Introduction
Endostatin (ES) is an endogenous angiogenesis
inhibitor that was initially identified by O'Reilly in 1997
(1). ES inhibits endothelial cell
proliferation, migration and angiogenesis in the chorioallantoic
membrane, and thus inhibits the growth and metastasis of tumors
(2,3). Furthermore, ES is able to reduce the
resistance of tumors to chemotherapeutic agents with long-term
repeated treatment. ES has previously attracted interest due to its
capacity to treat retinal, choroidal and corneal neovascularization
(CNV) in experimental settings (4).
For example, the inhibition of CNV by intravenous injection of
ES-expressing adenoviral vectors has been investigated, and
microvessel development was observed to be inhibited (5). In September 2005, the State Food and
Drug Administration of China approved the use of ES as an
angiogenesis inhibitor for the treatment of patients with
non-small-cell lung cancer (6).
However, since ES is a protein, there are numerous obstacles
preventing its effective clinical use as a drug, including the
necessity for the administration of high doses (7,8), short
half-life, poor stability and high cost.
Recently, the chemical modification of protein drugs
has become a topic of increased interest (9,10).
Modifications have been made with the aim of overcoming the
inherent disadvantages of proteins and improving their
effectiveness by prolonging half-life, lowering immunogenicity and
increasing stability. In previous studies, ES has been chemically
modified using polyethylene glycol (PEG) and polysulfated heparin
(PSH), and the modified products have been found to exhibit
enhanced heat stability, in addition to a high percentage of
retained activity and limited alteration of the secondary structure
(11,12). PEG-ES and PSH-ES represent potential
therapeutic agents for the treatment of cancer and other disorders
that may have certain advantages in comparison with ES alone.
However, to the best of our knowledge, the anti-angiogenesis
effects of these modified proteins in the context of CNV have not
yet been reported. Therefore, the present study investigated the
efficacy of these compounds as inhibitors of endothelial cell
proliferation and CNV, and compared their effects with those of
ES.
Materials and methods
Materials
PEG-6000 was purchased from Bio Basic, Inc.
(Amherst, NY, USA). PSH was purchased from Yantai Dongcheng
Biochemicals Co., Ltd. (Yantai, China). Pichia yeast
containing the human ES gene was provided by the Medical School of
Shandong University (Jinan, China).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
was purchased from Sigma-Aldrich (St. Louis, MO, USA). Human
umbilical vein endothelial cells (HUVECs) were provided by the
Institute of Pharmacology of Shandong University. Ready-to-use SP
immunohistochemistry kits were purchased from Zhongshan Chuangyi
Biochemical Engineering Co., Ltd. (Guangzhou, China). Basic
fibroblast growth factor (bFGF) was purchased from Lanzhou Yisheng
Biochemical Technology Co., Ltd. (Shanghai, China). New Zealand
albino rabbits were obtained from the Laboratory Animal Department
of Shandong University.
Preparation of ES
ES was prepared by a previously described method
(11). In brief, ES was expressed in
engineered Pichia yeast containing the human ES gene. The
culture supernatant of the Pichia yeast was purified using
carboxymethylcellulose-II exchange column chromatography (Whatman;
GE Healthcare, Piscataway, NJ, USA) and Superdex 75 column
chromatography (Pharmacia; GE Healthcare, Uppsala, Sweden). The
resulting ES was assayed using SDS-PAGE and exhibited a single
protein band at 20 kD.
