Introduction
The motor nerve terminal has been proposed as a
potential target for the binding of anti-ganglioside antibodies in
Guillain-Barré syndrome (GBS) (1,2). In
cases of axonal GBS, autoantibodies against the ganglioside-like
molecules, GM1, GM1b, GD1a and GalNAc-GD1a, are frequently
detected, and these gangliosides may be targeted by autoantibodies.
Anti-ganglioside antibodies are hypothesized to mediate the
pathogenic mechanism of GBS, leading to axonal neuropathy.
Voltage-dependent calcium channels (VDCCs) serve a
key function in neuromuscular transmission by facilitating calcium
ion influx into motor nerve terminals, thereby affecting
acetylcholine release. Previous studies have reported that
anti-ganglioside antibodies induce alterations in calcium channels
at neuromuscular junctions (NMJs) (3,4). In
addition, an IgG anti-GM1 monoclonal antibody (mAb) has been
previously demonstrated to reduce the rate of spontaneous muscle
action potential (SMAPs) in spinal cord-muscle co-culture systems
and the VDCC current in cerebellar Purkinje cells (5,6). Lipid
microdomains, which contain gangliosides, are involved in the
clustering of P/Q-type VDCCs and the organization of presynaptic
membrane sites for synaptic exocytosis (7,8). In a
previous study, IgG anti-GalNAc-GD1a antibodies, purified from a
rabbit immunized with GalNAc-GD1a, were shown to inhibit VDCC
currents in differentiated PC12 pheochromocytoma cells (9). Collectively, these observations
indicate that the inhibition of nerve conduction in patients with
GBS may be associated with the dysfunction of VDCCs in motor nerve
terminals.
In the present study, the effects of an IgG anti-GM1
mAb on SMAPs were evaluated using pretreatment with N-type and
P/Q-type calcium channel blockers in a rat spinal cord-muscle
co-culture system. Immunohistochemical analysis using an IgG
anti-GM1 mAb, calcium channel antibodies and triple fluorescence
labeling were used to show the colocalization of IgG anti-GM1 mAb
and calcium channels in the NMJs of the rat hemidiaphragm.
Materials and methods
IgG anti-GM1 mAb
An IgG anti-GM1 mAb was provided by Dr Nobuhiro Yuki
(Departments of Microbiology and Medicine, National University of
Singapore, 2 Science Drive, Singapore 17597), and all samples were
stored at −80°C until required. Using an enzyme-linked
immunosorbent assay, reactivity was detected with the IgG anti-GM1
mAb, which was in accordance with previous observations by Hotta et
al (5).
Drugs
Drugs were dissolved in the co-culture medium
bathing the preparation. ω-conotoxin GVIA and ω-agatoxin IVA
(Alomone Labs Ltd., Jerusalem, Israel) were dissolved in a stock
solution containing cytochrome c (1 mg/ml; Sigma-Aldrich;
St. Louis, MO, USA) to prevent the non-specific binding of the
peptide to the chamber walls and tubing.
Spinal cord-muscle co-culture
Pregnant Wistar rats (n=35) were purchased from
Japan Laboratory Animals, Inc. (Tokyo, Japan) individually housed
under controlled conditions, with a 12-h light-dark cycle and free
access to food and water. Experiments were conducted in accordance
with the Guidelines for Animal Care of Showa Pharmaceutical
University (Tokyo, Japan), in addition to the guidelines for animal
use published by the National Institutes of Health (https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-use-of-laboratory-animals.pdf).
This study was conducted with the approval of the Showa
Pharmaceutical University Research Ethics Committee (approval no.
H18).
A spinal cord-muscle co-culture system was
established according to a previously described method by Taguchi
et al (10). Briefly, muscle
was extracted from prenatal day 17 fetal rats and separated into
~3-mm sections in Tyrode's solution (Sigma-Aldrich) containing 100
µg/ml streptomycin and 100 µg/ml penicillin. The sections were
subsequently incubated at 37°C for 20 min in Ca2+- and
Mg2+-free Tyrode's solution containing 1 mg/ml
collagenase. For the innervation experiments, explants of the
entire transverse slices of fetal spinal cord, including the dorsal
root ganglia, were placed onto a collagen-coated 35-mm Petri dish.
