Introduction
Gastric cancer is among the most common types of
cancer, and is a leading cause of cancer-related mortality in Asia
(1). The disease is difficult to
cure, even with surgical resection and chemotherapy. Currently,
although chemotherapy has become a main treatment for gastric
cancer, its therapeutic effect is dissatisfactory. Multidrug
resistance is one of the leading factors underlying chemotherapy
failure (2). It is therefore
necessary to develop a new drug that can reverse chemotherapy drug
resistance or enhance the sensitivity to chemotherapy.
Huaier has been used for the treatment of several
diseases, such as viral hepatitis, in China for a number of years
(3); however, it has only been used
as a supplementary anti-cancer therapy since the recent discovery
of its anti-tumor effect (4). The
effective ingredient of Huaier, which is isolated from the extract
of the fermented Huaier fungus, has been confirmed to be a
proteoglycan with the following constituents: Water, 8.72%; amino
acids, 12.93%; and polysaccharides, 41.53% (5,6). Huaier
has been shown to be effective against several types of cancer,
exerting its anti-cancer activity through the inhibition of tumor
growth, the induction of apoptosis and through anti-angiogenic
effects (7,8); however, to the best of our knowledge,
there have been no studies to date about the effects of Huaier on
gastric cancer cells.
The phosphatidylinositol 3-kinase (PI3K)/AKT
signaling pathways plays a vital role in the resistance to
chemotherapy and radiotherapy, as it is associated with a number of
processes involved in cell survival, including cell growth,
proliferation, movement, apoptosis and angiogenesis. In this study,
the anti-proliferative and apoptosis-promoting effects of Huaier on
gastric cancer cells and the associated mechanisms were
investigated.
Materials and methods
Ethics statement
All study methods were approved by the Ethics
Committee of the First Affiliated Hospital of Zhejiang Chinese
Medical University (Hangzhou, China).
Materials
Aqueous Huaier extract was purchased from Gaitianli
Pharmacy Co., Ltd. (Qidong, China). A total of 1 g electuary
ointment was dissolved in 10 ml complete medium and sterilized with
a 0.22-mm filter to obtain the 100 mg/ml stock solution, which was
stored at −20°C in RPMI-1640 medium (Gibco®, Hangzhou MultiSciences
Biotech Co., Ltd., Hangzhou, China). Fetal bovine serum (FBS) was
provided by Gibco, and MTT and dimethyl sulfoxide (DMSO) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). The BD
Cycletest™ Plus DNA Reagent kit, FACSCalibur™ flow cytometer,
annexin V/fluorescein isothiocyanate (FITC) kit and propidium
iodide (PI) were supplied by BD Biosciences (San Jose, CA,
USA).
Cell culture
The MKN45 and SGC7901 human gastric cancer cell
lines were obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The two cell lines were cultured in
RPMI-1640 medium supplemented with 10% (v/v) FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in a humidified
atmosphere with 5% CO2. The cells were subcultured every
2 days.
MTT assay
The effect of Huaier on MKN45 and SGC7901 gastric
cancer cell proliferation was assessed using MTT. Exponentially
growing cells were seeded into 96-well plates at 4×103
cells/well for viability measurements and incubated for 24 h.
Different concentrations of Huaier (1, 2, 4, 8 and 16 mg/ml) were
added to wells and incubated for various durations at 37°C. On the
day of collection, the cell number was measured 24, 48 and 72 h
after incubation using a standard MTT-based assay. A total of 100
µl MTT (working concentration, 1 mg/ml) was added to each well, and
the cells were subsequently incubated at 37°C for 4 h. Following
the removal of the supernatant, 200 µl DMSO was added to dissolve
the formazan crystals, and the optical density was detected at 570
nm using a microplate spectrophotometer (SpectraMax®; Molecular
Devices, LLC, Sunnyvale, CA, USA). The data represent the mean of
three readings, and each dose was tested in triplicate.
Apoptosis
The level of cell apoptosis was evaluated by
assessing annexin V-positive staining using flow cytometry. A total
of 1×105 cells/well was seeded in six-well plates. The
cells were incubated with Huaier (2.5 and 5 mg/ml) or in
Huaier-free medium as a control. After 24 h of incubation, the
cells were collected using gentle agitation and washed twice with
phosphate-buffered saline (PBS). The cells were resuspended in 100
µl binding buffer at a density of 0.5×106 cells/100 µl.
Annexin V/FITC (5 µl) was added to the cell suspension, which was
incubated for a further 10 min at room temperature. The cells were
washed and resuspended in 200 µl binding buffer, and PI staining
solution was added prior to flow cytometry being performed. In
total, 5,000–10,000 events were analyzed using a FACScan™ machine
(BD Biosciences). The apoptosis rate was determined using
CellQuest™ software (BD Biosciences).
