day five. The differences were
statistically significant (P<0.05) compared with the percentages
for the control and treatment control groups. At all the indicated
time points, the percentages of CD34+ cells in the
treatment group were higher compared with those in the model group,
and the differences on postoperative day 5 and 10 were
statistically significant (P<0.05). For the two groups, the
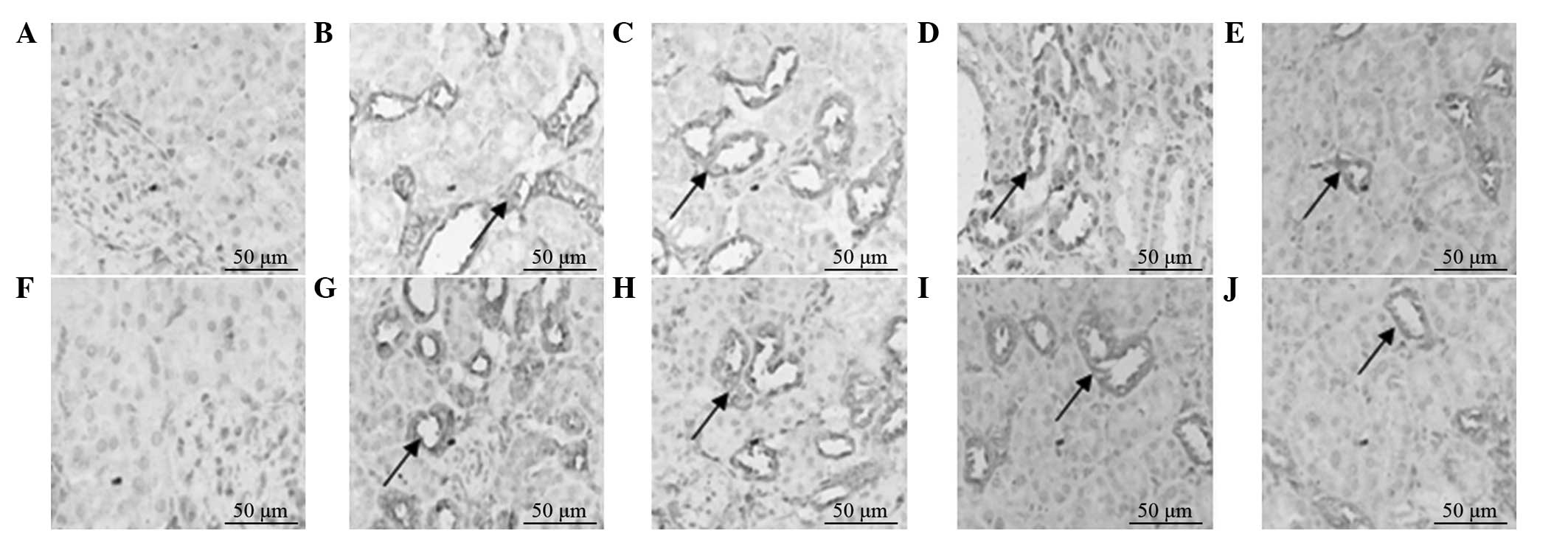
expression of CD34 gradually decreased with time (Fig. 1).
Expression levels of HGF and EGF
mRNA
The mRNA expression levels of HGF and EGF in the
renal tissues of the model and treatment groups increased on
postoperative day five, and peaked on postoperative day 10 and 17
in the treatment and model groups, respectively. The mRNA
expression levels of HGF in the treatment group on days 5 and 10
were significantly higher compared with those in the model group.
In addition, the mRNA expression levels of HGF and EGF in the
treatment group on days 5, 10 and 17 were significantly higher
compared with those in the model group (P<0.05). Following the
peak mRNA expression of HGF and EGF, the levels gradually decreased
to a normal level over time. The mRNA expression levels of HGF and
EGF were low in the control and treatment control groups (Fig. 2).