Individual muscle cells were derived from trituration and placed on
the slices of spinal cord for culture. The muscle cells and spinal
cords were co-cultured in 67% DMEM (Gibco Life Technologies,
Carlsbad, CA, USA), 23% medium 199 (Gibco Life Technologies) and
10% fetal calf serum (Roche Diagnostics, Basel, Switzerland), which
was supplemented with 25 ng/ml fibroblast growth factor
(Sigma-Aldrich) and 20 µg/ml insulin (Gibco Life Technologies). The
spinal cord-muscle co-culture systems were stored in a
CO2 incubator with 5% CO2 and 95%
O2 at 37°C.
Measurement of SMAPs
After 1 week of co-culture, the innervated muscle
specimens were placed in an experimental chamber on the stage of an
inverted microscope (IX-70; Olympus Corporation, Tokyo, Japan). The
1-ml experimental chamber was continuously perfused with medium
(67% DMEM and 23% medium 199) at a rate of 1–2 ml/min, with
continuous bubbling of 5% CO2/95% O2. Glass
microelectrodes (GD-1; Narishige Group, Tokyo, Japan) filled with 3
M KCl, with a tip resistance of 20–40 MΩ, were used to record the
SMAPs. Each electrode that was connected to a microelectrode
amplifier (MEZ-8301; Nihon Koden Corporation, Tokyo, Japan)
recorded the electrical activity, which was displayed on an
oscilloscope (VC-11; Nihon Koden Corporation). After the stability
of muscle action potentials was observed for ~4 min, 10 µl anti-GM1
mAb antibody was applied using a micropipette. Data were
transferred to and stored in a computer, using pCLAMP6 software
(Molecular Devices, Sunnyvale, CA, USA). All experiments were
conducted at 33±1°C.
Immunohistochemical analysis
Hemidiaphragms were used for triple fluorescence
labeling to determine the localization of GM1. Tissue samples were
incubated in 10% normal goat serum (NGS; Funakoshi Co., Ltd.,
Tokyo, Japan) in Block Ace blocking agent (Dainippon Sumitomo
Pharma Co., Ltd., Tokyo, Japan) for 30 min at room temperature to
block non-specific binding, as previously described (6). After blocking, the tissues were
incubated for 5 h at 4°C with IgG anti-GM1 antibodies (1:100;
provided by Dr Nobuhiro Yuki) in 10% NGS and 10% Block Ace in
phosphate-buffered saline (PBS). In order to detect the IgG
anti-GM1 mAb, the tissues were incubated for 1 h at 4°C with a
fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (1:100;
Sigma-Aldrich). Subsequently, the hemidiaphragms were incubated
with primary anti-Cav2.1 (α1A; 1:1,000;
#ACC-001) and anti-Cav2.2 (α1B; 1:1,000;
#ACC-002) antibodies (Alomone Labs Ltd., Jerusalem, Israel) for 5 h
at 4°C. To detect the primary antibodies, the hemidiaphragms were
incubated for 1 h at 4°C with a monoclonal Cy5-conjugated
anti-rabbit IgG (1:100; AP182SA6; Chemicon International, Inc.,
Temecula, CA, USA). Hemidiaphragms were subsequently incubated with
rhodamine-α-bungarotoxin (α-BuTx; 1:300; Molecular Probes;
Invitrogen Life Technologies, Grand Island, NY, USA) for 5 h at
4°C. Each reaction was terminated by numerous washes with PBS, and
the tissue was mounted using Aqua Poly/Mount (PolySciences, Inc.,
Warrington, PA, USA). All the immunostained sections were observed
under an Olympus laser-scanning confocal microscope (Fluoview BW50;
Olympus Corporation, Tokyo, Japan) at wavelengths of 488 nm (FITC),
543 nm (rhodamine) or 568 nm (Cy5).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean (SEM). A paired t-test was used to compare the effects of
the IgG anti-GM1 mAb, where P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
conducted using Microsoft Excel and statistical add-on software
(Microsoft Corporation, Redmond, WA, USA).