Preparation of the PSH-ES
conjugate
PSH was prepared and activated by periodate
oxidation. In brief, heparin was dissolved in formamide at 60°C
with constant stirring. A mixture of formamide and chlorosulfonic
acid with a volume ratio of 2:1 was added to the heparin solution
at 25°C, and constantly stirred for 6 h. The reaction was
terminated by alcohol precipitation. Then, 0.5% NaHSO3
was added to a 10% solution of the precipitate and the pH was
adjusted to 9.0 with Na2CO3. The solution was
then kept at 65°C for 3 h, after which 1.0% NaCl was added and the
pH was adjusted to 6.5. The PSH (0.3 g) was dissolved in distilled
water (4.5 ml) and stirred while 12% sodium periodate solution (0.5
ml) was added. The solution was adjusted from pH ~5.4 to ~5.0 by
the addition of hydrochloric acid (0.1 mol/l). The solution was
stirred in the dark for 20 h. The activation reaction was then
terminated by the dropwise addition of 5% NaHSO3
solution. In the conjugation step, 90 mg ES was dissolved in 1 ml
0.3 M sodium carbonate buffer (pH 9.5), 6 ml activated PSH (pH 9.0)
was added and the solution was agitated slowly in the dark for 48 h
at 4°C. The reaction was terminated by adding glycine and the
solution was subjected to Superdex 75 column chromatography
(12). The eluent containing PSH-ES
was subjected to filtration with a TX004 cellulose bag (Whatman)
and then desalted with phosphate buffer (10 mM, pH 8.0) for 48 h.
Finally, the PSH-ES was subjected to concentration at 4°C and then
was lyophilized.
Preparation of the PEG-ES
conjugate
A mixture of 18 g PEG-6000, 2.2 g anhydrous
Na2CO3 and 2.75 g cyanuric chloride was added
to 75 ml anhydrous benzene at room temperature and constantly
stirred overnight. The solution was filtered and the product was
precipitated with ethyl ether. The dissolution and precipitation
step was repeated many times until no unreacted cyanuric chloride
was detected by ultraviolet scanning. After vacuum drying, a white
powder comprising activated PEG-6000 was obtained. ES and activated
PEG-6000 were subsequently dissolved in 10 mM sodium tetraborate
buffer (pH 9.0) at a molar ratio of 1:40. The reaction was allowed
to continue at 4°C under slow agitation for 24 h, then glycine was
added to terminate the reaction (13). The reaction solution was purified
using carboxymethylcellulose-II exchange column chromatography and
Superdex 75 column chromatography.
HUVEC proliferation assay
Culture was conducted as previously described
(14). HUVECs were protected in
Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA),
which included 10% heat-inactivated calf serum (Hyclone), 1%
benzylpenicillin-streptomycin (Boehrvet, Germany) and 3 ng/ml bFGF.
The inhibitory activity of ES, PSH-ES and PEG-ES on HUVEC
proliferation in vitro was analyzed by MTT colorimetric
analysis (15). Log-phase HUVECs
were gathered and plated in a 96-well plate at 1.0×104
cells/well in a volume of 200 µl. These cells were incubated in a
humidified atmosphere of 95% air/5% CO2 at 37°C.
Following the addition of ES, PSH-ES or PEG-ES (5, 10 or 15 µg/ml)
to the wells, the cells were incubated at 37°C for 48 h in DMEM
media with 10% calf serum. The supernatant was removed and HUVECs
were washed twice with phosphate-buffered saline. Cells were
resuspended in DMEM media with 10% calf serum and incubated with 20
µl MTT solution (5 mg/ml) for 4 h at 37°C. Subsequently, the
supernatant was removed, 150 µl dimethyl sulfoxide was added to
each well and the plate was agitated for 10 min. Absorbance was
measured at 570 nm using a microplate reader (Model 680, Bio-Rad
Laboratories, Hercules, CA, USA), and the inhibitory ratios (IR)
were calculated as follows: IR (%) = (1 - absorbance of
experimental group/absorbance of blank control group) × 100.
CNV assays
A study population of 32 New Zealand albino
6-month-old rabbits, 18 male and 14 female, was used in this study.
Animals were treated in accordance with the Shandong University
Animal Experimentation Ethic Committee (AEEC) guidelines and the
study protocol was approved by the AEEC. All animal care, use and
treatment were in strict accordance with the ARVO Statement for the
use of Animals in Ophthalmic and Vision Research. Animals were
anesthetized using a mixture of ketamine hydrochloride (25 mg/kg)
and chlorpromazine (25 mg/kg) that was administered
intramuscularly. Following the induction of sufficient anesthesia,
as determined by corneal response, the central cornea of the right
eye was burned by placing a NaOH-soaked (1 mol/l) circular piece of
filter paper on the corneal surface for 60 sec. Following the
removal of the filter paper, the ocular surface and the
conjunctival sac were immediately rinsed with 20 ml saline for 1
min and animals were allocated at random into four treatment groups
(n=8 per group). Following ocular washing, 0.2 ml PSH-ES (50
µg/ml), PEG-ES (50 µg/ml), ES (50 µg/ml) or physiological saline
(as a control) were injected into the subconjunctival tissue. The
animals received injections once every other day for 14 days, which
was a total of 7 injections. Following each injection, topical
antibiotic ointment was administered to the burned eye to minimize
the risk of infection.