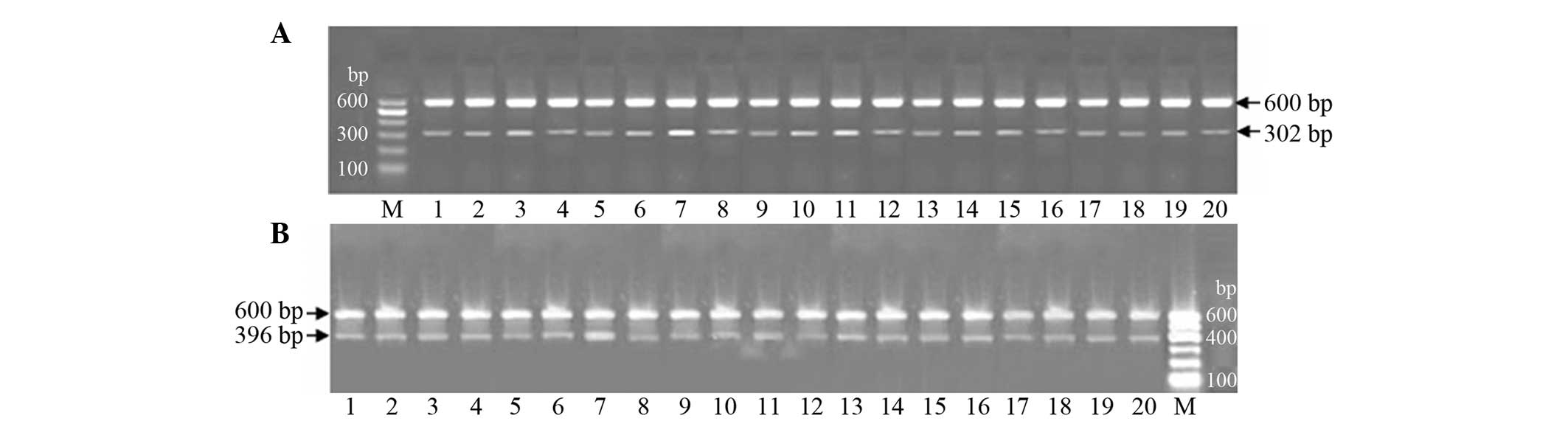 | Figure 2.mRNA expression levels of (A)
hepatocyte growth factor (HGF) and (B) epidermal growth factor
(EGF) in the four experimental groups. HGF mRNA was found to be 302
bp in length, while EGF mRNA was shown to be 396 bp. β-actin was
used as the internal reference (length, 600 bp). M, marker; 1–4,
control, model, treatment and treatment control groups on day five,
respectively; 5–8, control, model, treatment and treatment control
groups on day 10, respectively; 9–12, control, model, treatment and
treatment control groups on day 17, respectively; 13–16, control,
model, treatment and treatment control groups on day 24,
respectively. The mRNA expression levels of HGF and EGF in the
model and treatment groups increased on postoperative day five, and
peaked on postoperative day 10 and 17 in the treatment and model
groups, respectively. The mRNA expression of HGF was significantly
higher in the treatment group compared with the model group. |
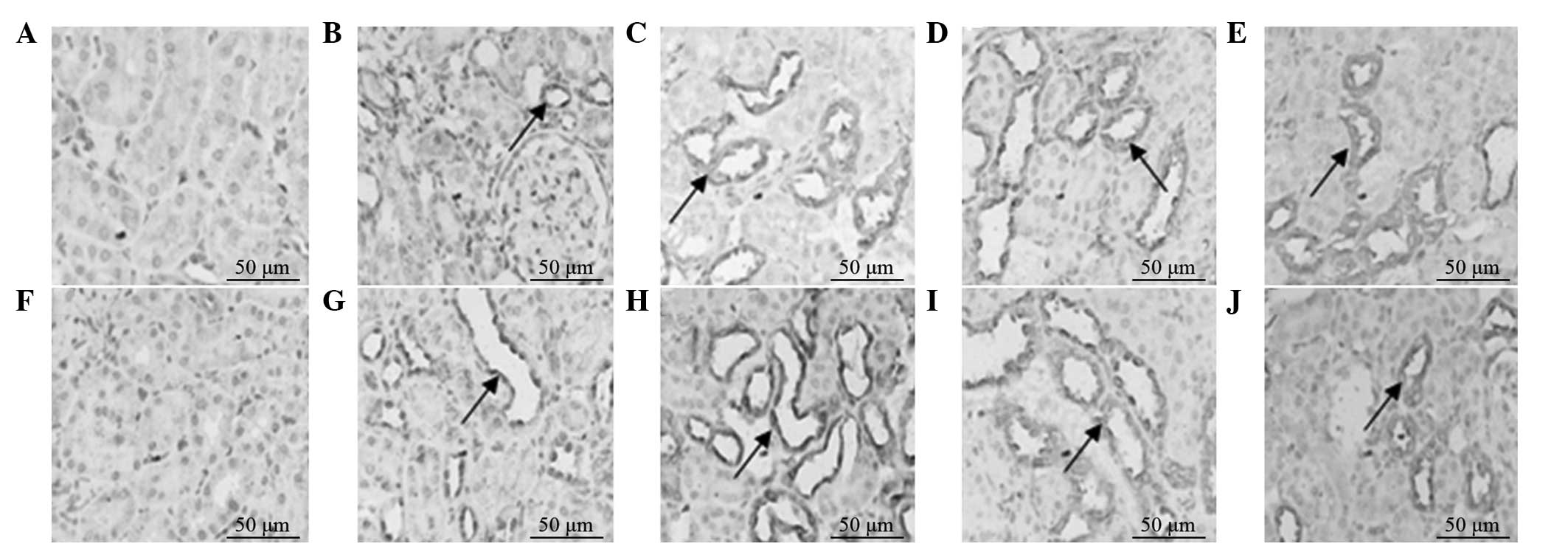
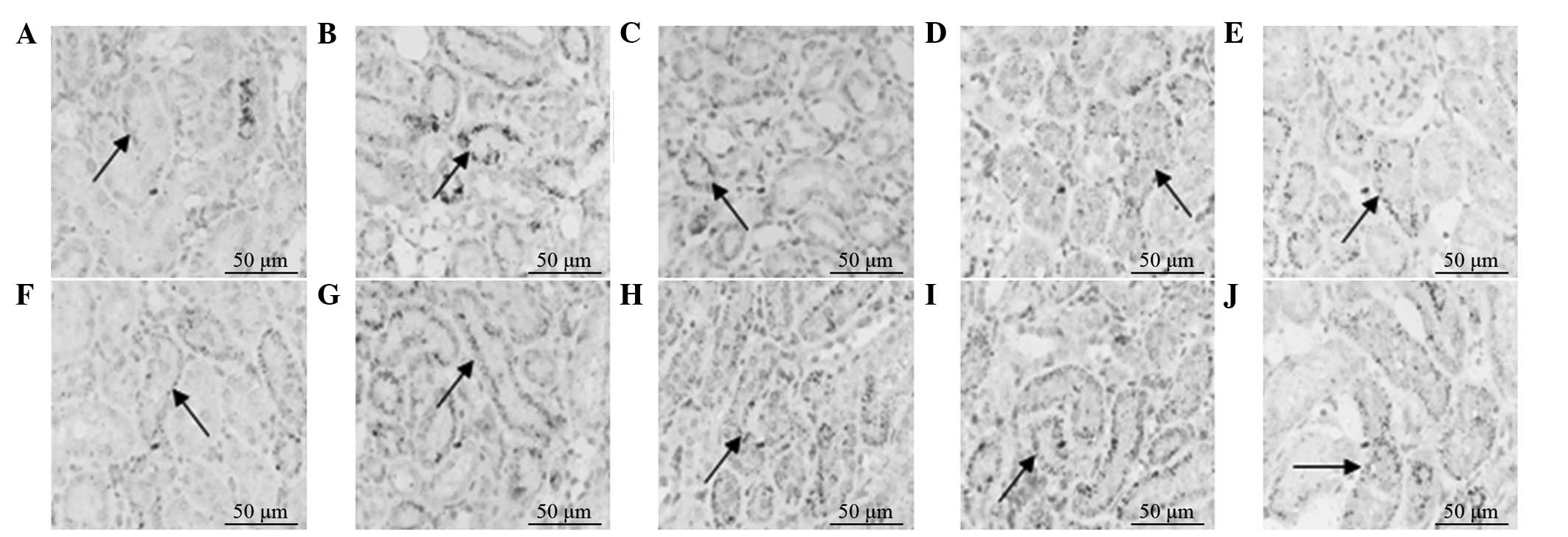
Protein expression levels of HGF and
EGF
The protein expression levels of HGF and EGF in the
model and treatment groups increased on postoperative day five, and
peaked on postoperative day 10 and 17 in the treatment and model
groups, respectively. The protein expression levels of HGF and EGF
in the treatment group on days 5 and 10 were significantly higher
compared with those in the model group (P<0.05). Following peak
expression of HGF and EGF protein, the levels gradually decreased