Results
Effects of IgG anti-GM1 mAb on
SMAPs
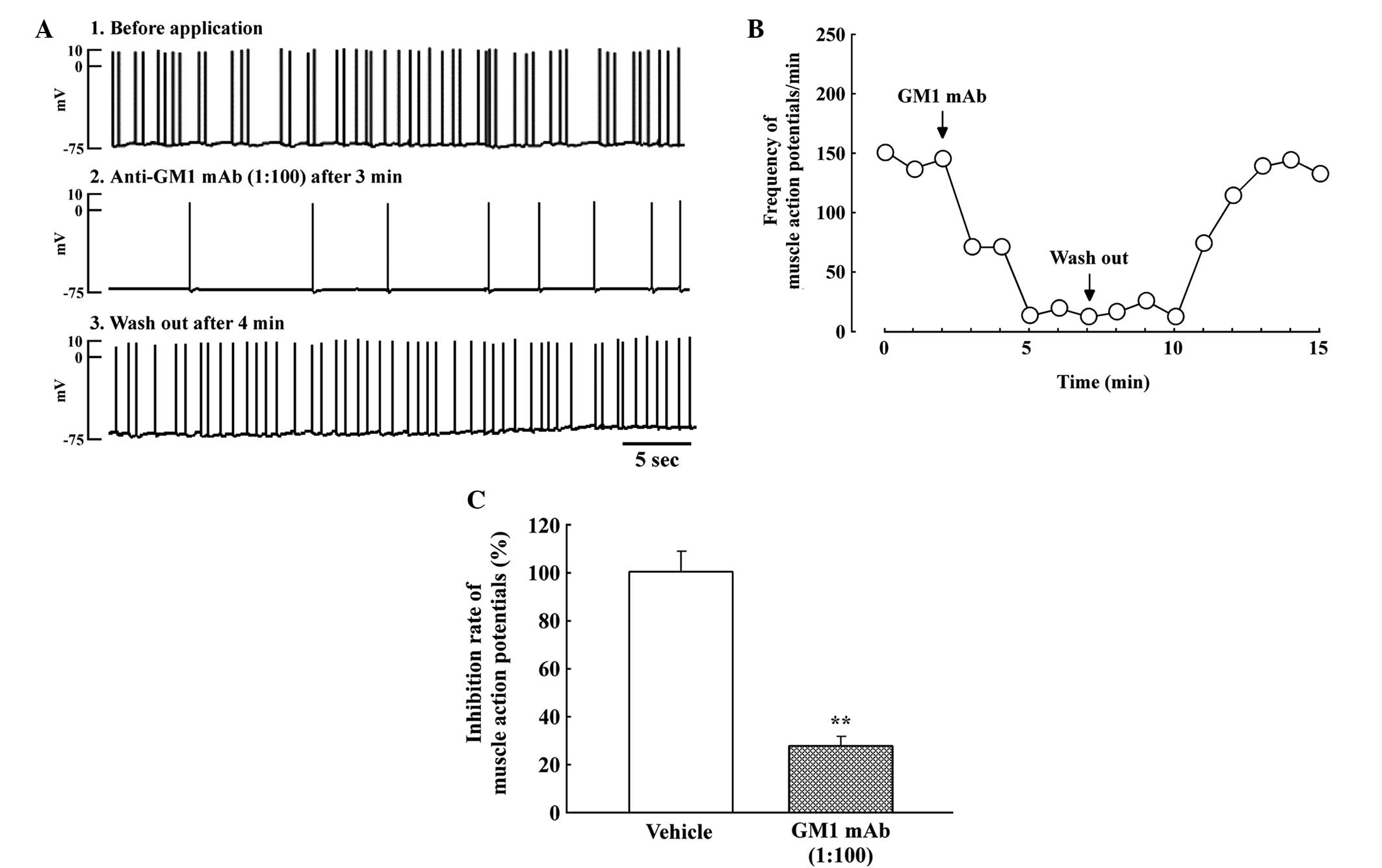
After 5–7 days of co-culture, asynchronous
contraction of numerous individual muscle fibers was observed at
the newly developed NMJs. SMAPs within the innervated muscle cells
were recorded with a frequency of 12.7±4.8 sec per 5 sec and an
amplitude of 65.4±8.4 mV (n=8, mean ± SEM). IgG anti-GM1 mAb
rapidly reduced the number of SMAPs at the NMJs within 1 min
(Fig. 1A). Subsequently, SMAPs at
the NMJs were blocked completely (Fig.
1B). The inhibitory effect of the IgG anti-GM1 mAb was reversed
by washing with the co-culture medium. Thus, IgG anti-GM1 mAb
significantly reduced the rate of SMAPs at the NMJs (n=4,
70.8±4.8%; Fig. 1C).
