Introduction
γ-aminobutyric acid (GABA) is the principal
inhibitory neurotransmitter that activates GABAA,
GABAB and GABAC receptors in the mammalian
brain (1,2). The inhibitory synaptic transmission is
decreased or terminated by the reuptake of the released GABA in the
synaptic cleft through GABA transporters, which is considered to be
the key action of the current termination. GABA transporters are,
therefore, involved in maintaining a low extracellular GABA
concentration throughout the brain, preventing the tonic phenomenon
caused by excessive activation of synaptic and extrasynaptic
receptors (2).
GAT is highly expressed in the vesicle membrane, the
presynaptic membrane and the glial cell membranes, and belongs to a
family of electrogenic sodium-dependent transporters (3). Four GABA transporters (GAT-1-4) have
been identified and cloned (4,5). The
affinity of the receptors for GABA is as follows:
GAT-1>GAT-3>GAT-2>GAT-4 (6); thus, GAT-1 has the largest ability to
uptake GABA in the brain (5). GAT-1
is particularly abundant in areas rich in GABAergic neurons, such
as the cerebellum, hippocampus, neocortex and retina (7). GAT-1 is primarily responsible for the
removal of GABA from the synaptic cleft and the termination of
GABAergic neurotransmission; therefore, the transporter plays an
important role in the metabolism of GABA (8).
Increasing evidence has demonstrated that the GABA
system is important in the pathogenesis of anxiety (9,10). In
humans and animals, stimulation of GABA receptors generally
produces anxiolytic activity, while antagonists produce
anxiogenic-like effects (11). The
GABA system is also known to be involved in the modulation of
memory and learning (12,13); however, the underlying mechanisms are
yet to be fully elucidated. In the present study, GAT-1 knockout
(GAT-1−/−) mice were used as a model. Three types of
behavioral tests, namely the open field test, elevated 0-maze (EZM)
and Morris water maze, were used to evaluate anxiety-like behaviors
and cognitive function in the GAT-1−/− and wild-type
(WT) mice.
Materials and methods
Animals
Experimental protocols and the use of animals were
performed in compliance with the Guidelines for Animal Experiments
of the Chinese Academy of Medical Sciences (Beijing, China) and
with approval from the Ethics Committee for Animal Care at Jinshan
Hospital (Shanghai, China). In total, 20 adult male
GAT-1−/− and WT mice were obtained from
Shanghai South Biomodel Organism Co., Ltd. (Shanghai, China) and
housed in the animal center of Jinshan Hospital. The animals were
maintained under a 12-h light/dark cycle at 22°C and 50% humidity.
The animal rooms were kept neat and uncluttered. The drinking water
was autoclaved and changed everyday, and the animal cages were
disinfected by ultraviolet light. The padding used in the cages was
also changed everyday. Food and water were available ad
libitum, with the exception of during the tests. The mice (age,
6–8 weeks) were divided into two groups (n=10): GAT-1−/−
and WT.
Open-field test
The open-field test is widely used to test
anxiety-like behavior and activity in animals (14). Mice have an innate tendency to escape
bright, open new surroundings. The open-field test can cause
anxiety-like behavior; thus, the test is applicable to the
assessment of motor behavior caused by anxiety. The open field
(DuoYi animal behavior analysis system; Shanghai Mobile Datum Co.,
Shanghai, China) was a square arena (50 cm3) with white
plastic walls and floor (ABS engineering plastic; Shanghai Mobile
Datum Co., Shanghai, China). The mice were placed in the center of
the box and allowed to freely explore for a 5-min period. The mice
were recorded using a camera (Shanghai Mobile Datum Co.,) fixed
above the floor, which was analyzed with a video tracking system
(Shanghai Mobile Datum Co.) that divided the arena into ‘margin’
and ‘center’ fields. The center field was defined as the central
25% area of the open field. The software (Shanghai Mobile Datum
Co.) automatically recorded a mice motion curve, identifying the
movement and stationary state of the mice. The arenas were cleaned
with a 70% alcohol solution between trials to remove excrement and
odor.