Cell cycle analysis
The cell cycle analysis was performed using the
standard method with certain modifications (9). Following digestion using trypsin, the
cells were resuspended and washed twice with PBS, and then
1×105 cells/well were seeded in six-well plates for
overnight incubation. At the end of the incubation period, once the
cells had adhered to the side wall, the Huaier extract stock
solution was diluted to the final concentrations of 0, 2.5 and 5
mg/ml, respectively. Each concentration was used in three parallel
control wells. Following the addition of the stock solution, 1 ml
was centrifuged and the supernatant was discarded. The three
solutions of the BD Cycletest Plus DNA Reagent kit (BD Biosciences)
were then added in sequence: i) 250 µl solution A, which contained
trypsin in a spermine tetrahydrochloride detergent buffer for the
enzyme-mediated disaggregation of the solid tissue fragments and
the digestion of cell membranes and cytoskeletons, was first added
for 10 min of incubation at room temperature; ii) 250 µl solution B
(BD Biosciences), which contained trypsin inhibitor and
ribonuclease A in citrate-stabilizing buffer with spermine
tetrahydrochloride for the inhibition of trypsin activity and the
digestion of RNA, was then added for a further 10 min of incubation
at room temperature; and iii) 200 µl cold solution C, which
contained PI and spermine tetrahydrochloride in citrate stabilizing
buffer, was added for the stoichiometric binding of the PI to the
DNA (final concentration, ≥125 µg/ml). Following the addition of
solutions A, B and C, a mesh filter was used to filter the
resulting solution, which was then analyzed after 48 and 72 h using
flow cytometry. The cell cycle was analyzed using ModFit software
obtained from CellQuest (BD Biosciences).
Western blot analysis
The cells were plated in 3.5-cm dishes at a density
of 2×105 and collected following treatment. The cells
were placed in lysis buffer (Biyuntian Biotech Co., Ltd, Shanghai,
China) according to the manufacturer's instructions. Using 12%
SDS-PAGE, equal quantities of protein (20 mg per lane) were
separated and transferred to polyvinylidene membranes (Millipore,
Billerica, MA, USA). Following the blocking of the membranes,
primary monoclonal antibodies against a number of proteins
associated with the PI3K/AKT signaling pathway were added and the
membranes were incubated overnight at 4°C. Primary monoclonal
antibodies against AKT (1:1,000; 10176-2-AP), PDK1 (1:1,000;
10026-1-AP), Bcl-2-associated death promoter (BAD; 1:1,000;
10435-1-AP) and GAPDH (1:4,000; 60004-1-Ig) were purchased from
Proteintech (Manchester, UK), while monoclonal antibodies against
p-AKT (The-308; 1:800; 2118-1), p-AKT (Ser-473; 1:800; 2214-1),
PI3K (1:1,000; 1593-S), PTEN (1:1,000; 5171-1), p-PTEN (1:1,000;
2134-1), Bcl-2 (1:1,000; 10026-1-AP), pro-caspase-9 (1:1,000;
1023-1) and cyclin B1 (1:2,000; 1495-1) were obtained from Abcam
(Cambridge, UK), and monoclonal anti-cleaved-caspase-9 (1:1,000;
sc-22182) was from Santa Cruz Biotechnology, Inc. (Shanghai,
China). Subsequently, the membranes were labeled with the
appropriate secondary antibodies conjugated with horseradish
peroxidase (1:5,000; GB23301), purchased from Wuhan Gugeshengwu
Technology Co., Ltd. (Wuhan, China). GAPDH was used as the loading
control. An enhanced chemiluminescence system (Pierce, Rockford,
IL, USA) was used to visualize the immunoreactive bands.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical treatment was performed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). The one-tailed Student's t-test was used
to analyze differences in drug response between groups, and data
were collected from three different experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibitory effects of Huaier on
gastric cancer cell growth
To evaluate the biological activity of Huaier in
cancer cells, cell viability was measured using the MTT assay
following treatment with various concentrations of Huaier. Cell
survival decreased with the increase in the concentration of
Huaier, and the IC50 was determined to be 2.104 mg/ml
for MKN45 cells and 3.579 mg/ml for SGC7901 cells (Fig. 1). The results indicated that Huaier
had an anti-proliferative effect against gastric cancer cells.
