Introduction
Human cystic echinococcosis, also known as cystic
hydatid disease (CHD), affects humans and livestock and is caused
by infection with the larval stage of Echinococcus
granulosus. CHD can be seriously harmful to human health and
occurs worldwide (1,2). In China, the prevalence of CHD is more
extensive than that of alveolar echinococcosis, with
600,000–1,300,000 individuals suffering from CHD (3). At present, the primary methods of
treating CHD include early prevention via annual check-ups,
medication and surgical operation. However, these approaches are
frequently prohibitively expensive, particularly in undeveloped
countries and remote areas. Therefore, there is an urgent
requirement for highly effective CHD treatments that are relatively
inexpensive.
Vaccination of livestock may provide a novel
approach for the control of CHD. Previous studies have reported the
use of vaccines to effectively protect certain animals, including
sheep, goats and bovines, against CHD induced by the cysts of E.
granulosus (4–9).
In our previous study, a Chinese strain of E.
granulosus glutathione S-transferase (EgGST) was cloned and
sequenced (10), and the capacity of
EgGST to induce an immune response and immunoprotection was tested
in an experimental model of hydatidosis in mice. In the present
study, recombinant EgGST (rEgGST) was expressed in Escherichia
coli and purified for antigen preparation (11). Following the vaccination of mice with
rEgGST, the resulting immunoprotection was analyzed and the
protective mechanisms were investigated to assess the potential of
rEgGST as a novel molecular vaccine.
Materials and methods
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from freshly isolated E.
granulosus protoscoleces (PSCs) using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The E.
granulosus protoscoleces were extracted aseptically from
fertile E. granulosus cysts from the livers and lungs of
infected sheep. The EgGST gene was amplified by RT-PCR (Promega
Corporation, Madison, WI, USA) using two primers according to the
sequence of E. granulosus. Primer I: EcoRI
recognition site, 5′-ATG AAT TCA TGG CTC CCA CTC TGG CTT-3;
Primer II: NotI recognition site, 5′-GTG CGG CCG CGT
CAC CTA ACA GTC ACC AC-3. Each primer contained
EcoRI/NotI restriction enzyme sites and was
synthesized by Beijing SBS Genetech, Co., Ltd. (Beijing, China).
RT-PCR was performed in a 50-µl reaction mixture, containing 10 µl
5X buffer (Promega Corporation), 2 µl Mg2+ (25 mmol/l),
1 µl dNTP (10 mmol/l), 1 µl avian myeloblastosis virus reverse
transcriptase (Promega Corporation), 1 µl Thermus flavus DNA
polymerase (Promega Corporation), 5 µl of each primer, 5 µl RNA and
20 µl diethylpyrocarbonate-treated water. The reaction protocol for
RT-PCR was as follows: 48°C for 45 min, 94°C for 2 min, followed by
40 cycles of 30 sec at 94°C, 60 sec at 60°C and 2 min at 68°C, with
a final extension for 7 min at 68°C. RT-PCR products were
identified using 1% agarose gel electrophoresis (Liuyi Instrument
Factory, Beijing, China).
Subcloning of EgGST gene into
expression plasmid vector
The target fragment was purified using a gel cleanup
kit (SBS Genetech, Co., Ltd.) and inserted between the EcoRI
and NotI sites of the expression vector pET-28a (Novagen;
Merck KGaA, Darmstadt, Germany). The recombinant vector
EgGST/pET-28a was identified by restriction digestion using
EcoRI and NotI and the EgGST insert was verified by
sequencing, performed by Sangon Biotech Co., Ltd. (Shanghai,
China). E. coli BL21 (DE3) pLysS, provided by Dr Xiao
Wei (University of Saskatchewan, Saskatoon, Canada) was transformed
for induced expression of His6-tagged EgGST protein.
Expression and purification of
rEgGST
Protein expression was induced at 25°C by
cultivation of the transformed E. coli BL21 overnight in the
presence of isopropyl-β-D-thiogalactoside (IPTG; Promega
Corporation) at a final concentration of 0.6 mmol/l. The
recombinant His6-tagged rEgGST was purified from the extract of
transformed E. coli BL21 (DE3) by Ni2+ chelate
affinity chromatography (Novagen) according to the manufacturer's
instructions. Purified His6-tagged protein was analyzed using 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gel. Protein concentrations were determined using the
Bradford method (12).
Immunization
A total of 84 male 6-week old ICR mice were obtained
from the Experimental Animal Centre of Ningxia Medical University
(Yinchuan, China). Mice were allocated at random into two groups
containing 42 mice each. Mice in group A received three
subcutaneous immunizations with 10 µg rEgGST in 100 µl
phosphate-buffered saline (PBS) emulsified in Freund's adjuvant
(Sigma-Aldrich, St. Louis, MO, USA). The three immunizations were
delivered at 2-week intervals, starting at week 0 in Freunds
complete adjuvant and followed by two booster immunizations in
Freund's incomplete adjuvant at weeks 2 and 4. Mice in the control
group B were injected with the corresponding adjuvant and PBS. This
study was approved by the Ningxia Medical University Ethical
Committee.