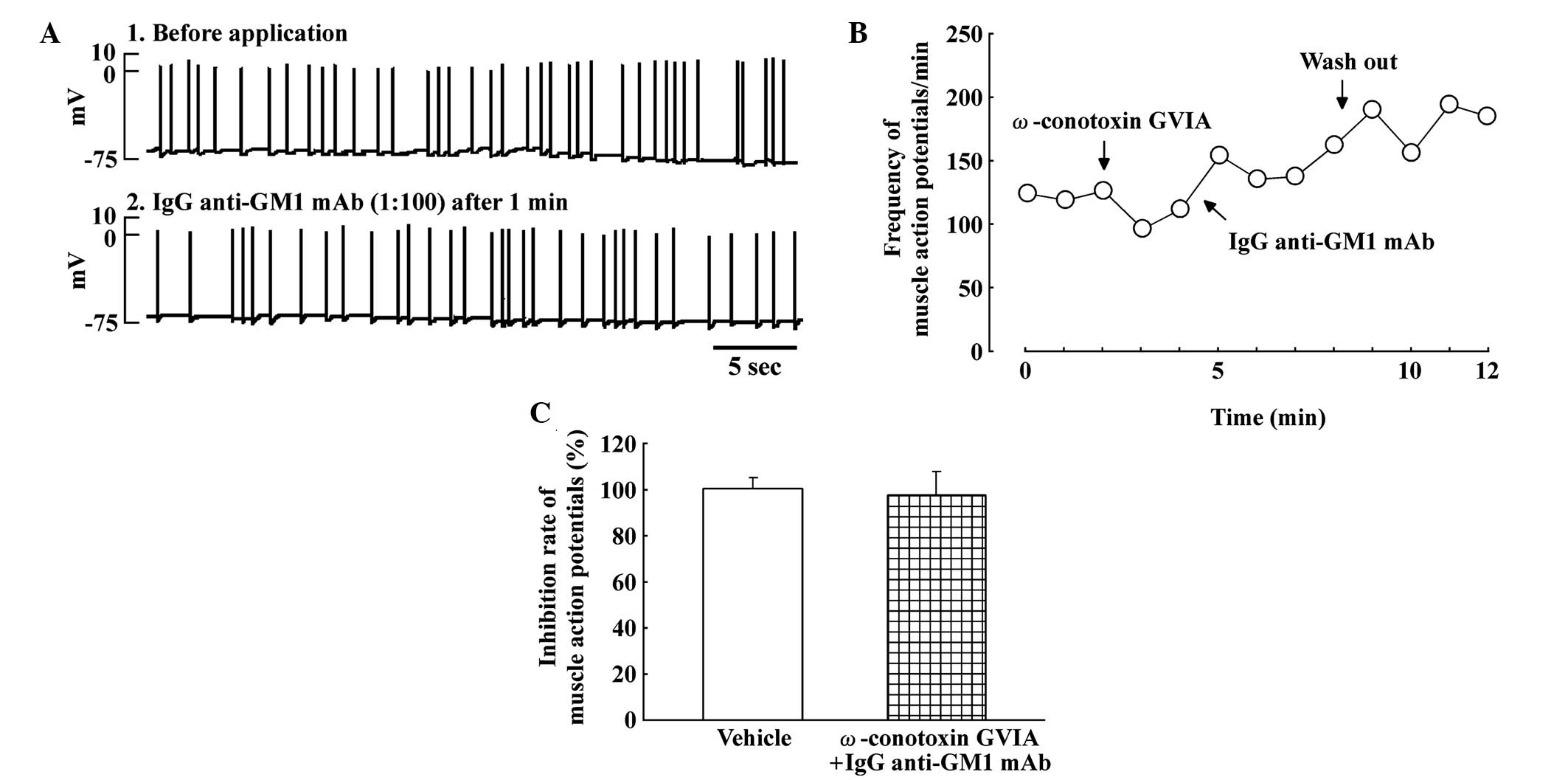
Effects of pretreatment with
ω-conotoxin GVIA on SMAPs in the spinal cord-muscle co-culture
system
Fig. 2 shows the
effect of pretreatment with the N-type calcium channel blocker,
ω-conotoxin GVIA (30 nM), and IgG anti-GM1 mAb on the SMAPs. The
inhibitory effect of IgG anti-GM1 mAb was completely blocked by
pretreatment with ω-conotoxin GVIA in the spinal cord-muscle
co-culture systems (Fig. 2A).
Fig. 2B shows the time course of the
reversal of the IgG anti-GM1 mAb-induced inhibition of SMAPs by
pretreatment with ω-conotoxin GVIA. Thus, pretreatment with
ω-conotoxin GVIA attenuated the inhibitory effect on SMAPs induced
by IgG anti-GM1 mAb (Fig. 2C).