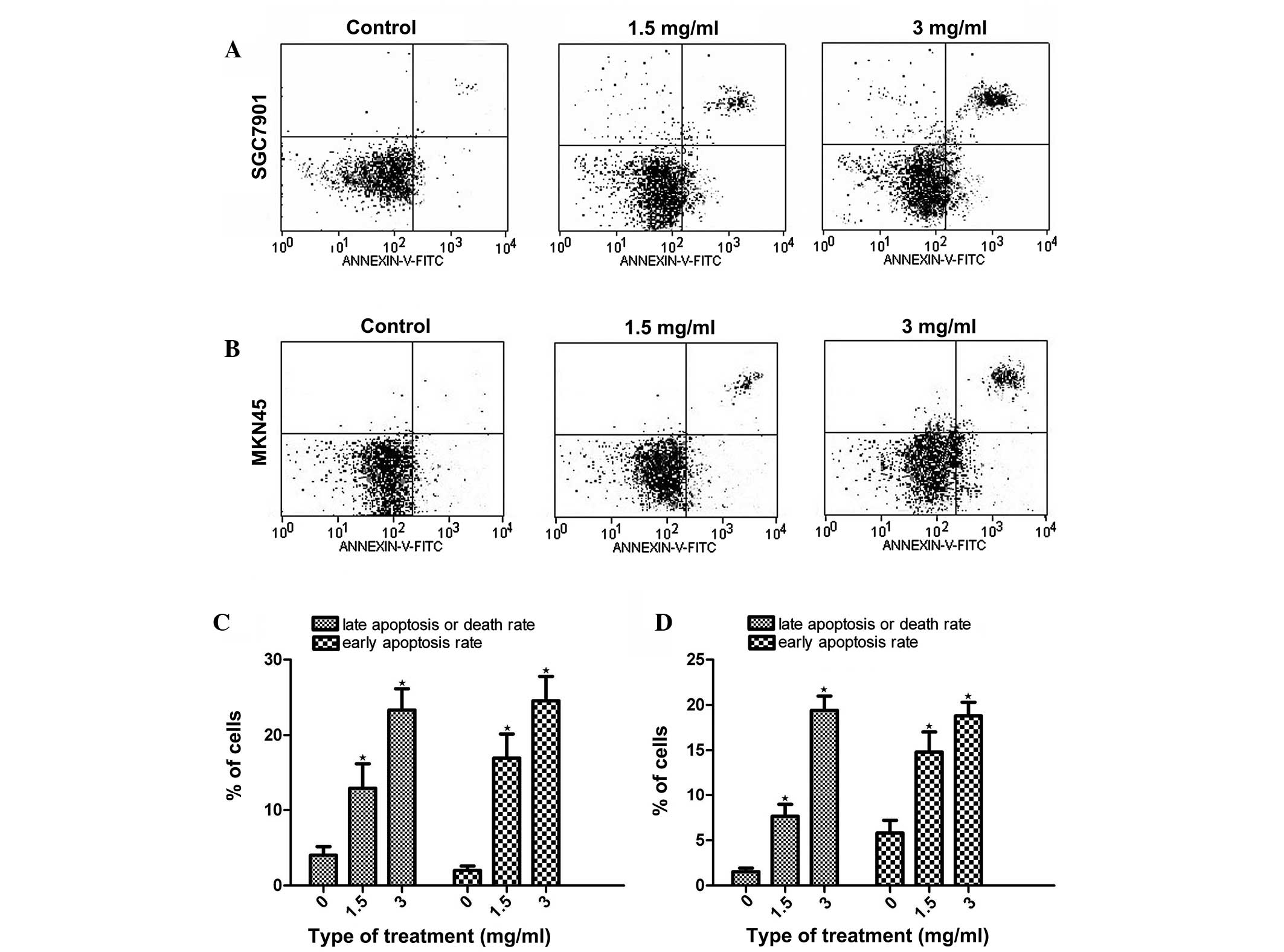
Analysis of cell apoptosis using
PI-annexin V staining
As Huaier significantly inhibited cell growth, it
was investigated whether this effect was achieved by inducing
apoptosis. The PI-annexin V staining assay showed that, following
the treatment of the gastric cancer cells with Huaier for 24 h, the
late-apoptosis or cell death rate (upper right quadrant) and the
early-apoptosis rate (lower right quadrant) were increased in a
dose-dependent manner, both in the MKN45 and SGC7901 cells
(Fig. 2).