Challenge infection and protective
immunity
Six weeks after the final vaccination, on week 10, a
challenge infection was induced in the mice via the intraperitoneal
injection of 1,500 PSCs. Six mice in each group were sacrificed at
different 0, 2, 4, 6, 10, 18 and 30 weeks following the initial
vaccination, in order to obtain sera and spleen cells. Mice were
sacrificed by cervical vertebra dislocation. Subsequently, the
carcasses were dissected and examined superficially for visible
hydatid cysts. The percentage of protection was determined
according to the method and formula described by Dempster et
al (13): Protective immunity in
vaccinated mice (%) = (average number of cysts in the test
group/average number of cysts in the control group) × 100.
Serum collection
Six mice in each group were sacrificed at 0, 2, 4,
6, 10, 18 and 30 weeks following the initial vaccination in order
to observe the macroscopic and microscopic effects of parasite
development. Serum samples were collected and stored individually
at −84°C.
Cytokine measurements using ELISA
The optical density values of a number of cytokines
were determined using ELISA. Spleens were isolated from mice and
splenocytes were harvested. A suspension of single splenocytes was
prepared after removing erythrocytes via hypotonic lysis and
resuspending the samples in RPMI 1640 (Gibco Life Technologies,
Carlsbad, CA, USA) by vigorous pipetting. Viable cells counted by