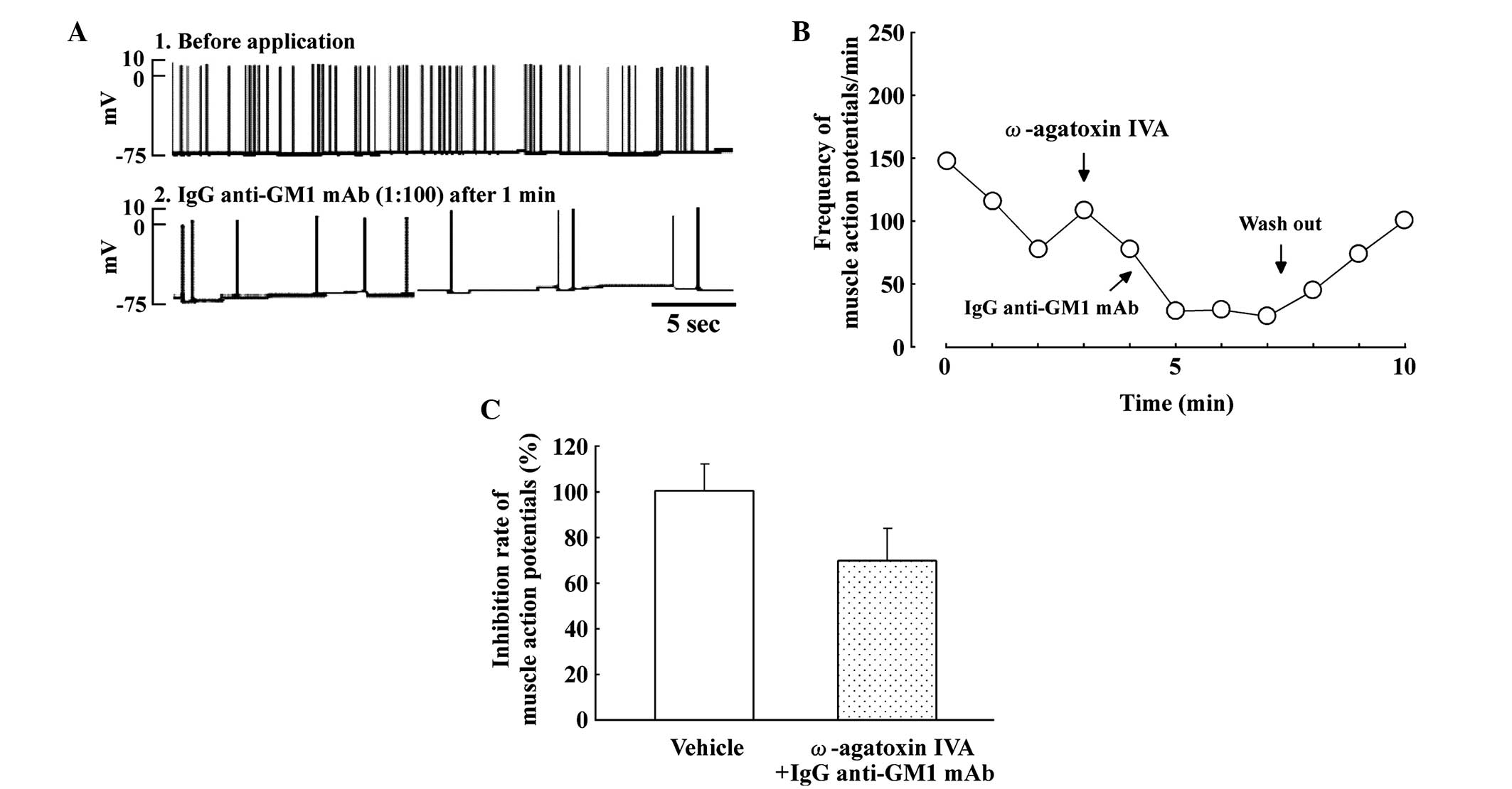
Effects of ω-agatoxin IVA on SMAPs in
the spinal cord-muscle co-culture system
Fig. 3 shows the
effect of the P/Q-type calcium channel blocker, ω-agatoxin IVA (10
nM), and IgG anti-GM1 mAb on SMAPs. ω-agatoxin IVA slightly
decreased the frequency of SMAPs in the spinal cord-muscle
co-culture systems (Fig. 3A). In
addition, the inhibitory effect of the IgG anti-GM1 mAb was
partially blocked by pretreatment with ω-agatoxin IVA in spinal
cord-muscle co-cultures. Fig. 3B
shows the time course of the partial reversal of the inhibitory
effect on SMAPs produced by IgG anti-GM1 mAb following pretreatment
with ω-agatoxin IVA. Therefore, pretreatment with ω-agatoxin IVA
slightly attenuated the inhibitory effect on SMAPs induced by IgG
anti-GM1 mAb (Fig. 3C).