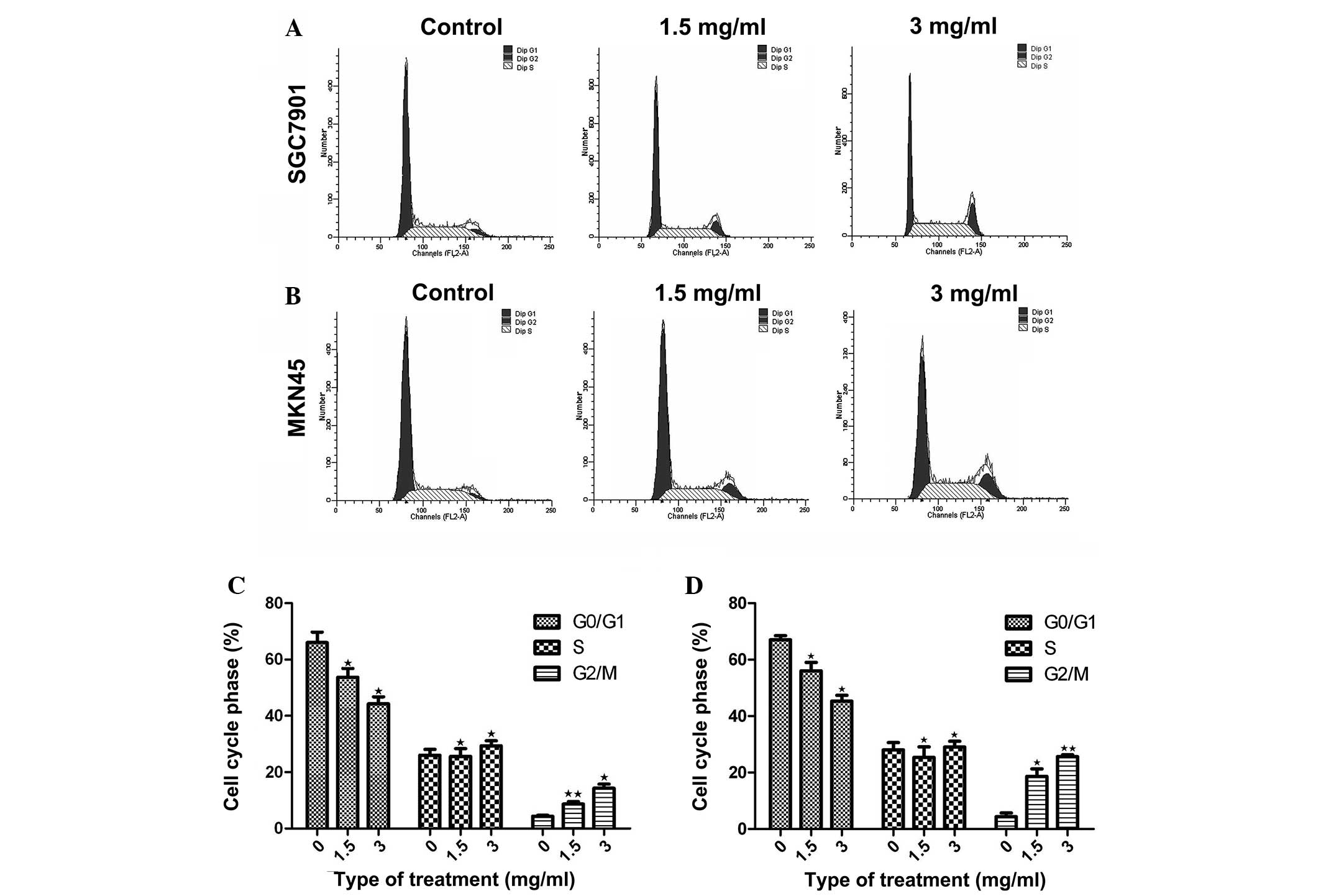
Huaier induces cell-cycle arrest
To investigate whether the inhibition induced by
Huaier extract was a result of cell-cycle arrest, the distribution
of the MKN45 and SGC7901 gastric cancer cells across the cell cycle
was analyzed using flow cytometry. Prior to analysis, the cells
were exposed to Huaier for 24 h at concentrations of 1.5 and 3
mg/ml. Compared with the untreated cells, an increased proportion
of Huaier-treated MKN45 and SGC7901 gastric cancer cells was found
to be in G2/M phase, indicating cell-cycle arrest (P<0.05).
Furthermore, the number of cells in G2/M phase in the
Huaier-treated groups was increased in a dose-dependent manner
compared with that in the control group (Fig. 3). These results indicated that the
inhibitory effect of Huaier on MKN45 and SGC7901 gastric cancer
cell proliferation occurred via cell-cycle arrest at G2/M phase
(Fig. 3).
Huaier downregulates proteins
associated with the apoptosis signaling pathway
The activation of the PI3K/AKT signaling pathway can
lead to cell apoptosis; therefore, the expression of proteins
associated with the PI3K/AKT signaling pathway, including AKT1,
pyruvate dehydrogenase kinase isoform 1 (PKD1), PI3K and
phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and
dual-specificity protein phosphatase (PTEN) was assessed using
western blotting. To further investigate the mechanisms through
which Huaier induces apoptosis and cell-cycle arrest, the
expression of cyclin B1, B-cell lymphoma 2 (Bcl-2) and caspase-9
was measured using western blotting. The results indicated that
Huaier inhibited cyclin B1, phosphorylated- (p-)AKT1, PI3K, PDK1,
p-PTEN and Bcl-2 expression and upregulated cleaved-caspase-9
expression in a dose-dependent manner (Figs. 4 and 5). Caspase-9 activation was significantly
increased following treatment with Huaier extract, resulting in
increased cleaved caspase-9 expression and decreased pro-caspase-9
expression.