to a normal level over time. The protein expression levels of HGF
and EGF were low in the control and treatment control groups
(Figs. 3 and 4).
Discussion
Herrera et al (16) established a mouse ARF model using
intramuscularly-injected glycerol, and the results demonstrated
that BMSCs were able to migrate into the damaged kidney and
differentiate into the tubular epithelium, thereby promoting the
morphological and functional recovery of the injured kidney. In
addition, Morigi et al (17)
established a cisplatin-induced ATN model, and the results revealed
that the infusion of BMSCs was able to improve renal function. The
in situ hybridisation method revealed that BMSCs may be
directly involved in renal reconstruction through differentiation
into renal tubular cells.
Under normal conditions, the BMSC count in the
peripheral blood is extremely low; however, when the body is under
stress, ischaemia or injury, the number of BMSCs in the peripheral
circulation can increase significantly. Kale et al (1) demonstrated that renal ischaemic injury
can increase the BMSC count in the blood circulation significantly,
although the number of BMSCs was not sufficient for repair
following a serious injury. Orlic et al (18) combined SCF and G-CSF for the
mobilisation of BMSCs to treat mice with myocardial infarction, and
found that the number of BMSCs in the peripheral blood was as much
as 250 times more compared with the normal amount. In clinical
studies, using a combination of SCF and G-CSF has resulted in a