The extent of CNV was quantified by the same
observer using a slit lamp every day after the first injection.
Furthermore, the ocular surface was examined for corneal ulceration
and bulbar conjunctival hyperemia and edema. Rabbit eyes were
photographed using a digital camera (Canon Inc., Tokyo, Japan) on
days 4, 7, 10 and 13 and vessel growth from the corneoscleral
limbus into the clear cornea was automatically quantified in terms
of vessel area (mm2) using Image-Pro Plus software
(Media Cybernetics, Inc., Rockville, MD, USA) (16–18).
Rabbits were sacrificed via an overdose of
intravenous pentobarbital sodium 16 days after the alkali corneal
burning. Eyes were enucleated and the cornea, including the
adjacent 2 mm scleral tissue, was removed and immediately fixed in
formalin. After 24 h, tissue specimens were dehydrated, infiltrated
and embedded in paraffin, and sectioned using a microtome. Serial
olefin sections (5 µm) of each eye were prepared and the expression
of vascular endothelial growth factor (VEGF) was analyzed by
immunohistochemical methods using goat anti-human VEGF (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). To visualize the vascular
endothelial cells and determine the degree of induced angiogenesis,
sections were stained with goat anti-rabbit CD34 antibody (Valeant
Pharmaceuticals, Laval, Canada). The immunostaining process was
performed according to the avidin-biotin complex (ABC) method. Thin
paraffin sections (5 µm) were mounted on silanized slides, dried
overnight and deparaffinized with descending concentrations of
ethanol and xylene. The slides were placed into citrate buffer (pH
6.0) and boiled for 5 min. After cooling for 30 min, the specimens
were incubated with 5% normal bovine serum for 30 min, followed by
incubation with the primary antibody in a humidified chamber for 2
h at room temperature. The primary antibodies used were goat
anti-human VEGF antibody (1:400) and goat anti-rabbit CD34 antibody
(1:400), plus 1% normal rabbit serum, respectively. For detection,
the 3-step ABC method was used. The secondary biotinylated antibody
was a mouse anti-goat immunoglobulin G (Abcam) antibody diluted
1:200. Finally, the slides were incubated with streptavidin and
alkaline phosphatase and the samples were counterstained with
Hoechst (1:1000; Sigma-Aldrich). Cell staining results were graded
as 1 (yellow), 2 (brown) or 3 (sepia) based on the color of the
cytoplasm. A Luzex-F image analyzer (Nireco Corporation, Tokyo,
Japan) was used to obtain VEGF levels from the staining results, as
indicated by grayscale levels. Three sections were selected for
each eye, and the color of the cytoplasm was detected by the
software at x400 magnification. Microvascular densities were
determined using previously established methods (19). Three sections were selected for each
eye, and new corneal vessels were counted in the 5 areas of highest
vascular density at x400 magnification. Microvascular density is
expressed as the mean number of vessels per field of view area.
Sections were also stained with hematoxylin and eosin for
histological investigation.
Statistical analysis
Statistical analyses were performed using SPSS
statistical software, version 15.0 (SPSS, Inc., Chicago, IL, USA).
The statistical significance of the differences between the PSH-ES,
PEG-ES, ES and control groups was determined using one-way analysis
of variance and unpaired Student's t-tests. Data are presented as
the mean ± standard deviation and P<0.05 was considered to
indicate a statistically significant difference.
Results
Preparation of ES, PSH-ES and
PEG-ES
The molecular weights of ES and its modified forms
were determined using SDS-PAGE. ES, PSH-ES and PEG-ES presented
with single bands at 20, 35 and 38 kD, respectively, following
purification. Final solutions of ES, PSH-ES and PEG-ES did not
include visible fine particles or precipitate and remained clear at
4°C and after thawing from −20°C.