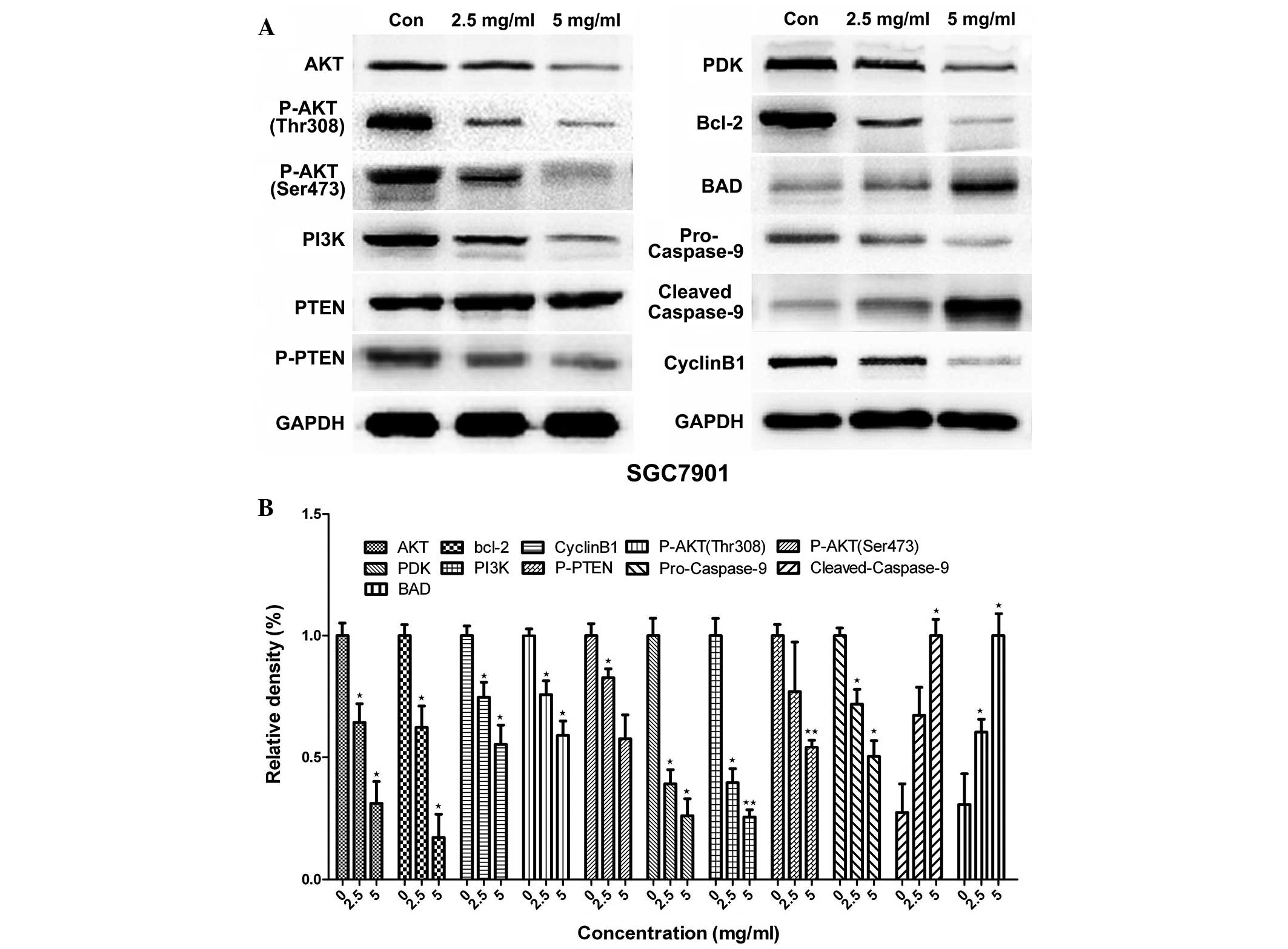 | Figure 4.Effect of Huaier extract on the
protein expression of AKT1, PI3K, PTEN, PDK1, caspase-9 and Bcl-2
in SGC7901 cells. The expression of GAPDH was used as an internal
control. The SGC7901 cells were treated with 0, 2.5 and 5 mg/ml
Huaier for 24 h, and (A) western blotting was used to analyze the
protein expression. (B) Quantification of the data. The experiment
was performed in triplicate, and the data are expressed as the mean
± standard deviation of the three separate experiments. *P<0.05
and **P<0.01, compared with the control. p-, phosphorylated-;
PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatidylinositol
3,4,5-trisphosphate 3-phosphatase and dual-specificity protein
phosphatase; PDK1, pyruvate dehydrogenase kinase isoform 1; Bcl-2,
B-cell lymphoma 2; BAD, Bcl-2-associated death promoter. |
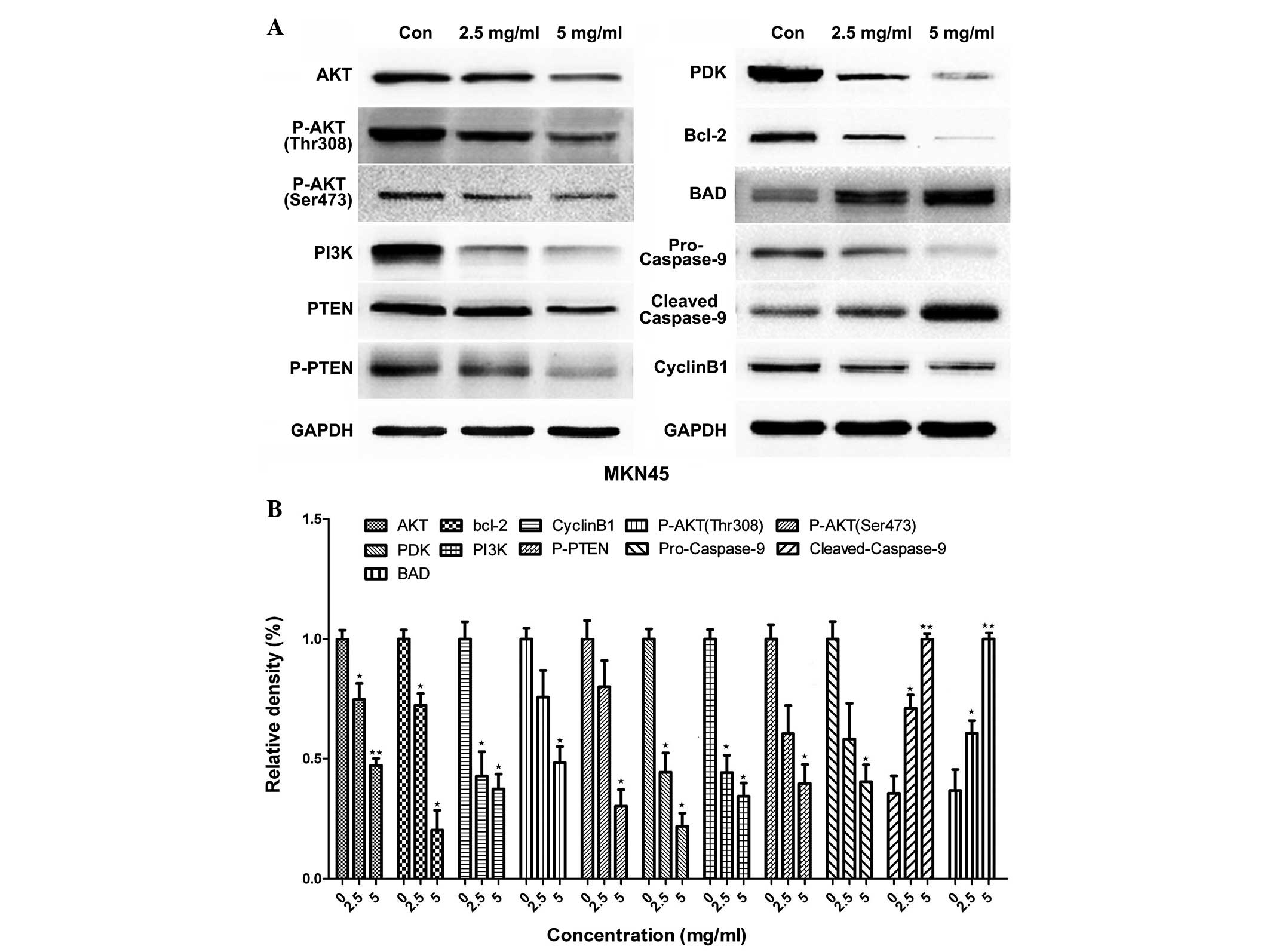 | Figure 5.Effect of Huaier extract on the
protein expression of AKT1, PI3K, PTEN, PDK1, caspase-9 and Bcl-2.
The expression of GAPDH was used as an internal control. MKN45
cells were treated with 0, 2.5 and 5 mg/ml for 24 h, and (A)
western blotting was used to analyze the protein expression. (B)
Quantification of the data. The experiment was performed in
triplicate, and the data are expressed as the mean ± standard
deviation of the three separate experiments. *P<0.05 and
**P<0.01, compared with the control. p-, phosphorylated-; PI3K,
phosphatidylinositol 3-kinase; PTEN, phosphatidylinositol