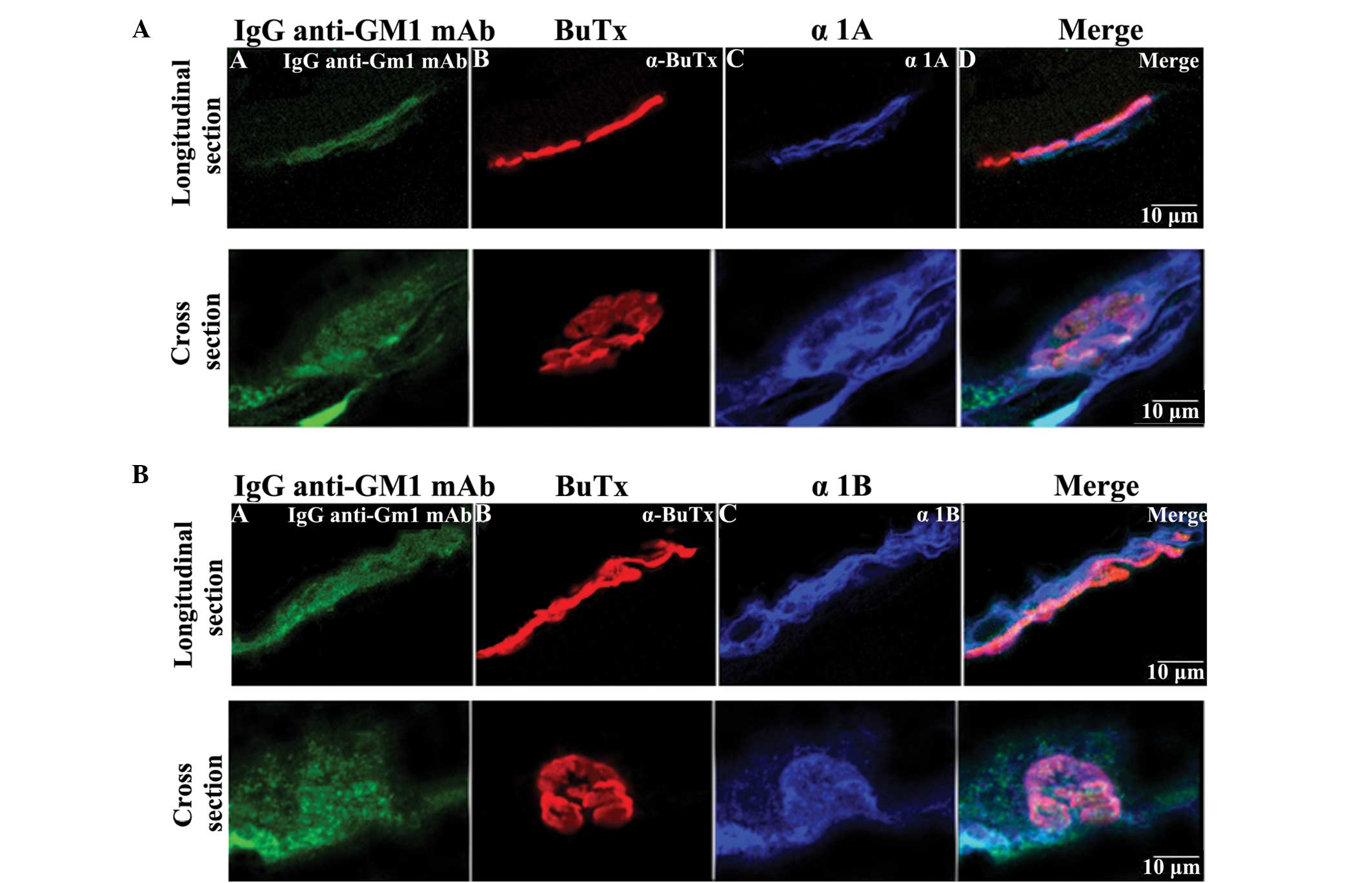
Immunohistochemical staining using IgG
anti-GM1 mAb and calcium channel antibodies in hemidiaphragm
sections
The localization of IgG anti-GM1 mAb and various
calcium channels in the nerve terminal was determined using
immunohistochemistry. As shown in Fig.