trypan blue exclusion (5×106 cells/ml) were exposed to
medium, 5 µg/ml concanavalin A (Sigma-Aldrich) and 10 µg/ml rEgGST
and incubated for 72 h. Supernatants of lymphocyte cultures were
added to a pre-coated microplate ELISA kit (Jingmei Biotech Co.,
Ltd., Shenzhen, China) and maintained at 37°C for 2 h. After
washing with PBS and 0.05% Tween 20 (PBST), 25–50 ng goat
biotin-conjugated antibodies against mouse IL-2 (F10092-A), IL-4
(F7695-A), IL-10 (F7701-A) and IFN-γ (F10077-A; 1:1000; Jingmei
Biotech Co. Ltd.) were added per well and the plates were incubated
for 2 h at 37°C. Following four washes in PBST, peroxidase-labeled
streptavidin was added for 1.5 h at 37°C. Plates were washed and
incubated with substrate for 0.5 h at 37°C. Finally, the reaction
was stopped with 100 ml sulfuric acid (2 M). Optical density was
measured at 450 nm using an ELISA reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Antibody measurement using ELISA
Serum antibody levels were quantified using ELISA at
0, 2, 4, 6, 10, 18 and 30 weeks after the first of the three
immunizations (14). In this assay,
96-well microtiter plates (Sino-American Biotechnology Co., San
Diego, CA, USA) were coated with rEgGST (10 µg/100 µl/well) and
incubated overnight in 0.1 M carbonate buffer (pH 9.6) at 4°C.
Serum samples were diluted 1:100 in PBST and tested in duplicate.
Bound antibodies were detected using HRP-conjugated goat anti-mouse