EZM
The exploratory drive of mice and their natural
avoidance of heights and open spaces were used in the EZM to
investigate the anxiety-like behavior of the mice (14). The EZM is modelled on the
elevated-plus-maze. The advantage of the EZM is that it removes the
ambiguous central square of the traditional elevated-plus-maze. The
EZM (Shanghai Mobile Datum Co.) consisted of a circular platform
(46 cm in outer diameter, 5.5 cm in runway width) that was elevated
40 cm above the floor. On the top of the platform, there were two
open and two enclosed segments. The closed segments were enclosed
by walls extending 20 cm above the surface of the platform. Each
test started by placing the mouse in any closed sector, and the
test session lasted for 5 min. Performance was recorded using a
video-camera placed above the EZM. The video tracking software
recorded the path moved, the percentage of time spent in the open
and closed segments, and the number of open and closed segment
entries. The EZM was cleaned with water between trials.
Morris water maze
The water maze was used to measure spatial learning
and memory ability (15,16). The apparatus (Shanghai Mobile Datum
Co.) was a circular swimming pool (100 cm in diameter, 50 cm high)
with black plastic walls and floor (ABS engineering). The swimming
pool was filled with water maintained at 24–26°C to a depth of 30
cm. Water was added with milk powder to enable clear observations
of the black mice and record their movement curve. The pool was
divided into four equal quadrants by four entry points marked on
the pool wall and a white escape platform was set in the center of
the target quadrant (1 cm below the water level). Each quadrant was
marked with a different shape in order to provide visual clues to
aid the mice in finding the escape platform. The position of the
platform was fixed throughout the place navigation test. The
platform was the only escape route for the mice in the water; thus,
the mice were required to search for the hidden underwater
platform. This task consisted of place navigation tests four times
a day for five consecutive days, with intertrial intervals of 15–20
min, followed by probe trials on the sixth day. In each trial, a
mouse was released into the water, facing the pool wall, from one
of the quadrants with the exception of the target quadrant. The
mice were allowed to swim for a maximum of 60 sec until they found
the platform. If the mouse failed to find the platform in 60 sec,
the mouse was gently placed on the platform and allowed to stay on
it for 10 sec prior to the next trial. On the probe trial day, the
platform was removed and each mouse was released into the pool from
the same position. The swimming paths of the mice were recorded for
60 sec and monitored by a camera mounted above the center of the
pool. Following the trial, the mice were placed in clean padding
and allowed to warm up and dry. The room was maintained at 22–24°C
and the water in the pool was changed everyday.
Data collection from the open-field
test
Rodents prefer to move around the periphery of an
apparatus than explore the central area when they are placed in a
novel environment (17). This
feature can protect animals from the invasion of outsiders. The
time spent in and the number of visits to the central area of the
open field is considered to be inversely correlated with the level
of anxiety-like behavior of mice, while the movement distance and
the kinematic velocity reflect the motility and active degree
(18).
The following parameters were assessed: Total
distance moved, velocity, the rest time during observation periods
and time spent in the central area, number of visits to the central
area and the distance traveled in the central area.
Data collection of EZM
During an EZM, based on their natural avoidance of
heights and open spaces, mice usually avoid the two open arms and
spend the majority of time in the two closed arms, while the search
for novel, open environment drives them into the open areas
(19). The time spent in the open
spaces and the total number of open arm entries and closed arm
entries are inversely correlated with the level of anxiety-like
behaviors (20). In addition, the
distance traveled in the maze reflects the motility and the degree
of activity.
The following parameters were assessed in the EZM
test: The number of total entries to the open arms and closed arms,
the proportion of time spent in the open arms and the distance
traveled in the maze.
Data collection of Morris water
maze
Although mice are natural swimmers, they dislike the
state of being in the water. Furthermore, swimming is physically
exhausting, and mice instinctively seek the rest area in the water.
This behavior involves a complex process of memory, including
collecting visual information associated with the spatial
orientation, dealing with, sorting, memorizing and strengthening
the information, with the purpose of finding the hidden platform in
the water (21) and finally escaping
from the water. In the training session, mice with a shorter escape
latency are considered to have stronger abilities of spatial
learning and memory. While in the probe trial, mice that spent a
longer time in the target quadrant had a more accurate location and
spatial memory (15).