two- to three-fold increase in CD34+ cells compared with
that for the single application of G-CSF (19).
The majority of studies have considered
CD34+ cells as BMSCs (20); thus, CD34+ cells have been
treated as the source cell marker of biological activities and
clinical applications in BMSC mobilisation.
The percentage of CD34+ cells in the
peripheral blood of the control group was ~0.1%, indicating that
under physiological conditions, only a small number of BMSCs are
circulating. At day five following treatment, the percentage of
CD34+ mononuclear cells in the peripheral blood of the
model group was higher compared with the control group, which was
consistent with previous observations (21). The results indicate that stress
factors, such as ischaemia and injury, are able to mobilise the
autologous BMSCs. Therefore, if the body has a self-healing
mechanism that allows the regeneration of ischaemic tissues, the
mechanism may involve certain cytokines, including G-CSF and
endothelial cell growth factor, which are locally secreted
following ischaemia (22,23). The percentage of CD34+
cells in the treatment control group was significantly higher
compared with that in the model group, indicating that SCF and
G-CSF have a strong ability to mobilise BMSCs. The ratio of
CD34+ cells in the treatment group reached the highest
level on postoperative day five; thus, the effect of BMSC
mobilisation was the strongest at this time point and may be the
synergic result of kidney damage and mobilisation agents.