IgG (ab97265) and IgG subclass antibodies against IgG1 (ab97240),
IgG2a (ab97245) and IgG3 (ab97260; Abcam, Cambridge, UK) at a
1:1,000 dilution in PBST. Antibody titers were measured at 450 nm
using an ELISA reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data comparisons were tested for significance
using one-way analysis of variance. SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA) was used to perform statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression and purification of
rEgGST
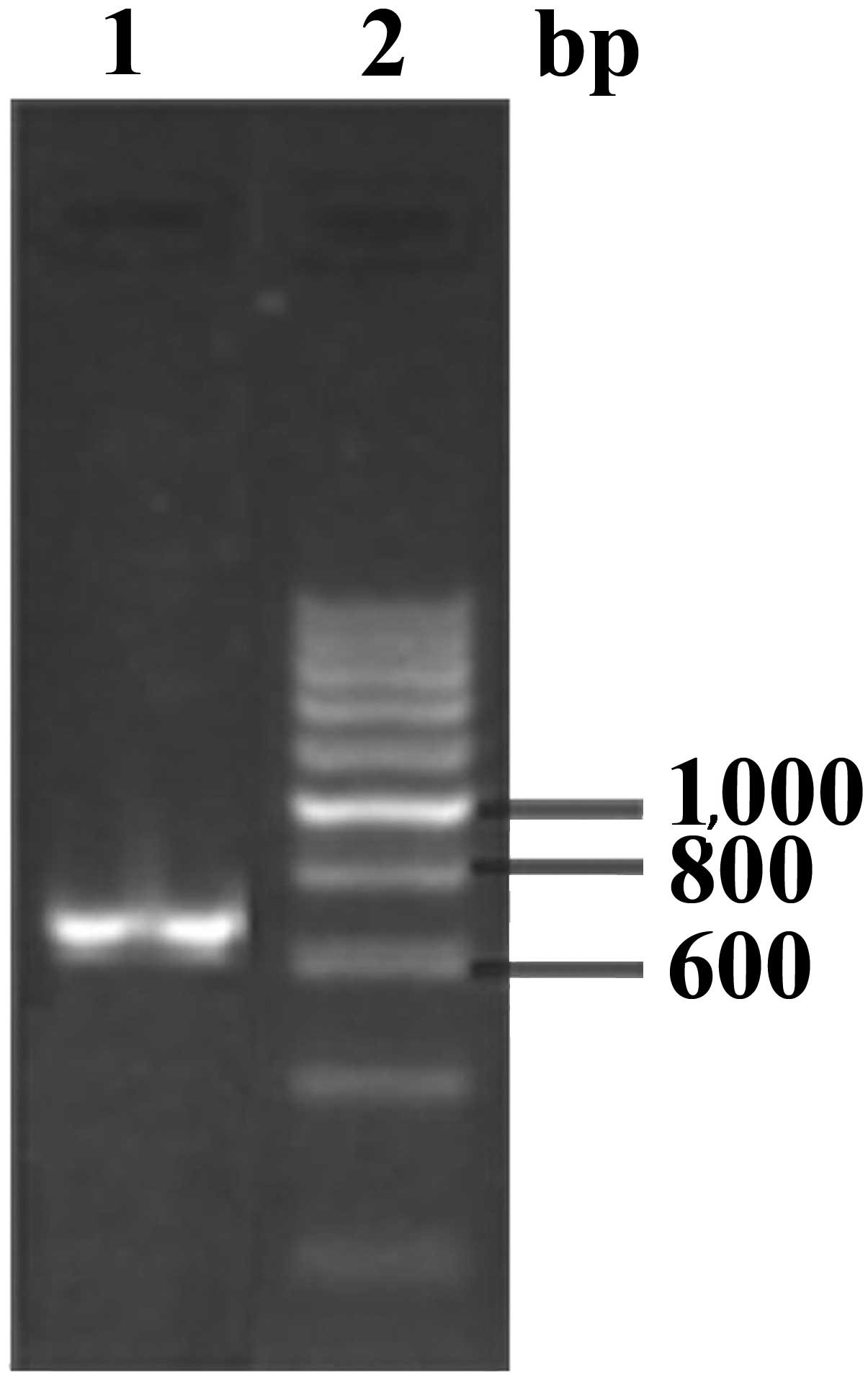
The EgGST gene was amplified using RT-PCR,
generating an amplified product of ~660 bp (Fig. 1). The recombinant expression plasmid
EgGST/pET-28a was constructed and transformed into E. coli
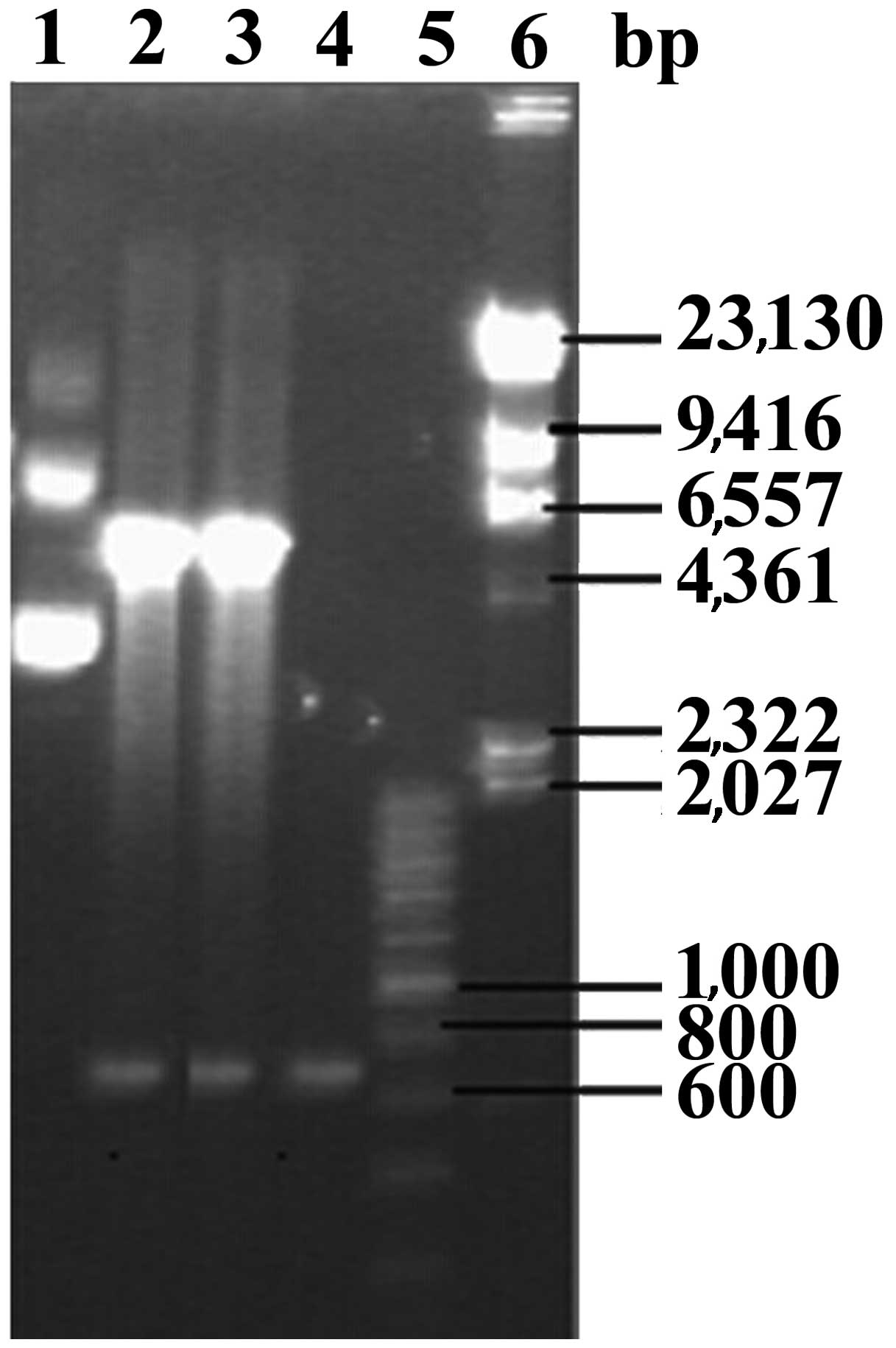
BL21 (DE3). The plasmids extracted from transformed E. coli
BL21 (DE3) were digested using the restriction enzymes EcoRI
and NotI (Fig. 2). The insert
sequence was analyzed using DNA sequencing and confirmed to be the
EgGST gene, suggesting that the recombinant plasmid EgGST/pET-28a
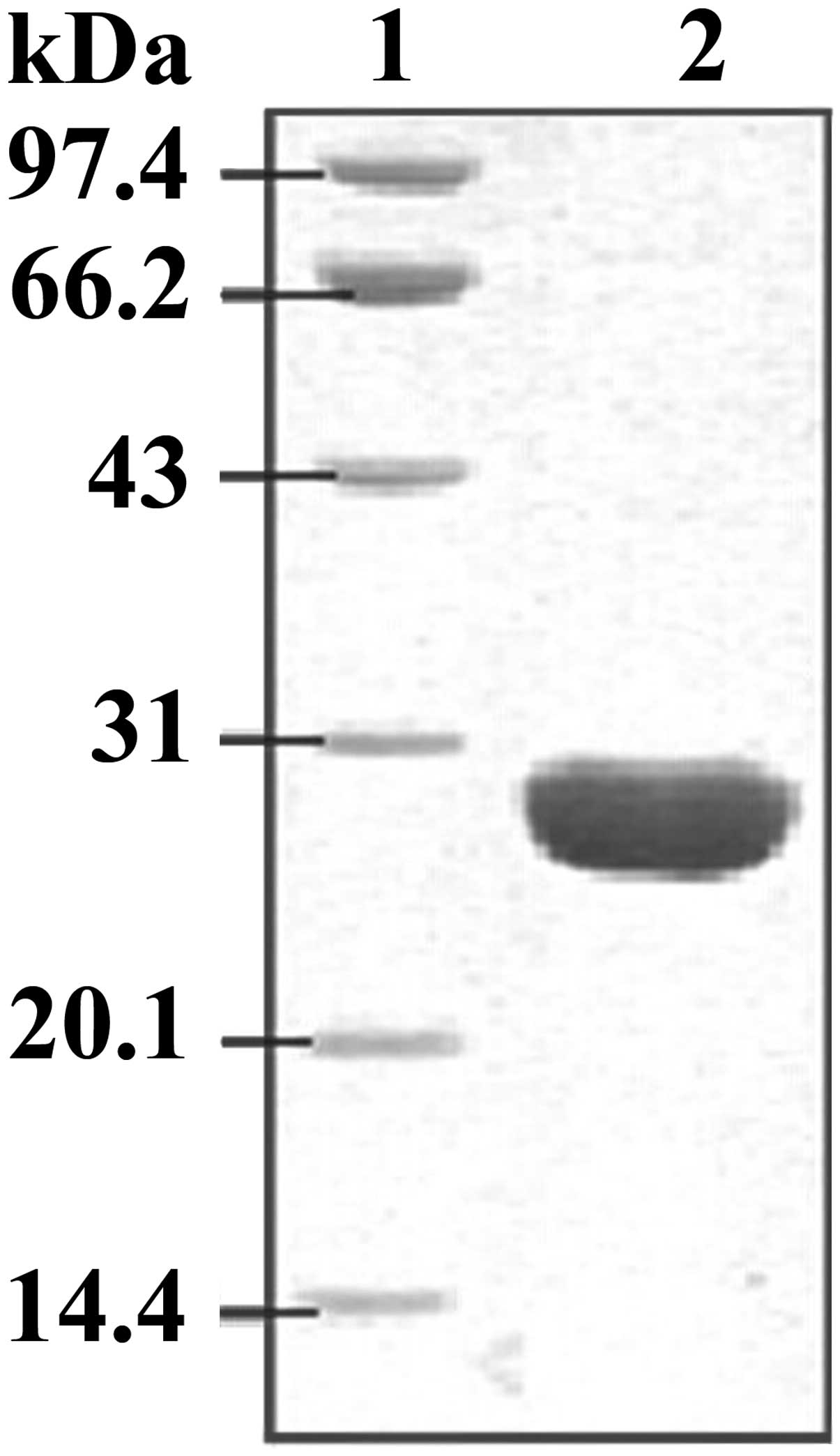
had been constructed successfully. Following IPTG induction, the
His6-labeled recombinant protein was purified using a
Ni2+-chelating column. SDS-PAGE staining results
demonstrated that the His6-tagged EgGST protein had been
successfully expressed in E. coli BL21 (DE3) and purified
efficiently from the E. coli lysate, with a molecular weight
of ~28 kD (Fig. 3).