The following parameters were assessed during the
Morris water maze: Average escape latency in the navigation test,
the proportion of time spent in each quadrant and the swimming
trace in the probe trial.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences between the two groups in the navigation test
in the Morris water maze were compared using repeated-measures
one-way analysis of variance (ANOVA). Other data comparisons were
analyzed using an independent sample t-test. P<0.05 was
considered to indicate a statistically significant difference in
all the statistical evaluations. Statistical analyses were
performed using SPSS 19.0 statistical software (IBM SPSS, Inc.,
Armonk, NY, USA).
Results
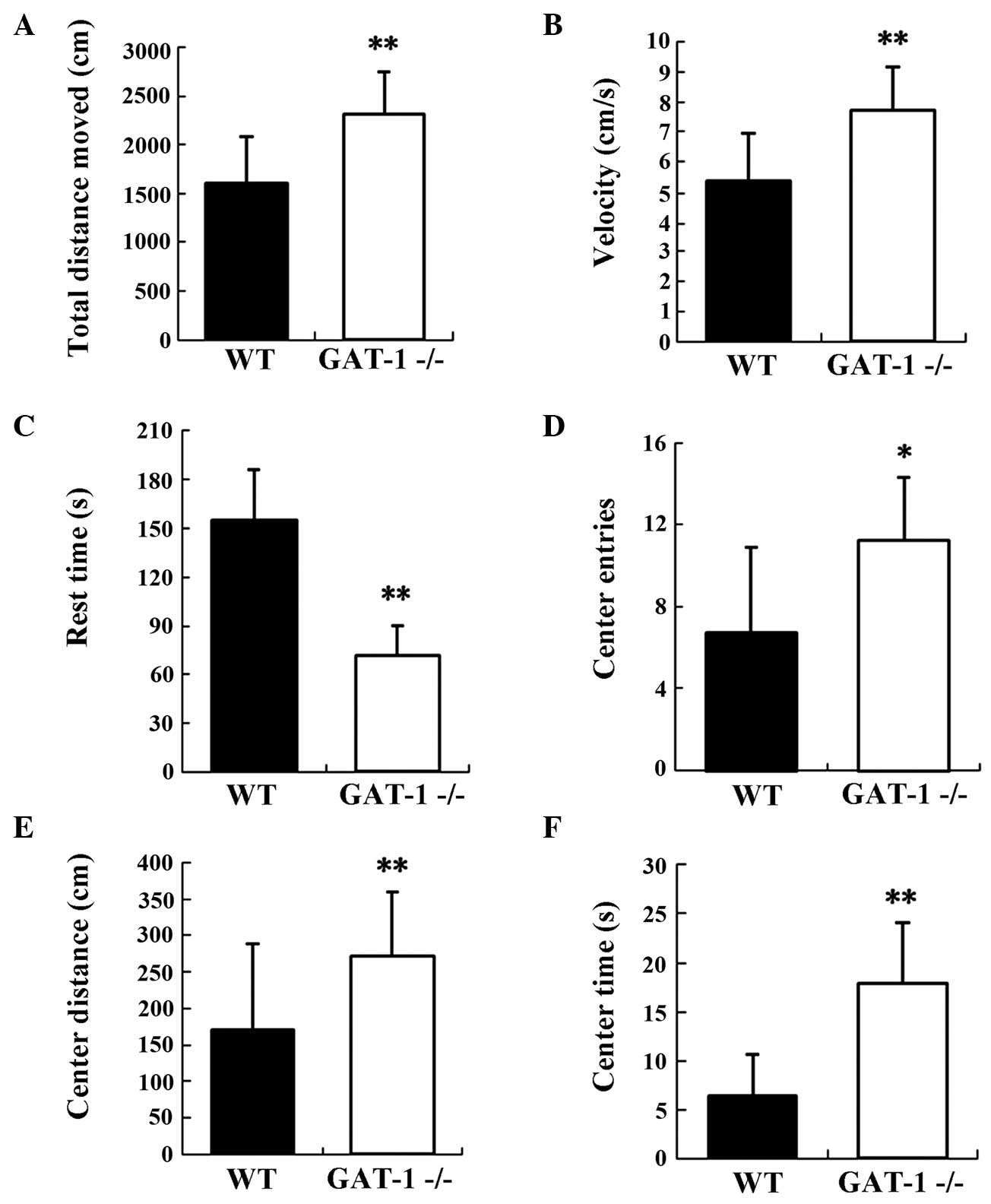
Assessment of the open-field test
In the open-field test,
GAT-1−/− mice traveled greater distances
compared with the WT mice (GAT-1−/−, 2,312.98±439.58 cm;
WT, 1,607.78±476.26 cm; P<0.01), and exhibited enhanced
kinematic velocity (GAT-1−/−, 7.70±1.46
cm/sec; WT, 5.36±1.59 cm/sec; P<0.01), with a significant
reduction in rest time (GAT-1−/−, 71.97±18.42 sec; WT,
155.30±30.32 sec; P<0.01). The GAT-1−/−
mice manifested hyperactivity and enhanced motility compared with
the WT mice. In addition, the GAT-1−/− mice spent more
time in the central area (GAT-1−/−,
17.87±6.16 sec; WT, 6.43±4.20 sec; P<0.01) and made more entries
into the central area when compared with the WT mice
(GAT-1−/−, 11.22±3.0; WT, 6.70±4.22; P<0.05).