In the present study, the combination of SCF and
G-CSF was demonstrated to mobilise a large number of BMSCs into
circulation; however, the mobilised BMSCs had to directionally
migrate to the kidney to complete the damage repair. A number of
studies (24–26) have demonstrated that BMSCs exhibit a
‘homing’ characteristic, whereby they migrate to the
ischaemia-damaged tissues. Inflammatory damage has been
hypothesised to be an initiating factor of BMSC homing (26). Helmuth (27) described this process as follows:
‘BMSC heard the call of the damaged tissues’.
On postoperative day five, the expression levels of
CD34 on the cells in the renal tissues of the model group
increased. The level of CD34 expression exhibited a statistically
significant difference when compared with that for the treatment
control group, which confirms the hypothesis that the
microenvironment formed by the tissue damage is one of the
essential conditions to induce BMSC homing. On days 5, 10 and 17,
the CD34 expression levels in the renal tissue cells of the
treatment group were significantly higher compared with those of
the model group, indicating that under simple injury factors, the
number of BMSCs that undergo differentiation and homing to the
damaged tissue may be smaller. By contrast, the combination of SCF
and G-CSF significantly increased the number of BMSCs in the
peripheral blood over a relatively short time period. These
conditions may cause various factors in the bone marrow to migrate
back to the necrotic lesions and participate in the regeneration of
the necrotic tissues and blood vessels. Therefore, a sufficient
number of BMSCs and the microenvironment around the injury are two
key factors required for BMSC homing and differentiation.
Having undergone mobilisation and migration to the
renal necrotic areas, the autologous BMSCs may undergo
differentiation into ‘environment-dependent’ cells. These cells may
differentiate from the renal tubular epithelial cells to
participate in the regeneration of tissues and blood vessels.
Therefore, BMSC mobilisation agents may be used to mobilise BMSCs
into the blood circulation, increasing the cell count in the
peripheral blood in order to promote the repair of renal tissues
(28).
A number of scholars have hypothesised that during
the repair process of ATN, the surviving cells are dependent on an
‘atavistic’ process to enter the cell cycle and proliferate as the
new epithelial cells (29).
Subsequently, the cells would stretch, migrate and recover the
basement membrane. A number of studies have hypothesised that
certain growth factors or specific hormones, including HGF, EGF and
IGF-1, are able to promote this transformation process, and
accelerate the differentiation of nascent epithelial cells
(30–32). In addition, several scholars have
demonstrated that BMSCs are able to secrete a number of cytokines,
thereby promoting the proliferation and repair of the endogenous
renal tubular epithelial cells (33,34).
In the present study, HGF and EGF expression levels
in the model group were shown to increase slowly, reaching a peak
on postoperative day 17 (P<0.05). A possible explanation for
this observation may be that when ATN occurs, the body mobilises
BMSCs in a compensatory response, which affects the secretion and
synthesis of growth factors, thereby reducing the renal tubular
necrosis and promoting tubular regeneration and proliferation. This
condition may be a self-protective mechanism of the body following
ATN. In the treatment group, the expression levels of HGF and EGF
were significantly increased following the application of SCF and
G-CSF. Thus, it was hypothesised that this condition may be
associated with an increased number of BMSCs, as a result of the
application of mobilisation agents. When the expression levels of
HGF and EGF genes and proteins are upregulated, the renal tubular
damage is reduced and the repair of renal tissues is
accelerated.
In conclusion, following the occurrence of ATN, the
repair mechanisms of the renal tubular epithelial cells can
mobilise the autologous BMSCs to migrate into the injured area of
renal tubule necrosis and differentiate into epithelial cells or
fuse with the residual cells, directly contributing to the repair
of the renal tissues. On the other hand, the functions of autocrine
and paracrine secretion can increase the gene and protein
expression levels of HGF, EGF, IGF-1 and other growth factors in
the kidney, thereby regulating the microenvironment of the kidney,
as well as stimulating DNA synthesis, cell proliferation and
division. In addition, various growth factors are able to provide a
good microenvironment to promote the self-repair of the kidney
tissues (35). By contrast, the
protective mechanism of the body, which mobilises the autologous
BMSCs following kidney damage, is limited. Therefore, the results
of the present study have demonstrated that a combination of BMSC
mobilisation agents, namely G-CSF and SCF, can fully mobilise the
BMSCs and increase their cell count in the peripheral blood, which
accelerates and promotes the regeneration and repair of renal
tubular epithelial cells. However, the experimental period was
short and future studies should allow a longer time period to
observe any side-effects of BMSCs, SCF and G-CSF. In addition,
further experiments are required to observe the differentiation of
BMSCs in ATN.