3,4,5-trisphosphate 3-phosphatase and dual-specificity protein
phosphatase; PDK1, pyruvate dehydrogenase kinase isoform 1; Bcl-2,
B-cell lymphoma 2; BAD, Bcl-2-associated death promoter. |
Discussion
Gastric cancer is one of the most common types of
malignant tumor in the world. The early prognosis associated with
gastric cancer is poor, as the condition is characterized by early
lymphatic metastasis with high malignancy. The current main
treatment for gastric cancer is surgery, in conjunction with
chemotherapy and other comprehensive treatments; however, the drug
resistance of gastric cancer has become a problem in the clinic,
and certain patients experience pain with chemotherapy. The
development of safe and effective drugs to inhibit cancer cell
proliferation is therefore currently an area of particular
interest. In recent years, Huaier, which is extracted from
officinal fungi, has been shown to inhibit the proliferation of
various types of cancer cells: Hepatocellular carcinoma (8), breast cancer (10), cholangiocarcinoma (11), melanoma (12), colorectal cancer (13) and ovarian cancer (14). However, the antitumor mechanism of
Huaier has yet to be fully elucidated; therefore, the aim of the
present study was to investigate the mechanism underlying the
Huaier-induced cell apoptosis. The results indicated that, in
addition to inhibiting cell proliferation, Huaier could induce cell
apoptosis by modulating the PI3K/AKT signaling pathways.