GAT-1−/− mice also showed a significant
increase in the distance traveled in the central area
(GAT-1−/−, 272.36±87.09 cm; WT, 170.39±117.68 cm;
P﹤0.01). These parameters are inversely correlated with the level
of anxiety-related proneness, indicating that
GAT-1−/− mice showed decreased anxiety-like
behaviors in comparison with the WT mice (Fig. 1).
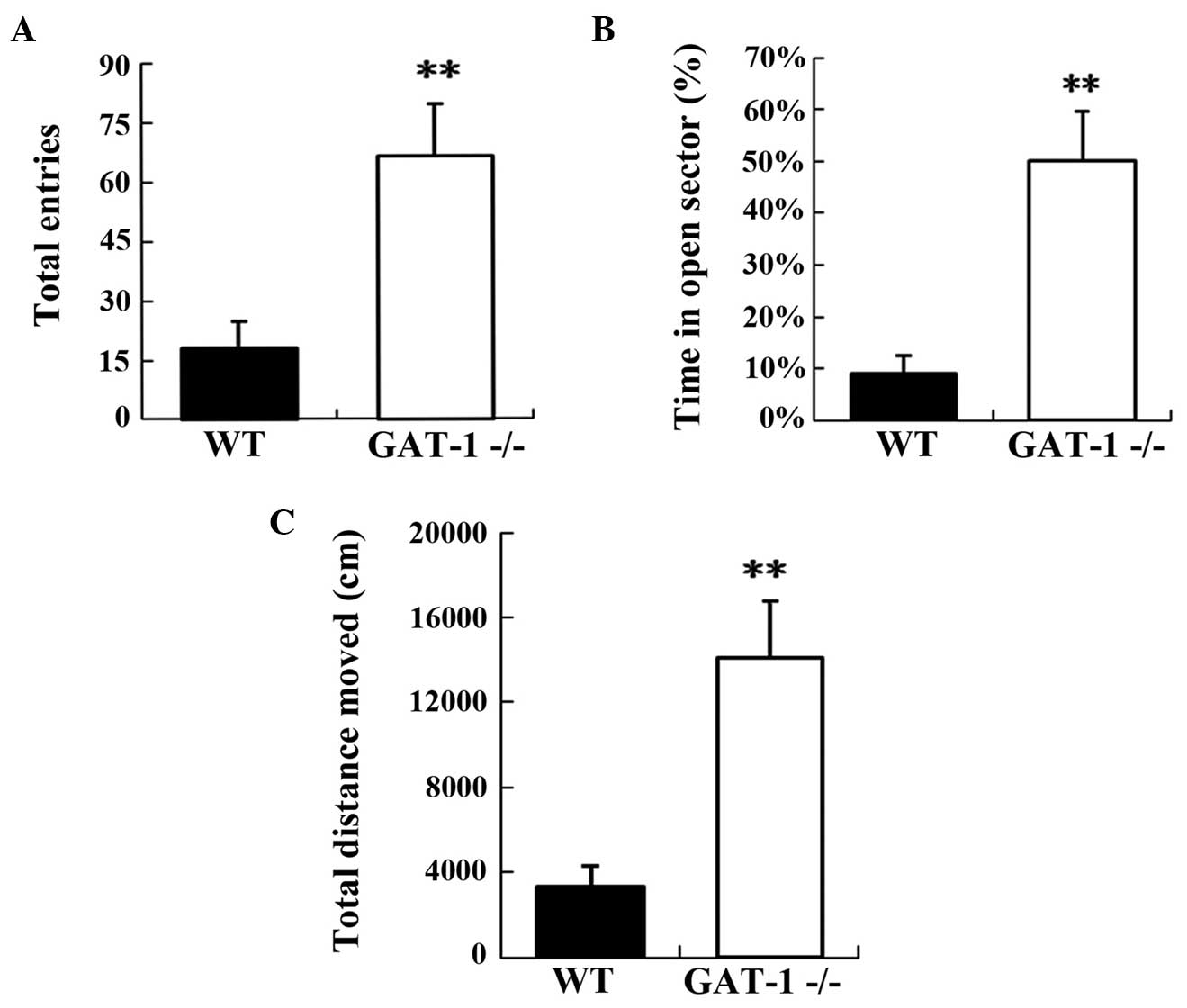
Assessment of the EZM test
GAT-1−/− mice manifested hyperactivity
and enhanced motility in the EZM test, as compared with the WT
mice, and traveled greater distances
(GAT-1−/−, 14,097.96±2,775.40 cm; WT,
3,356.12±968.37 cm; P<0.05). The results revealed that
GAT-1−/− mice had a significantly higher total number of
entries into the open and closed sectors compared with the WT mice
(GAT-1−/−, 66.78±13.07; WT, 18.00±6.83;
P<0.01). In addition, the GAT-1−/− mice exhibited an
increased percentage of time spent in the open sectors when
compared with the WT mice (GAT-1−/−,
50.00±0.097%; WT, 9.00±0.036%, P﹤0.01). Therefore, the results
confirmed that GAT-1−/− mice demonstrated reduced
anxiety-like behaviors (Fig. 2).
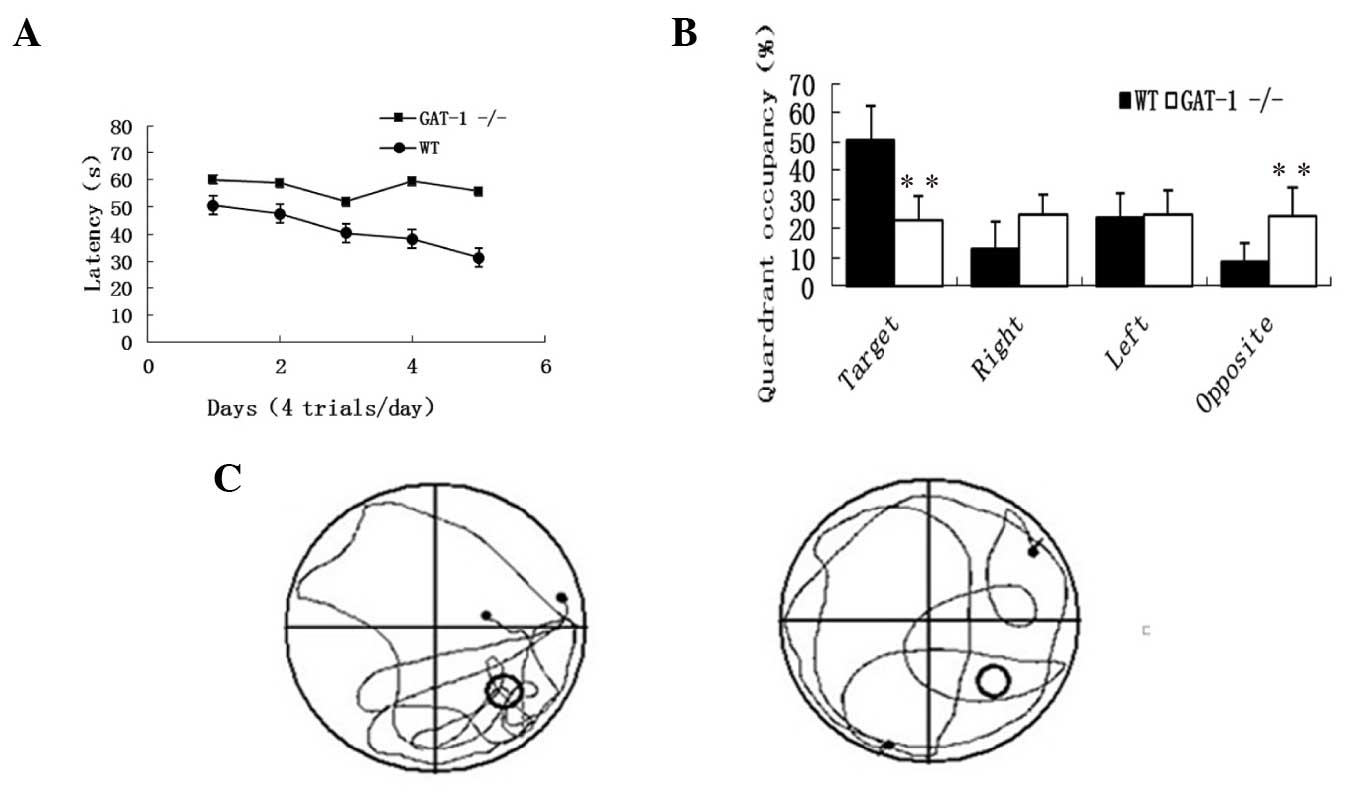
Assessment of the Morris water maze
test
During the learning session, repeated-measures ANOVA
indicated a significant difference between the WT and
GAT-1−/− mice (F=14.48, P=0.001), with
GAT-1−/− mice exhibiting significantly longer latencies
compared with the WT mice (F=3.23, P<0.05). Evidently, the
GAT-1−/− mice learned at a slower pace in