References
|
1
|
Kale S, Karihaloo A, Clark PR, et al: Bone
marrow stem cells contribute to repair of the ischemically injured
renal tubule. J Clin Invest. 112:42–49. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin F, Cordes K, Li L, et al:
Hematopoietic stem cells contribute to the regeneration of renal
tubules after renal ischemia-reperfusion injury in mice. J Am Soc
Nephrol. 14:1188–1199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia X, Xie X, Feng G, et al: Bone
marrow-derived cells can acquire renal stem cells properties and
ameliorate ischemia-reperfusion induced acute renal injury. BMC
Nephrol. 13:1052012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barile L, Cerisoli F, Frati G, et al: Bone
marrow-derived cells can acquire cardiac stem cells properties in
damaged heart. J Cell Mol Med. 15:63–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osada T, Watanabe M, Hasuo A, et al:
Efficacy of the coadministration of granulocyte colony-stimulating
factor and stem cell factor in the activation of intrinsic cells
after spinal cord injury in mice. J Neurosurg Spine. 13:516–523.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takami T, Terai S and Sakaida I: Advanced
therapies using autologous bone marrow cells for chronic liver
disease. Discov Med. 14:7–12. 2012.PubMed/NCBI
|
|
7
|
Shen WY, Li JY, Hong M, et al: Clinical
study on high-dose etoposide with granulocyte colony-stimulating
factor for mobilization of autologous peripheral blood stem cells
in patients with hematologic malignancies. Zhonghua Xue Ye Xue Za
Zhi. 33:628–631. 2012.(In Chinese). PubMed/NCBI
|
|
8
|
Devarajan P: Cellular and molecular
derangements in acute tubular necrosis. Curr Opin Pediatry.
17:193–199. 2005. View Article : Google Scholar
|
|
9
|
Rauen U and de Groot H: New insights into
the cellular and molecular mechanisms of cold storage injury. J
Investig Med. 52:299–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zweier JL and Talukder MA: The role of
oxidants and free radicals in reperfusion injury. Cardiovasc Res.
70:181–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tögel F, Hu Z, Weiss K, et al:
Administered mesenchymal stem cells protect against ischemic acute
renal failure through differentiation-independent mechanisms. Am J
Physiol Renal Physiol. 289:F31–F42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Supavekin S, Zhang W, Kucherlapati R, et
al: Differential gene expression following early renal
ischemia/reperfusion. Kidney Int. 63:1714–1724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Bai H, Yi Z, et al: Effect of
stem cell factor and granulocyte-macrophage colony-stimulating
factor-induced bone marrow stem cell mobilization on recovery from
acute tubular necrosis in rats. Ren Fail. 34:350–357. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martino M, Console G, Irrera G, et al:
Harvesting peripheral blood progenitor cells from healthy donors:
retrospective comparison of filgrastim and lenograstim. J Clin
Apher. 20:129–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cappellesso-Fleury S, Rage C, Tschaggeny
F, et al: Erythrocyte removal from bone marrow by density gradient
separation using the COBE 2991 cell processor with the triple-bag
processing set. Transfus Clin Biol. 16:43–49. 2009.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herrera MB, Bussolati B, Bruno S, et al:
Mesenchymal stem cells contribute to the renal repair of acute
tubular epithelial injury. Int J Mol Med. 14:1035–1041.