Effects of ES, PSH-ES and PEG-ES on
HUVEC proliferation
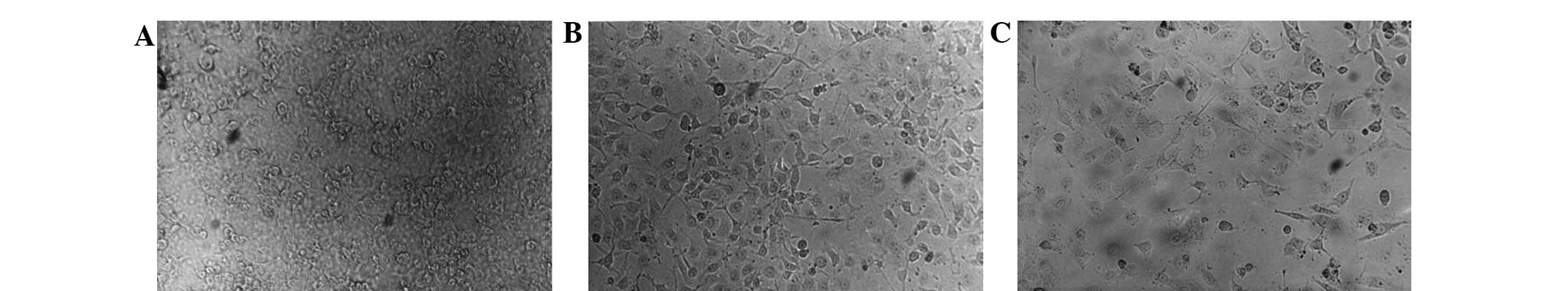
In the cell proliferation assay, ES, PSH-ES and
PEG-ES inhibited HUVEC proliferation in a concentration-dependent
manner (Fig. 1 and Table I). The inhibitory rates of ES, PSH-ES
and PEG-ES were 48.6, 42.2 and 40.1%, respectively, at a
concentration of 5 µg/ml; 59.6, 45.4 and 43.2%, respectively, at 10
µg/ml and 67.3, 56.8 and 52.3%, respectively, at 15 µg/ml. ES
produced more marked inhibition of HUVEC proliferation compared
with PSH-ES and PEG-ES (P<0.05); however, no significant
difference was observed between the effects produced by PSH-ES and
PEG-ES (P<0.05).
 | Table I.Inhibitory effects of ES, PSH-ES, and
PEG-ES on HUVEC proliferation. |
Table I.
Inhibitory effects of ES, PSH-ES, and
PEG-ES on HUVEC proliferation.
|
| Inhibitory rates
(%) |
|---|
|
|
|
|---|
| Group | 5 µg/ml | 10 µg/ml | 15 µg/ml |
|---|
| ES | 48.6 | 59.6 | 67.3 |
| PSH-ES | 42.2 | 45.4 | 56.8 |
| PEG-ES | 40.1 | 43.2 | 52.3 |
Effects of ES, PSH-ES and PEG-ES in
the CNV assay
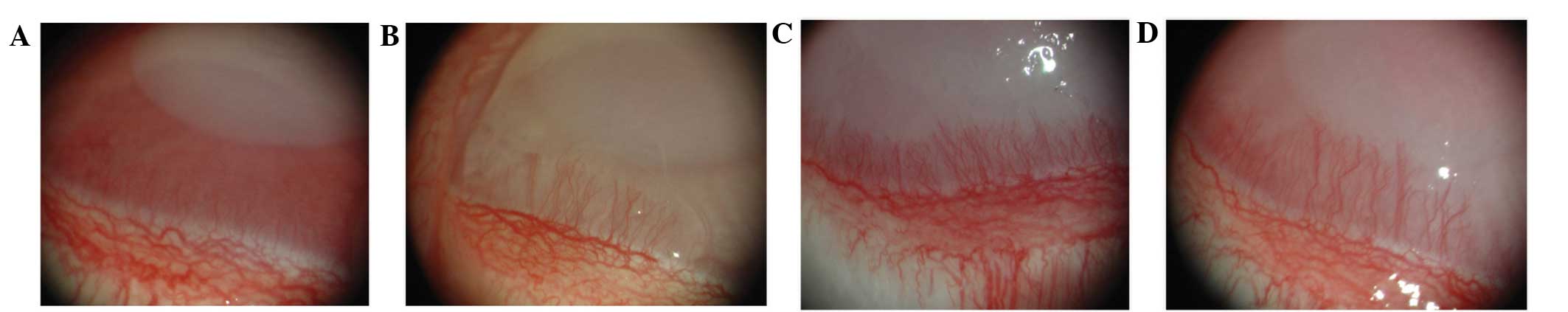
Following alkali-induced corneal burning, the eyes
of the rabbits were examined every day and photographic images
captured under a surgical microscope. Corneal opacity and