comparison to the WT mice. With regard to the GAT-1−/−
mice, the time spent between the four quadrants during the probe
test was not significantly different, while the WT mice spent 50.7%
of the total time on the target quadrant and only 8.82% on the
opposite quadrant. Compared with the WT mice,
GAT-1−/− mice spent less time in the target
quadrant (P<0.01). These results confirmed that
GAT-1−/− mice exhibited impaired spatial learning
ability and memory (Fig. 3).
Discussion
Increasing evidence has demonstrated that the
GABAergic system is involved in the pathogenesis of anxiety. Drugs
and GABA analogs can significantly reduce the anxiety-like effects
through affecting the neurotransmitter metabolism (22–24). The
open-field and EZM tests were used in the current study to evaluate
the anxiety-like behaviors of GAT-1−/− mice. When
compared with the WT mice, the experimental results indicated that
the GAT-1−/− mice showed decreased anxiety-like
behaviors (P<0.05). The results obtained are consistent with a
previous study which demonstrated that in tests for anxiety-like
behaviors, such as the light-dark exploration test, emergence test
or elevated-plus maze, the GAT-1−/− mice were prone to
exhibit reduced anxiety (25). A
GAT-1 deletion is hypothesized to cause an enhanced concentration
of intracephalic GABA, which results in hyperactivity of GABAergic
neurons and a consequent reduction in anxiety-like behaviors. A
mutant of GAT-1 (SCL6A1) has been previously reported to be