Antibody measurement using ELISA
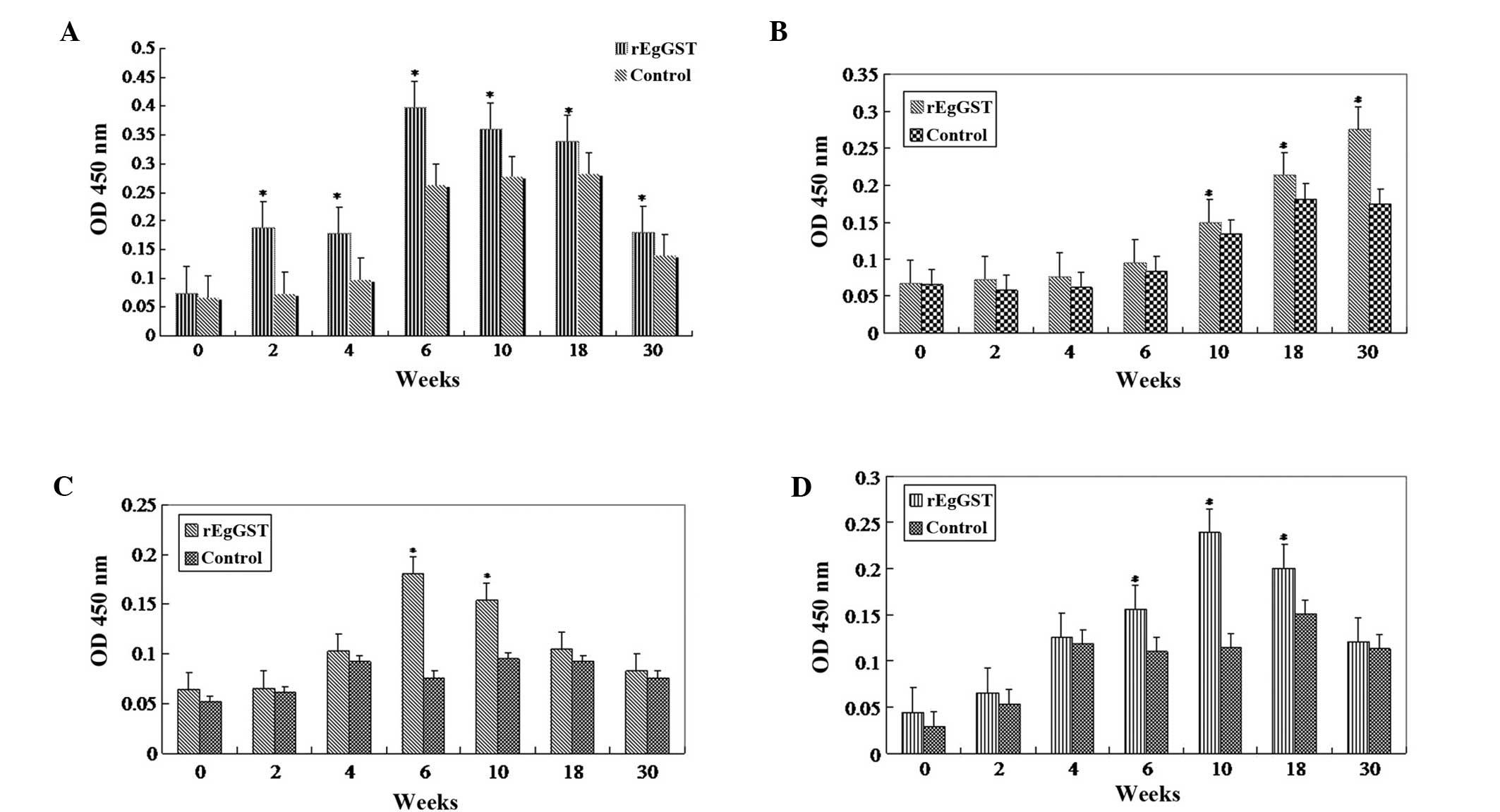
Sera from animals treated with PBS or rEgGST were
tested using the ELISA method. The levels of IgG were increased
following rEgGST immunization, typically from about week 4 after
the initial immunization until week 10. The animals were
challenge-infected at week 10. Following the challenge infection,
the levels of total IgG remained elevated (Fig 4A), the levels of IgG2a and IgG3
declined gradually (Fig. 4C and D),
and the levels of IgG1 continued to increase until week 30
(Fig. 4B).