4A, the rat hemidiaphragm sections were found to stain
positively for the anti-Cav2.1 (α1A; P/Q-type
calcium channel; 1:1,000; blue) antibody, and the staining
overlapped partially with the IgG anti-GM1 mAb staining (green) and
α-BuTx staining (red). In addition, anti-Cav2.2
(α1B; N-type calcium channel; 1:1,000; blue) antibody
staining overlapped with IgG anti-GM1 mAb staining and α-BuTx
staining, similarly to the anti-α1A antibody staining
(Fig. 4B). Thus, IgG anti-GM1 mAb
binding was localized at the motor nerve terminal, and the staining
corresponded with N-type calcium channels at the motor nerve
terminal and partially overlapped with P/Q-type calcium channel
staining.
Discussion
The results of the present study indicated that the
inhibitory effects on SMAPs induced by IgG anti-GM1 mAb were
completely blocked by pretreatment with ω-conotoxin GVIA, an N-type
calcium channel blocker, in spinal cord-muscle co-culture systems.
However, pretreatment with the P/Q-type calcium channel blocker,
ω-agatoxin IVA, only partially blocked the IgG anti-GM1 mAb-induced
inhibition of SMAPs. Previous studies have demonstrated that
calcium channels are targets for anti-ganglioside antibody-mediated
attack (11,12); however, the mechanism through which
the IgG anti-GM1 mAb inhibits neurotransmitter release and the
activity of calcium channels has not been fully clarified. Buchwald
et al (13) and Ortiz et
al (14) proposed that the sera
of patients with GBS may block calcium channels located on axon
terminals. In addition, a number of studies have indicated that
calcium ion influx via VDCCs triggers the release of acetylcholine
from motor nerve terminals at NMJs (15–17).
Furthermore, prior incubation with ω-agatoxin IVA has been shown to
completely block the inhibitory effect of IgM mAbs against GM2,
GalNAc-GD1a and GalNAc-GM1b on neurotransmitter release (14). Thus, the present results indicate
that the IgG anti-GM1 mAb blocks calcium influx via N-type and
P/Q-type calcium channels. In addition, the IgG anti-GM1 mAb
antibody was previously demonstrated to inhibit VDCC currents
(6). The present data may be
explained by the involvement of VDCC currents in the effects of IgG
anti-GM1 mAb on neurotransmitter release.
IgG anti-GM1 mAb staining was observed to overlap
with P/Q-type and N-type calcium channel staining, indicating that
the IgG anti-GM1 mAb binds to the two types of calcium channels at
the motor nerve terminal of the rat hemidiaphragm. GM1, GD1 and
GD1b ganglioside epitopes exist in presynaptic membranes or the
nodal region, which is crucial for peripheral nerve transmission.
Previous studies have demonstrated the rapid uptake of
anti-ganglioside antibodies at the presynaptic motor nerve
terminal, as compared with the axolemmal membrane at the node of
Ranvier (18,19). Thus, P/Q-type and N-type calcium
channels are implicated as targets for autoantibodies in patients
with GBS. However, GBS is associated with antibodies against
numerous gangliosides, including GM1b, GD1a, GD1b, GalNAc-GD1a and
GQ1b; thus, whether SMAPs are additionally inhibited by other
anti-ganglioside antibodies is yet to be determined.
In conclusion, the results of the present study
demonstrated that the binding site for the IgG anti-GM1 mAb is
present on P/Q-type and N-type calcium channels in the nerve
terminals of NMJs. Thus, IgG anti-GM1 antibodies may be among the
factors that result in muscle weakness in patients with GBS through
binding to calcium channels.
Acknowledgements
This study was supported in part by a grant from the
Japan Society for the Promotion of Science Grants-in-Aid for
Scientific Research (no. 22590087).
References
|
1
|
Buchwald B, Toyka KV, Zielasek J,
Weishaupt A, Schweiger S and Dudel J: Neuromuscular blockade by IgG
antibodies from patients with Guillain-Barré syndrome: A
macro-patch-clamp study. Ann Neurol. 44:913–922. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Hanlon GM, Bullens RW, Plomp JJ and
Willison HJ: Complex gangliosides as autoantibody targets at the
neuromuscular junction in Miller Fisher syndrome: A current
perspective. Neurochem Res. 27:697–709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taguchi K, Ren J, Utsunomiya I, Aoyagi H,
Fujita N, Ariga T, Miyatake T and Yoshino H: Neurophysiological and
immunohistochemical studies on Guillain-Barre syndrome with IgG
anti-GalNAc-GD1a antibodies - effects on neuromuscular
transmission. J Neurol Sci. 225:91–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quattrini A, Lorenzetti I, Sciorati C,
Corbo M, Previtali SC, Feltri ML, Canal N, Wrabetz L, Nemni R and
Clementi E: Human IgM anti-GM1 autoantibodies modulate
intracellular calcium homeostasis in neuroblastoma cells. J
Neuroimmunol. 114:213–219. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hotta S, Nagaoka T, Taguchi K, Nakatani Y,
Utsnomiya I, Masuda Y, Abe K and Yuki N: Neurophysiological and
immunohistochemical studies of IgG anti-GM1 monoclonal antibody on
neuromuscular transmission: Effects in rat neuromuscular junctions.