angiogenesis gradually increased in all groups; however, the ES,
PSH-ES and PEG-ES groups exhibited significantly inhibited
angiogenesis compared with the saline-treated control. The average
CNV length in the ES, PSH-ES and PEG-ES groups was reduced compared
with that in the saline group. Furthermore, the average CNV area in
the ES, PSH-ES and PEG-ES groups was reduced compared with that in
the saline group (Fig. 2 and
Table II). The results indicate
that the PSH-ES and PEG-ES derivatives inhibited angiogenesis in
the CNV model more effectively than ES did.
 | Table II.Microvascular length and corneal
neovascularization (n=8 eyes per group). |
Table II.
Microvascular length and corneal
neovascularization (n=8 eyes per group).
|
| Day 4 | Day 7 | Day 10 | Day 13 |
|---|
|
|
|
|
|
|
|---|
| Group | Length (mm) | Area
(mm2) | Length (mm) | Area
(mm2) | Length (mm) | Area
(mm2) | Length (mm) | Area
(mm2) |
|---|
| PSH-ES |
0.42±0.12 |
2.58±1.04 |
1.38±0.16a |
4.39±1.27a |
2.15±0.96a |
5.08±1.97a |
2.32±1.46a |
10.18±3.26a |
| PEG-ES |
0.51±0.16 |
2.92±1.16 |
1.56±0.19a |
5.02±1.18a |
2.34±0.93a |
6.88±2.02a |
2.97±1.54a |
12.37±3.92a |
| ES |
0.88±0.21 |
3.67±1.28 |
1.73±0.28a |
5.82±1.24a |
2.68±1.07a |
8.59±2.08a |
3.49±1.68a |
16.89±4.15a |