involved in the pathogenesis of anxiety (26).
A definite association between the GABAergic system
and cognitive function has been established (12,13);
however, the mechanism remains unclear. Clinical evidence suggests
that tiagabine, a GABA reuptake inhibitor, can improve verbal
memory when used as an adjunctive therapy in the treatment of
convulsions (27). Similarly,
NNC-711 (an analog of tiagabine) can also enhance cognitive
function (28); however, there is
contradictory evidence showing that tiagabine impaired the spatial
learning of rats in the Morris water maze (29). At present, whether GAT-1 inhibitors
are able to enhance or impair cognitive function remains
controversial. In the present study, GAT-1 gene deletion was shown
to result in impaired spatial learning and memory ability in
GAT-1−/− mice. Cognitive behavioral tests, such as
passive avoidance and contextual fear conditioning, have previously
demonstrated that GAT-1−/− mice exhibit impaired
hippocampus-dependent learning and memory (12). In addition, the cognitive function of
GAT-1 overexpressing transgenic mice was found to be impaired in
conditioned avoidance and novel object recognition tasks (30). These results indicated that GAT-1 can
antiport and release GABA in normal and pathological conditions
(31,32); however, the specific mechanism of
GAT-1 antiport is not clear. Further research may help to clarify
the function of GAT-1 in the modulation of excitatory and
inhibitory amino acids in the brain.