2004.PubMed/NCBI
|
|
17
|
Morigi M, Imberti B, Zoja C, et al:
Mesenchymal stem cells are renotropic, helping to repair the kidney
and improve the function in acute renal failure. J Am Soc Nephrol.
15:1794–1804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orlic D, Kajstura J, Chimenti S, et al:
Mobilized bone marrow cells repair the infracted heart, improving
function and survival. Proc Natl Acad Sci USA. 98:10344–10349.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toth ZE, Leker RR, Shahar T, et al: The
combination of granulocyte colony-stimulating factor and stem cell
factor significantly increases the number of bone marrow-derived
endothelial cells in brains of mice following cerebral ischemia.
Blood. 111:5544–5552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wright DE, Wagers AJ, Gulati AP, et al:
Physiological migration of hematopoietic stem and progenitor cells.
Science. 294:1933–1936. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Li XR and Yuan JQ: Effects of
bone-marrow mesenchymal stem cells transplanted into vitreous
cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin
Exp Ophthalmol. 247:503–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huls M, Russel FG and Masereeuw R:
Insights into the role of bone marrow-derived stem cells in renal
repair. Kidney Blood Press Res. 31:104–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bittira B, Kuang JQ, Al-Khaldi A, et al:
In vitro preprogramming of marrow stromal cells for myocardial
regeneration. Ann Thorac Surg. 74:1154–1160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu N, Patzak A and Zhang J:
CXCR4-overexpressing bone marrow-derived mesenchymal stem cells
improve repair of acute kidney injury. Am J Physiol Renal Physiol.
305:F1064–F1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Illien-Jünger S, Pattappa G, Peroglio M,
et al: Homing of mesenchymal stem cells in induced degenerative
intervertebral discs in a whole organ culture system. Spine (Phila
Pa 1976). 37:1865–1873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
González MN, Dey N, Garg NJ and Postan M:
Granulocyte colony-stimulating factor partially repairs the damage
provoked by Trypanosoma cruzi in murine myocardium. Int J Cardiol.
168:2567–2574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Helmuth L: Neuroscience. Stem cells hear
call of injured tissue. Science. 290:1479–1481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang M, Yan CY, Wu XR, et al: Changes of
biological characteristics of bone marrow stem cells homing to
infracted myocardium after mobilization by stem cell factor.
Zhongguo Lin Chuang Kang Fu. 9:67702005.(In Chinese).
|
|
29
|
Smeets B, Boor P, Dijkman H, et al:
Proximal tubular cells contain a phenotypically distinct, scattered
cell population involved in tubular regeneration. J Pathol.
229:645–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahmoud K, Opelz G, Pelzl S, et al:
Evaluation of hepatocyte growth factor as a sensitive marker for
early detection of acute renal allograft rejection.
Transplantation. 83:1035–1040. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grzelak P, Szymczyk K, Strzelczyk J, et
al: Perfusion of kidney graft pyramids and cortex in
contrast-enhanced ultrasonography in the determination of the cause
of delayed graft function. Ann Transplant. 16:48–53.
2011.PubMed/NCBI
|
|
32
|
Juarez JC, Manuia M, Burnett ME, et al:
Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated
oxidation and inactivation of phosphatases in growth factor
signaling. Proc Natl Acad Sci USA. 105:7147–7152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Villanueva S, Carreño JE, Salazar L, et
al: Human mesenchymal stem cells derived from adipose tissue reduce
functional and tissue damage in a rat model of chronic renal
failure. Clin Sci (Lond). 125:199–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Villanueva S, Ewertz E, Carrión F, et al:
Mesenchymal stem cell injection ameliorates chronic renal failure
in a rat model. Clin Sci (Lond). 121:489–499. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tasanarong A, Khositseth S and
Thitiarchakul S: The mechanism of increased vascular permeability
in renal ischemic reperfusion injury: potential role of
angiopoietin-1 and hyaluronan. J Med Assoc Thai. 92:1150–1158.
2009.PubMed/NCBI
|