| Control |
1.35±0.86 |
4.99±1.36 |
2.38±0.56 |
7.59±1.34 |
3.92±1.29 |
12.37±3.93 |
5.85±1.28 |
24.69±7.63 |
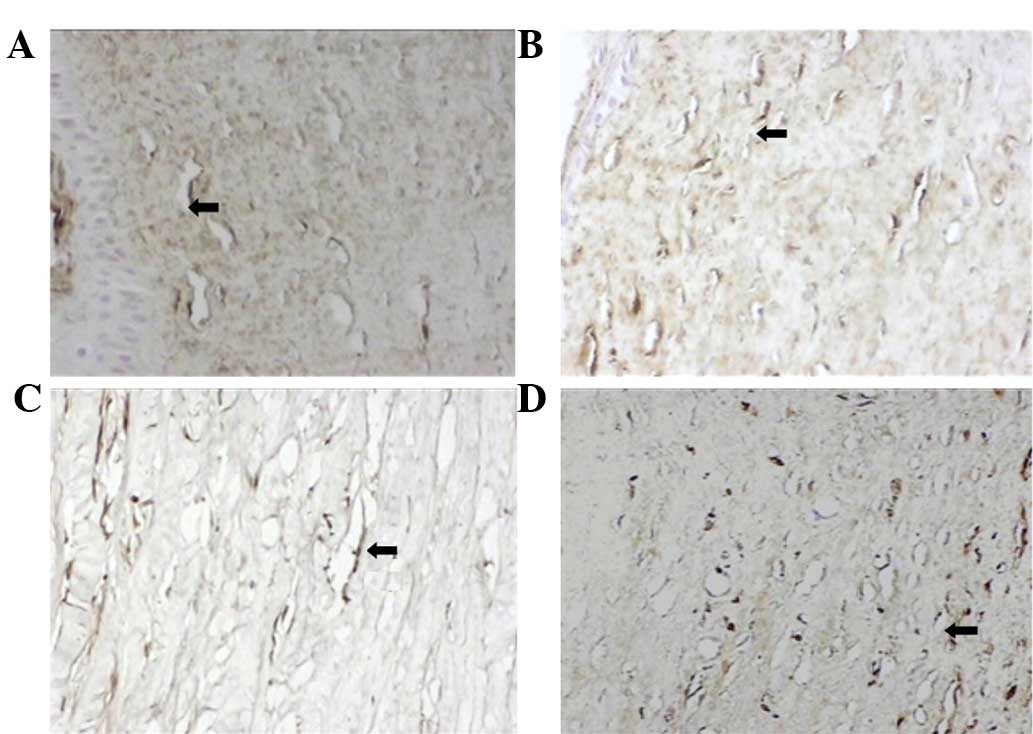
It was hypothesized that the effects of PSH-ES and
PEG-ES on CNV were mediated by the downregulation of VEGF, a known
mediator of CNV and inflammation. Therefore, immunohistochemical
analysis was used to measure corneal VEGF levels. In the
physiological saline group, inflammatory cell infiltration occurred
throughout the corneal hypothallus and the expression of VEGF in
the inflammatory cytoplasm was grade 3. In the ES group,
inflammatory cell infiltration occurred in the anterior half of the
corneal hypothallus and the expression of VEGF in the inflammatory
cytoplasm was grade 2. In the PSH-ES and PEG-ES groups,
inflammatory cell infiltration occurred in approximately one-third
of the anterior of the corneal hypothallus and the expression of
VEGF in the inflammatory cytoplasm was grade 1. VEGF expression was
significantly inhibited by ES and modified ES, with PSH-ES and
PEG-ES producing more marked effects compared with ES (P<0.05;
Fig. 3 and Table II).
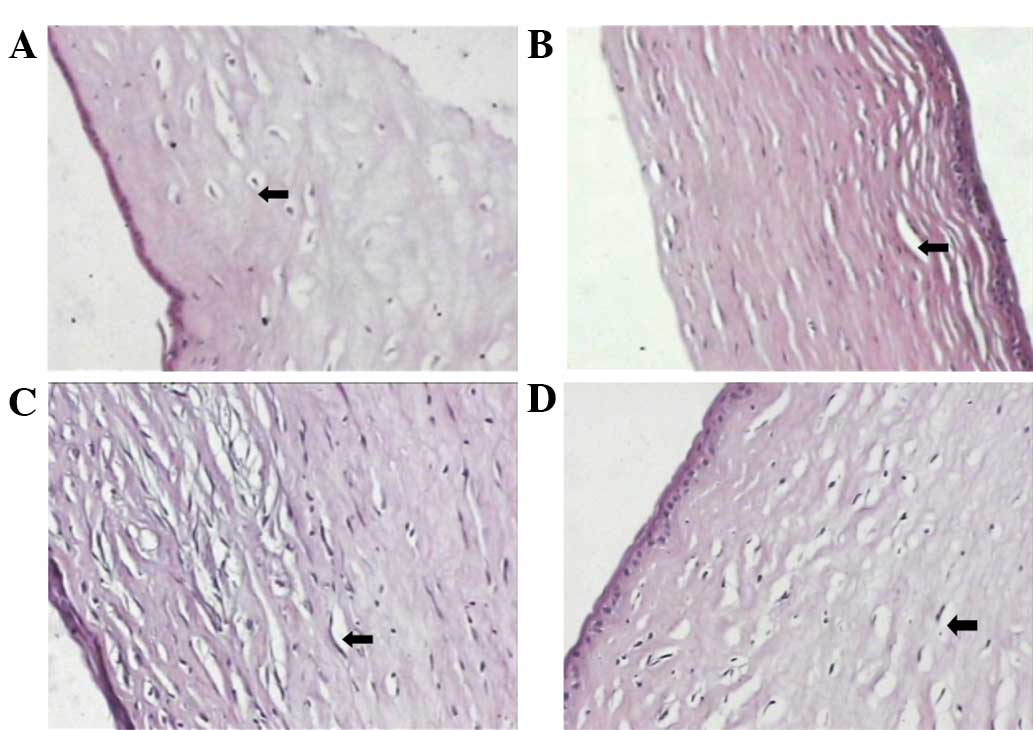
A limited number of new corneal vessels were
detected in the eyes of rabbits treated with PSH-ES and PEG-ES.