Synaptic plasticity is considered to be one of the
cellular mechanisms of learning and memory, and long-term
potentiation (LTP) is an important form of synaptic plasticity
(33). GAT-1 disruption has been
demonstrated to specifically impair theta-burst stimulation-induced
LTP and hippocampus-dependent learning and memory (15). Recent advances have revealed that
GAT-1 heterozygous mice (GAT-1+/−) manifested enhanced
learning and memory ability through two behavioral experiments,
namely the passive avoidance paradigm and the Morris water maze
(34). By recording the field
potential in the CA1 area of the hippocampus of three genetic
phenotypes (GAT-1+/+, GAT-1+/− and
GAT-1−/−), GAT-1−/− mice were found to have a
decreased LTP in the hippocampus, while GAT-1+/− mice
exhibited enhanced LTP. The results indicated that changing the
activity of GAT-1 can alter the LTP in the CA1 region of the
hippocampus (34). Therefore, the
differing extent of GAT-1 deficiency may result in distinct effects
on GABA metabolism. GAT-1+/− mice exhibited increased
learning and memory, while homozygous GAT-1−/− mice
exhibited impaired hippocampus-dependent learning and memory
(34). Only a moderate reduction in
GAT-1 activity caused an enhancement of learning and memory in
mice.
In conclusion, the present study demonstrated that
GAT-1 was involved in anxiety-like behaviors and cognitive
function; thus, the transporter may be a potential target for the
treatment of anxiety in the future.
Acknowledgements
This study was supported by grants from the Science
and Technology Commission of Jinshan District, Shanghai, China
(2012-3-3).
References
|
1
|
Hu J and Quick MW: Substrate-mediated
regulation of gamma-aminobutyric acid transporter 1 in rat brain.
Neuropharmacology. 54:309–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiu CS, Brickley S, Jensen K, et al: GABA
transporter deficiency causes tremor, ataxia, nervousness, and
increased GABA-induced tonic conductance in cerebellum. J Neurosci.
25:3234–3245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanner BI: Structure and function of
sodium-coupled GABA and glutamate transporters. J Membr Biol.
213:89–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu QR, Lόpez-Corcuera B, Mandiyan S,
Nelson H and Nelson N: Molecular characterization of four
pharmacologically distinct gamma-aminobutyric acid transporters in
mouse brain [corrected]. J Biol Chem. 268:2106–2112.
1993.PubMed/NCBI
|
|
5
|
Chiu CS, Jensen K, Sokolova I, et al:
Number, density and surface/cytoplasmic distribution of GABA
transporters at presynaptic structures of knock-in mice carrying
GABA transporter subtype 1-green fluorescent protein fusions. J
Neurosci. 22:10251–10266. 2002.PubMed/NCBI
|
|
6
|
Ueda Y and Willmore LJ: Hippocampal
γ-aminobutyric acid transporter alterations following focal
epileptogenesis induced in rat amygdala. Brain Res Bull.
52:357–361. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guastella J, Nelson N, Nelson H, et al:
Cloning and expression of a rat brain GABA transporter. Science.
249:1303–1306. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keros S and Hablitz JJ: Subtype-specific
GABA transporter antagonists synergistically modulate phasic and
tonic GABAA conductances in rat neocortex. J Neurophysiol.
94:2073–2085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lydiard RB: The role of GABA in anxiety
disorders. J Clin Psychiatry. 64:(Suppl 3). 21–27. 2003.PubMed/NCBI
|
|
10
|
Rudolph U and Möhler H: GABA-based
therapeutic approaches: GABAA receptor subtype functions. Curr Opin
Pharmacol. 6:18–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalueff AV: Neurobiology of memory and
anxiety: from genes to behavior. Neural Plast.
2007:781712007.PubMed/NCBI
|
|
12
|
Zarrindast MR, Bakhsha A, Rostami P and
Shafaghi B: Effects of intrahippocampal injection of GABAergic
drugs on memory retention of passive avoidance learning in rats. J
Psychopharmacol. 16:313–319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zarrindast MR, Noorbakhshnia M, Motamedi
F, Haeri-Rohani A and Rezayof A: Effect of the GABAergic system on
memory formation and state-dependent learning induced by morphine
in rats. Pharmacology. 76:93–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prut L and Belzung C: The open field as a
paradigm to measure the effects of drugs on anxiety-like behaviors:
a review. Eur J Pharmacol. 463:3–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong N, Li Y, Cai GQ, et al: GABA
transporter-1 activity modulates hippocampal theta oscillation and
theta burst stimulation-induced long-term potentiation. J Neurosci.