Neurol Sci. 35:205–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakatani Y, Hotta S, Utsunomiya I, Tanaka
K, Hoshi K, Ariga T, Yu RK, Miyatake T and Taguchi K: Cav2.1
voltage-dependent Ca2+ channel current is inhibited by
serum from select patients with Guillain-Barré syndrome. Neurochem
Res. 34:149–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davies A, Douglas L, Hendrich J, Wratten
J, Tran Van Minh A, Foucault I, Koch D, Pratt WS, Saibil HR and
Dolphin AC: The calcium channel alpha2delta-2 subunit partitions
with CaV2.1 into lipid rafts in cerebellum: Implications for
localization and function. J Neurosci. 26:8748–8757. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taverna E, Saba E, Rowe J, Francolini M,
Clementi F and Rosa P: Role of lipid microdomains in P/Q-type
calcium channel (Cav2.1) clustering and function in presynaptic
membranes. J Biol Chem. 279:5127–5134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakatani Y, Nagaoka T, Hotta S, Utsunomiya
I, Yoshino H, Miyatake T, Hoshi K and Taguchi K: IgG
anti-GalNAc-GD1a antibody inhibits the voltage-dependent calcium
channel currents in PC12 pheochromocytoma cells. Exp Neurol.
204:380–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taguchi K, Shiina M, Shibata K, Utsunomiya
I and Miyatake T: Spontaneous muscle action potentials are blocked
by N-type and P/Q-calcium channels blockers in the rat spinal
cord-muscle co-culture system. Brain Res. 1034:62–70. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka Y, Waki H, Kon K and Ando S:
Gangliosides enhance KCl-induced Ca2+ influx and
acetylcholine release in brain synaptosomes. Neuroreport.
8:2203–2207. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ledeen RW and Wu G: Ganglioside function
in calcium homeostasis and signaling. Neurochem Res. 27:637–647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buchwald B, Zhang G, Vogt-Eisele AK, Zhang
W, Ahangari R, Griffin JW, Hatt H, Toyka KV and Sheikh KA:
Anti-ganglioside antibodies alter presynaptic release and calcium
influx. Neurobiol Dis. 28:113–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ortiz N, Rosa R, Gallardo E, Illa I, Tomas
J, Aubry J, Sabater M and Santafé M: IgM monoclonal antibody
against terminal moiety of GM2, GalNAc-GD1a and GalNAc-GM1b from a
pure motor chronic demyelinating polyneuropathy patient: Effects on
neurotransmitter release. J Neuroimmunol. 119:114–123. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwasaki S, Momiyama A, Uchitel OD and
Takahashi T: Developmental changes in calcium channel types
mediating central synaptic transmission. J Neurosci. 20:59–65.
2000.PubMed/NCBI
|
|
16
|
Pagani R, Song M, McEnery M, Qin N, Tsien
RW, Toro L, Stefani E and Uchitel OD: Differential expression of
alpha 1 and beta subunits of voltage dependent Ca2+
channel at the neuromuscular junction of normal and P/Q
Ca2+ channel knockout mouse. Neuroscience. 123:75–85.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urbano FJ, Pagani MR and Uchitel OD:
Calcium channels, neuromuscular synaptic transmission and
neurological diseases. J Neuroimmunol. 201–202:136–144. 2008.
View Article : Google Scholar
|
|
18
|
Fewou SN, Rupp A, Nickolay LE, Carrick K,
Greenshields KN, Pediani J, Plomp JJ and Willison HJ:
Anti-ganglioside antibody internalization attenuates motor nerve
terminal injury in a mouse model of acute motor axonal neuropathy.
J Clin Invest. 122:1037–1051. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fewou SN, Plomp JJ and Willison HJ: The
pre-synaptic motor nerve terminal as a site for antibody-mediated
neurotoxicity in autoimmune neuropathies and synaptopathies. J
Anat. 224:36–44. 2014. View Article : Google Scholar : PubMed/NCBI
|