Additionally, the number of microvessels observed in the PSH-ES and
PEG-ES groups on day 16 was significantly lower compared with that
in the ES group. By contrast, numerous new corneal vessels were
detected in the eyes of the control group, and were observed
throughout the entire stroma (P<0.05; Fig. 4 and Table III). The results of the CNV assay
indicate that PSH-ES and PEG-ES exhibit more marked
anti-angiogenesis activity compared with ES, and that PSH-ES was
the most effective among the three proteins investigated.
 | Table III.VEGF levels and the number of
microvessels at day 16 (n=8 eyes per group). |
Table III.
VEGF levels and the number of
microvessels at day 16 (n=8 eyes per group).
| Group | VEGF levels
(grayscale) | Microvessels
(n) |
|---|
| PSH-ES |
101.32±17.46a |
1.04±0.82a |
| PEG-ES |
112.26±18.35a |
1.16±1.02a |
| ES |
123.56±20.13a |
3.59±1.75a |
| Saline |
153.15±24.54 |
6.32±2.75 |
Discussion
The anti-angiogenic activity of ES results in the
inhibition of endothelial cell adhesion, migration, and
proliferation, in addition to the induction of apoptosis (3). ES is among the most effective
endogenous angiogenesis inhibitors. It has exhibited notable
anti-angiogenesis and antitumor effects, for example in the therapy
of non-small-cell lung cancer (20).
In addition to its effects on tumors, ES has been observed to
inhibit CNV (4). However, the
clinical application of ES has been limited by the high dose
requirement, short half-life and poor stability (21).
Chemical modification is a commonly used method of
improving the properties of proteins. PEG is a modification agent
used in numerous drugs approved by the US Food and Drug
Administration, and is the most commonly used modifier of proteins.
PSH is a polysaccharide with anti-angiogenesis and antitumor
activity, which has also been reported to be a suitable modifier
for improving the properties of ES (12). Based on the results of our previous
research (10,12), the present study compared the
anti-angiogenesis effects of ES and two ES derivatives.
The results of the HUVEC proliferation assay
indicated that ES, PSH-ES and PEG-ES inhibited cell proliferation,
with ES producing the most notable effect. Thus, purified PSH-ES
and PEG-ES exhibited reduced HUVEC inhibitory activity compared
with ES, which may be due to alterations in its active domains
following chemical modification. In addition, PSH-ES exhibited more
pronounced antiproliferative activity compared with PEG-ES, which
indicated that the PSH modification reduced activity to a lesser
extent than the PEG modification.
The CNV assay indicated that PSH-ES produced the
most marked inhibition of CNV, while PEG-ES produced more notable
inhibitory effects compared with ES. Furthermore, VEGF expression
was significantly inhibited by PSH-ES and PEG-ES. A previous study
demonstrated that chemical modification is able to prolong protein
half-life (22), and in our previous
studies (10,12), the stability of ES was improved by
modification with PEG or PSH. It was hypothesized that the
conjugation of ES with PSH or PEG may extend the biological
half-life of ES (23) and thus
prolong its effects. In a previous study, a chicken chorioallantoic
membrane assay demonstrated that ES activity was prolonged by
conjugation with PSH or PEG, with purified PSH-ES and PEG-ES
exhibiting improved heat stability compared with ES at 25 and 37°C
(24). These modification effects
may underlie the notable in vivo anti-angiogenic properties
of PSH-ES and PEG-ES, as compared with ES. Furthermore, the marked
VEGF downregulation observed in the presence of PSH-ES and PEG-ES
as compared with ES may have contributed to the higher efficacy of
PSH-ES and PEG-ES in the inhibition of CNV.