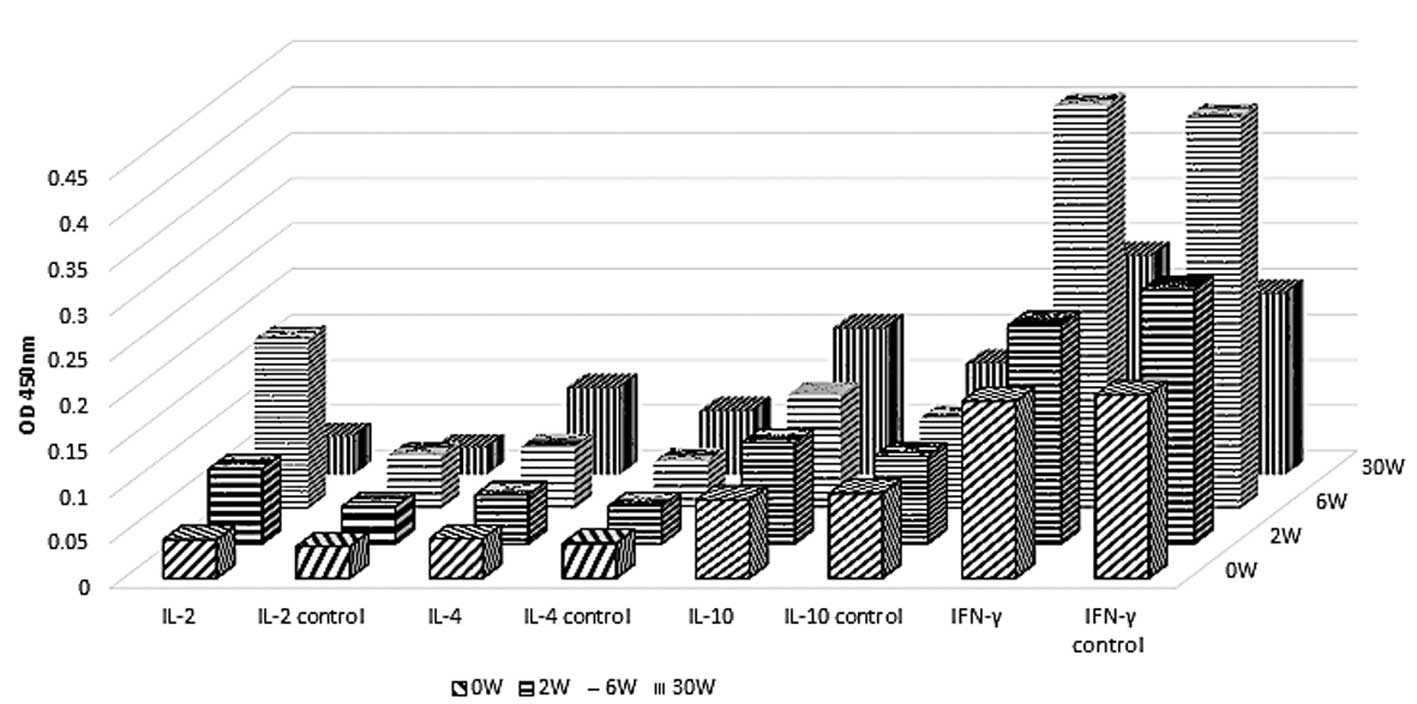
Analysis of cytokine levels in mice
immunized with rEgGST by ELISA
The animals' reaction to rEgGST was further
evaluated by measuring the levels of a number of cytokines,
specifically interferon (IFN)-γ, interleukin (IL)-2, IL-4 and
IL-10. The levels of IFN-γ and IL-2 in the immunized group were
significantly higher compared with those in the control group at
week 6 after the first immunization, while the levels of IL-4 and
IL-10 remained unaltered during the period of observation. After
the challenge infection at week 10, the levels of IFN-γ and IL-2
decreased to normal levels by week 30. The levels of IL-4 and IL-10
did not change significantly following the challenge infection in
the immunization group (Fig. 5).
These results indicate that type 1 T helper (Th1) cells mediate the
primary response following immunization.