29:15836–15845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y, Hu Z, Liu G, Zhou W and Zhang Y:
Cytokines induced by long-term potentiation (LTP) recording: a
potential explanation for the lack of correspondence between
learning/memory performance and LTP. Neuroscience. 231:432–443.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Treit D and Fundytus M: Thigmotaxis as a
test for anxiolytic activity in rats. Pharmacol Biochem Behav.
31:959–962. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shin J, Gireesh G, et al: Phospholipase C
beta 4 in the medial septum controls cholinergic theta oscillations
and anxiety behaviors. J Neurosci. 29:15375–15385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coutellier L, Friedrich AC, Failing K,
Marashi V and Würbel H: Effects of foraging demand on maternal
behaviour and adult offspring anxiety and stress response in
C57BL/6 mice. Behav Brain Res. 196:192–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coutellier L, Friedrich AC, Failing K,
Marashi V and Würbel H: Effects of foraging demand on maternal
behaviour and adult offspring anxiety and stress response in
C57BL/6mice. Behav Brain Res. 196:192–199. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morris RG, Garrud P, Rawlins JN and
O'Keefe J: Place navigation impaired in rats with hippocampal
lesions. Nature. 297:681–683. 1982. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Argyropoulos SV, Sandford JJ and Nutt DJ:
The psychobiology of anxiolytic drugs: Part 2: pharmacological
treatments of anxiety. Pharmacol Ther. 88:213–227. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beleboni RO, Carolino ROG, Pizzo AB, et
al: Pharmacological and biochemical aspects of GABAergic
neurotransmission: pathological and neuropsychobiological
relationships. Cell Mol Neurobiol. 24:707–728. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stahl SM: Anticonvulsants as anxiolytics,
part 1: tiagabine and other anticonvulsants with actions on GABA. J
Clin Psychiatry. 65:291–292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu GX, Cai GQ, Cai YQ, et al: Reduced
anxiety and depression-like behaviors in mice lacking GABA
transporter subtype 1. Neuropsychopharmacol. 32:1531–1539. 2007.
View Article : Google Scholar
|
|
26
|
Thoeringer CK, Ripke S, Unschuld PG, et
al: The GABA transporter 1 (SLC6A1): a novel candidate gene for
anxiety disorders. J Neural Transm. 116:649–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kälviäinen R: Cognitive effects of
GABAergic antiepileptic drugs. Electroencephalogr Clin Neurophysiol
Suppl. 50:458–464. 1999.PubMed/NCBI
|
|
28
|
O'Connell AW, Fox GB, Kjøller C, et al:
Anti-ischemic and cognition-enhancing properties of NNC-711, a
γ-aminobutyric acid reuptake inhibitor. Eur J Pharmacol. 424:37–44.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmitt U and Hiemke C: Tiagabine, a
γ-amino-butyric acid transporter inhibitor impairs spatial learning
of rats in the Morris water-maze. Behav Brain Res. 133:391–394.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu JH, Ma YH, Jiang J, et al: Cognitive
impairment in mice over-expressing [gamma]-aminobutyric acid
transporter I (GAT1). Neuroreport. 15:9–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Richerson GB and Wu Y: Dynamic equilibrium
of neurotransmitter transporters: not just for reuptake anymore. J
Neurophysiol. 90:1363–1374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Richerson GB and Wu Y: Role of the GABA
transporter in epilepsy. Recent Advances in Epilepsy Research.
Binder DK and Scharfman HE: Springer; New York, NY: pp. 76–91.
2004, View Article : Google Scholar
|
|
33
|
Whitlock JR, Heynen AJ, Shuler MG and Bear
MF: Learning induces long-term potentiation in the hippocampus.
Science. 313:1093–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi J, Cai Y, Liu G, et al: Enhanced
learning and memory in GAT1 heterozygous mice. Acta Biochim Biophys
Sin (Shanghai). 44:359–366. 2012. View Article : Google Scholar : PubMed/NCBI
|