PI3Ks, the second messengers produced by
phosphoinositide phosphorylate at the D-3 position of the inositol
ring, are involved in a number of cellular activities and can
promote several biological properties, including proliferation,
survival, motility and morphology (15). AKT plays vital roles in the signaling
pathways in response to growth factors and other extracellular
stimuli to regulate various cellular functions, including nutrient
metabolism and cell growth, survival and apoptosis (16). AKT, a downstream effector of PI3K, is
activated by Class 1A and 1B PI3Ks, while the Class 1A and 1B PI3Ks
are activated by tyrosine kinase and G-protein-coupled receptors,
respectively (17). Following the
activation of PI3K, phosphatidylinositol 4,5-biphosphate on the
3-OH group generates the second messenger phosphatidylinositol
3,4,5-trisphosphate (PIP3). PIP3 levels are regulated by
phosphatases such as PTEN, which removes the phosphate from the
3-OH position (16). The present
results suggested that Huaier inhibited the expression of AKT,
p-AKT, PTEN and p-PTEN by downregulating PI3K.
Human caspase-9 is an initiator of apoptosis
(18). The phosphorylation of
caspase-9 by PI3K/AKT has been found to lead to an attenuation of
its activity (19). Considerable
evidence has suggested that among the most important roles of AKT
are the enhancement of growth factor-induced cell survival and the
inhibition of cell apoptosis. Mammalian cell apoptosis is a process
occurring in a series of steps. An early event is the loss of
mitochondrial integrity, and this is followed by the release of
cytochrome c, which is fixed to and activates the apoptotic
protease-activating factor (Apaf-1). The activated Apaf-1 then
binds to, cleaves and activates cysteine protease and caspase-9.
The activated caspase-9 triggers a cascade amplification reaction
of proteins of the caspase family (20). In the present study it was found that
cleaved-caspase 9, the activated form of caspase-9, is
downregulated following treatment with Huaier. This may contribute
to the inhibition of p-AKT expression.
Members of the Bcl-2 family are important regulators
in the PI3K/AKT pathway. Among the Bcl-2 family are anti-apoptotic
effectors, such as Bcl-extra large (Bcl-XL) and Bcl-2, and
pro-apoptotic effectors, such as Bcl-associated X protein, BH3
interacting-domain death agonist and Bcl-2-interacting killer (the
BH3 subfamily), as well as BAD and Bcl-2 homologous
antagonist/killer (21). When BAD
binds to Bcl-2 or Bcl-XL and PTEN is phosphorylated by protein
kinase B (PKB)/AKT, the anti-apoptotic potential of Bcl-2 or Bcl-XL
is inhibited. As a result, BAD is regarded as one of the direct
targets of the PI3K/AKT pathway in the promotion of cell survival.
p-AKT can also mediate cell growth, survival and differentiation,
promote anti-apoptotic gene expression, inhibit the c-myc-induced
cell apoptosis and regulate the adaptability of cell survival. The
present results showed that Huaier could increase the expression of
BAD. We inferred that Huaier could promote BAD release from the
complex containing BAD and Bcl-2/Bcl-XL that is localized on the
mitochondrial membrane, leading to increased BAD in the
cytoplasm.
In this study, the results of the MTT assay, flow
cytometry and western blotting indicated that Huaier played a role
in the induction of cell proliferation and apoptosis. The
investigation into the effect of Huaier on MKN45 and SGC7901
gastric cancer cell proliferation indicated that Huaier had
powerful dose-dependent inhibitory effects and inhibited MKN45 and
SGC7901 human gastric cancer cell proliferation by arresting the
cell cycle at G2/M phase and by modulating the PI3K/AKT signaling
pathways. Huaier is capable of upregulating cleaved-caspase-9
expression and downregulating PI3K, p-AKT1, p-PTEN and PDK1
expression. We hypothesize that Huaier inhibits AKT phosphorylation
by inhibiting the expression of PI3K and PDK1 and that this
reduction in AKT phosphorylation leads to the downregulation of
pro-caspase-9 and Bcl-2 expression.