Protective immunity in mice
Mice in each group were sacrificed at 5 months (30
weeks) after the initial immunization. The internal organs of the
mice were examined for the presence of hydatid cysts. Mice
vaccinated with rEgGST exhibited significantly reduced numbers of
hydatid cysts compared with the control group (Table I; P<0.01). The protective immunity
induced by rEgGST was calculated to be 89.39%.
 | Table I.Number of hydatid cysts and protective
immunity in vaccinated and control groups. |
Table I.
Number of hydatid cysts and protective
immunity in vaccinated and control groups.
| Group | Mice (n) | Cysts (n) | Immunity (%) |
|---|
| Control | 6 | 3.67±3.14 | – |
| rEgGST | 6 |
0.33±0.51a | 89.39a |
Discussion
Previous studies have reported that the 14-3-3
protein (15), myophilin (16), ferritin (17) and P29 (18) that are secreted by E.
granulosus exhibit marked immunogenic properties. The aim of
the present study was to determine whether immunization with rEgGST
was able to induce effective protective immunity when compared with
a control group. A positive result may be used to provide an
experimental basis for the potential use of rEgGST as a
vaccine.
Glutathione S-transferases (GSTs) are a family of
detoxification enzymes (19), and
Schistosoma mansoni 28 kDa GST (Sj28GST) has been recognized
as an effective protective antigen against S. mansoni
(20). For E. granulosus, the
observation of GST protein expression at various stages of parasite
development provided an experimental basis for the investigation of
the potential of EgGST as a vaccine candidate against
echinococcosis (21). Therefore, GST
is considered to be a potential treatment for the prevention of
shistosomiasis infection (22).
In the present study, immunization with rEgGST
stimulated humoral immunity in mice following the third
immunization. Increased serum levels of total IgG and the IgG
subtypes IgG2a, IgG1 and IgG3 were observed in mice following
rEgGST immunization and challenge infection compared with the
control group. These results indicate that an immune response may
be effectively activated by treatment with rEgGST. In addition,
IgG, IgG2a, IgG1 and IgG3 may be involved in the early protective
immune response. As the time following the induction of the
challenge infection increased, IgG2a and IgG3 levels decreased
gradually while the levels of IgG1 increased continuously. This
observation may be related to the parasite escaping from host
immune surveillance. rEgGST was able to induce the production of
high levels of antibody following immunization, which remained
elevated following early infection. These results indicate that
rEgGST possesses notable immunogenicity and immunoreactivity.
Cytokines are commonly produced by T helper cells in
humoral and cellular immunity in order to mediate the regulation of
parasitic diseases. Th1 cells produce interleukin (IL)-2 and
interferon (IFN)-γ to promote IgG2a and IgG3 production. Th2 cells
produce IL-4 and IL-10 and promote a humoral response, in
particular via the induction of IgG1. The Th1 protective response
mediates protective immunity and helps the host to eliminate
hydatid disease, while the Th2 response promotes the humoral immune
response and mitigates parasitism (23–25). In
the present study, four cytokines were selected (IFN-γ, IL-2, IL-10
and IL-4) for examination of their dynamic changes in mice
following treatment with rEgGST and the induction of a challenge
infection using PSCs. Following rEgGST immunization, the levels of
Th1-type cytokines (IFN-γ and IL-2) significantly increased;
however, the levels of Th2-type cytokines (IL-4 and IL-10) remained
unaltered. Levels of Th1-type cytokines decreased following the
induction of the PSC challenge infection, and the levels of
Th2-type cytokines remained the same. These results suggest that
rEgGST induces the Th1-type immune response, which gradually
transitions into the Th2-type response at later stages of
infection. The changes in cytokines levels are typically associated
with the changes in the levels of specific antibodies. It was
observed that following rEgGST immunization, the Th1-dependent
increase of IgG2a and IgG3 levels was coordinated with the increase
of Th1-type cytokines. However, the increase of Th2-dependent IgG1
was not associated with an increase of Th2 type cytokines. These
results suggest that rEgGST immunization specifically stimulates
Th1 cells, which subsequently induce the production of
Th1-dependent antibodies and Th1-type cytokines. However, the
mechanism by which rEgGST stimulates the observed immune response
in animals remains unclear.
In conclusion, the results of the present study
demonstrate that 89.39% protection may be induced by administering
rEgGST in a murine model of echinococcosis. Therefore, rEgGST is
potentially competent to be used as the anti-hydatid component of a
novel vaccine candidate, with the aim of effectively preventing and
controlling of the development of hydatid disease. However, the
mechanism underlying the observed rEgGST-induced protection remains
unclear and requires further study.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81360249).
References
|
1
|
Zhang W and McManus DP: Vaccination of
dogs against Echinococcus granulosus: A means to control
hydatid disease? Trends Parasitol. 24:419–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Battelli G: Echinococcosis: Costs, losses
and social consequences of a neglected zoonosis. Vet Res Commun.
33:(Suppl 1). 47–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Craig PS: Echinococcosis Working Group in
China: Epidemiology of human alveolar echinococcosis in China.
Parasitol Int. 55:(Sul). S221–S225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heath DD and Lawrence SB: Antigenic
polypeptides of Echinococcus granulosus oncospheres and
definition of protective molecules. Parasite Immunol. 18:347–357.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lightowlers MW, Lawrence SB, Gauci CG,
Young J, Ralston MJ, Maas D and Health DD: Vaccination against
hydatidosis using a defined recombinant antigen. Parasite Immunol.
18:457–462. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dempster RP, Robinson CM and Harrison GB:
Parasite vaccine development: Large-scale recovery of immunogenic
Taenia ovis fusion protein GST-45W(B/X) from Escherichia
coli inclusion bodies. Parasitol Res. 82:291–296. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manderson D, Dempster R and Chisti Y: A
recombinant vaccine against hydatidosis: Production of the antigen
in Escherichia coli. J Ind Microbiol Biotechnol. 33:173–182.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larrieu E, Herrero E, Mujica G, et al:
Pilot field trial of the EG95 vaccine against ovine cystic
echinococcosis in Rio Negro, Argentina: Early impact and
preliminary data. Acta Trop. 127:143–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heath DD, Robinson C and Lightowlers MW:
Maternal antibody parameters of cattle and calves receiving EG95
vaccine to protect against Echinococcus granulosus. Vaccine.
30:7321–7326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Zhao J, Wang Y, et al: Cloning and
sequence analyzing of glutathione S-transferase gene on
Echinococcus granulosus of China. Ning Xia Yi Xue Yuan.
28:4–6. 2006.(In Chinese).
|
|
11
|
Gao Peng, Xiong Ying, Du Juan, et al:
Immune protection of recombinant protein glutathione S-transferase
(rEgGST) of Echinococcus granulosus (Chinese mainland
strain). Zhong Guo Wei Sheng Wu Xue Hui. 27:238–240. 2011.(In
Chinese).
|
|
12
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dempster RP, Berridge MV, Harrison GB and
Heath DD: Echinococcus granulosus: Development of an
intermediate host mouse model for use in vaccination studies. Int J
Parasitol. 21:549–554. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu JM, Wang AH, Chen YJ and Gu HM:
Studies on the antigenicity and protein pattern of soluble antigens
from a germinal cell line of Echinococcus granulosus. Nong
Ye Sheng Wu Ji Shu Xue Bao. 6:277–280. 1998.(In Chinese).
|
|
15
|
Li ZJ, Wang YN, Wang Q and Zhao W:
Echinococcus granulosus 14-3-3 protein: A potential vaccine
candidate against challenge with Echinococcus granulosus in
mice. Biomed Environ Sci. 25:352–358. 2012.PubMed/NCBI
|
|
16
|
Sun J, Wang Y, Li Z, et al:
Echinococcus granulosus: Immunoprotection accompanied by
humoral and cytokine response against secondary hydatidosis in mice
immunized with rEg.myophilin. Vet Res Commun. 35:193–200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Li ZJ, Li ZY, Bo Y and Zhao W:
Recombinant ferritin protects mice against challenge with
Echinococcus granulosus? Acta Parasitological. 54:335–340.
2009.
|
|
18
|
Shi Z, Wang Y, Li Z, et al: Cloning,
expression, and protective immunity in mice of a gene encoding the
diagnostic antigen P-29 of Echinococcus granulosus. Acta
Biochim Biophys Sin (Shanghai). 41:79–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhargavi R, Vishwakarma S and Murty US:
Modeling analysis of GST (glutathione-S-transferases) from
Wuchereria bancrofti and Brugia malayi.
Bioinformation. 1:25–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rezende CM, Goes TS, Goes VS, Azevedo V,
Leite MF and Goes AM: GM-CSF and TNF-alpha synergize to increase in
vitro granuloma size of PBMC from humans induced by Schistosoma
mansoni recombinant 28-kDa GST. Immunol Lett. 95:221–228. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng H, Zhang W, Zhang L, et al: The
genome of the hydatid tapeworm Echinococcus granulosus. Nat
Genet. 45:1168–1175. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilson MS, Mentink-Kane MM, Pesce JT, et
al: Immunopathology of schistosomiasis. Immunol Cell Biol.
85:148–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shainheit MG, Saraceno R, Bazzone LE, et
al: Disruption of interleukin-27 signaling results in impaired
gamma interferon production but does not significantly affect
immunopathology in murine schistosome infection. Infect Immun.
75:3169–3177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nono JK, Lutz MB and Brehm K: EmTIP, a
T-Cell immunomodulatory protein secreted by the tapeworm
Echinococcus multilocularis is important for early
metacestode development. PLoS Negl Trop Dis. 8:e26322014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boutennoune H, Qaqish A, Al-Aghbar M, et
al: Induction of T helper 1 response by immunization of BALB/c mice
with the gene encoding the second subunit of Echinococcus
granulosus antigen B (EgAgB8/2). Parasite. 19:183–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|