A previous study involving the tissue culture of
mammalian cells showed that the Cdc2-cyclin B1 complex is a vital
effector for the G2 checkpoint (22). In the present study, it was found
that Huaier could significantly decrease the expression of cyclin
B1, and the number of cells arrested in the G2/M-phase of the cell
cycle was reduced simultaneously. We therefore inferred that the
Huaier-induced G2/M-phase cell-cycle arrest and inhibition of cell
proliferation occurred through its regulation of cyclin B1
expression.
In conclusion, there are numerous signaling pathways
that are important in Huaier-induced apoptosis. Among them, the
PI3K/AKT pathway is one of the most critical. The present results
suggest that Huaier modulates the PI3K/AKT pathway by inhibiting
PI3K expression. This study may facilitate a clearer understanding
of the mechanism of Huaier-induced cell apoptosis, and provide a
theoretical basis for the antitumor effects of Huaier.
Acknowledgements
This study was supported by the Zhejiang Provincial
Natural Science Foundation (grant nos. LY12H16029 and LY13H160027)
and the Key Project of Provincial Administration of Traditional
Chinese Medicine (grant no. 2012ZZ002).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:359–386. 2015.
View Article : Google Scholar
|
|
2
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li LX, Ye SL, Wang YH and Tang ZZ:
Progress on experimental research and clinical application of
Trametes robiniophila. Zhong Guo Zhong Liu. 16:110–113.
2007.(In Chinese).
|
|
4
|
Xu X, Wei Q, Wang K, Ling Q, Xie H, Zhou L
and Zheng S: Anticancer effects of Huaier are associated with
down-regulation of P53. Asian Pac J Cancer Prev. 12:2251–2254.
2011.PubMed/NCBI
|
|
5
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo Y, Cheng P, Chen Y, Zhou X, Yu P, Li Y
and Zhuang Y: Isolation and analysis of the polysaccharide of
Huaier mycelium. Chin J Biochem Pharm. 63:56–59. 1993.
|
|
7
|
Ren J, Zheng C, Feng G, Liang H, Xia X,
Fang J, Duan X and Zhao H: Inhibitory effect of extract of fungi of
Huaier on hepatocellular carcinoma cells. J Huazhong Univ Sci
Technolog Med Sci. 29:198–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu X, Wei Q, Wang K, Ling Q, Xie H, Zhou L
and Zheng S: Anticancer effects of Huaier are associated with
down-regulation of P53. Asian Pac J Cancer Prev. 12:2251–2254.
2011.PubMed/NCBI
|
|
9
|
Cheng YL, Chang WL, Lee SC, Liu YG, Chen
CJ, Lin SZ, et al: Acetone extract of Angelica sinensis
inhibits proliferation of human cancer cells via inducing cell
cycle arrest and apoptosis. Life Sci. 75:1579–1594. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zhang N, Huo Q, Sun M, Lv S and
Yang Q: Huaier aqueous extract suppresses human breast cancer cell
proliferation through inhibition of estrogen receptor α signaling.
Int J Oncol. 43:321–328. 2013.PubMed/NCBI
|
|
11
|
Sun Y, Sun T, Wang F, Zhang J, Li C, Chen
X, Li Q and Sun S: A polysaccharide from the fungi of Huaier
exhibits anti-tumor potential and immunomodulatory effects.
Carbohydr Polym. 92:577–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang F, Zhang Z and Liu Z: Effects of
Huaier aqueous extract on proliferation and apoptosis in the
melanoma cell line A875. Acta Histochemica. 115:705–711. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang T, Wang K, Zhang J, Wang X, Chen Z,
Ni C, Qiu F and Huang J: Huaier aqueous extract inhibits colorectal
cancer stem cell growth partially via downregulation of the
Wnt/β-catenin pathway. Oncol Lett. 5:1171–1176. 2013.PubMed/NCBI
|
|
14
|
Yan X, Lyu T, Jia N, Yu Y, Hua K and Feng
W: Huaier aqueous extract inhibits ovarian cancer cell motility via
the AKT/GSK3β/β-catenin pathway. PLoS One. 8:e637312013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar A and Carrera AC: New functions for
PI3K in the control of cell division. Cell Cycle. 6:1696–1698.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hafsi S, Pezzino FM, Candido S, Ligresti
G, Spandidos DA, Soua Z, McCubrey JA, Travali S and Libra M: Gene
alterations in the PI3K/PTEN/AKT pathway as a mechanism of
drug-resistance (review). Int J Oncol. 40:639–644. 2012.PubMed/NCBI
|
|
18
|
Donepudi M and Grütter MG: Structure and
zymogen activation of caspases. Biophys Chem. 10:145–153. 2002.
View Article : Google Scholar
|
|
19
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allan LA and Clarke PR: Apoptosis and
autophagy: Regulation of caspase-9 by phosphorylation. FEBS J.
276:6063–6073. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stark GR and Taylor WR: Analyzing the G2/M
checkpoint. Methods Mol Biol. 280:51–82. 2004.PubMed/NCBI
|