The most commonly used protein modifier is currently
PEG, which is not bioactive and produces no side-effects. The
biological inactivity of PEG has resulted in its widespread use as
a chemical modifier of proteins and peptides. However, in the
present study the functional polysaccharide PSH was additionally
used as an ES modifier, which increased the stability and
bioactivity of ES, with no evident side-effects. This result may be
due to the relatively large size of PSH-ES as compared with ES. The
molecular weight of ES and the PSH modifier are 20 and 5.2 kD,
respectively, as measured with gel permeation chromatography
(12). SDS-PAGE has previously been
used to demonstrate that the molecular weight of PSH-ES is 35 kD;
therefore, it is presumed that, on average, one ES molecule
conjugates with three PSH molecules. PSH-ES is structured with PSH
on the compound exterior, where it protects ES and induces markedly
improved stability compared with native ES. Finally, PSH possesses
anti-angiogenic properties (24),
and PSH and ES may function synergistically. Animal studies are
currently underway to examine the immunogenicity and toxicity of
endostatin if used independently and in combination with
chemotherapeutic agents. Collectively, previously published results
(10) and the present study suggest
that PSH-ES and PEG-ES may be clinically applicable interventions
in the near future.
In the present study, ES and its derivatives PSH-ES
and PEG-ES significantly inhibited HUVEC proliferation and CNV.
Therefore, these conjugated ES compounds represent candidate
anti-angiogenesis drugs that may lead to the expanded clinical
application of ES derivatives for the treatment of
angiogenesis-related diseases.
Acknowledgements
The authors thank Dr. Fengshan Wang of the Institute
of Biochemical and Biotechnological Drugs at the Shandong
University School of Pharmaceutical Science for technical
support.
References
|
1
|
O'Reilly MS, Boehm T, Shing Y, et al:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Addison CL, Nör JE, Zhao H, et al: The
response of VEGF-stimulated endothelial cells to angiostatic
molecules is substrate-dependent. BMC Cell Biol. 6:382005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dkhissi F, Lu H, Soria C, et al:
Endostatin exhibits a direct antitumor effect in addition to its
antiangiogenic activity in colon cancer cells. Hum Gene Ther.
14:997–1008. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai LJ, Xiao X and Wu JH: Inhibition of
corneal neovascularization with endostatin delivered by
adeno-associated viral (AAV) vector in a mouse corneal injury
model. J Biomed Sci. 14:313–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mori K, Ando A, Gehlbach P, et al:
Inhibition of choroidal neovascularization by intravenous injection
of adenoviral vectors expressing secretable endostatin. Am J
Pathol. 159:313–320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Wang JW, Sun Y, et al: Randomized
phase II trial on escalated doses of rh-endostatin (YH-16) for
advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi.
28:138–141. 2006.(In Chinese). PubMed/NCBI
|
|
7
|
Zhuo W, Luo C, Wang X, et al: Endostatin
inhibits tumour lymphangiogenesis and lymphatic metastasis via cell
surface nucleolin on lymphangiogenic endothelial cells. J Pathol.
222:249–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdollahi A, Hlatky L and Huber PE:
Endostatin: The logic of antiangiogenic therapy. Drug Resist Updat.
8:59–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Ren Y, Pan L and Xu HM: In
vivo anti-tumor activity of polypeptide HM-3 modified by
different polyethylene glycols (PEG). Int J Mol Sci. 12:2650–2663.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan H, Yang S, Liu C, et al: Enhanced
anti-angiogenesis and anti-tumor activity of endostatin by chemical
modification with polyethylene glycol and low molecular weight
heparin. Biomed Pharmacother. 66:648–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan H, Yang S, Feng Y, et al:
Characterization and secondary structure analysis of endostatin
covalently modified by polyethylene glycol and low molecular weight
heparin. J Biochem. 144:207–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ning TH, Chao CJ, Ying MG, et al:
Preparation, characterization and anti-angiogenesis activity of
endostatin covalently modified by polysulfated heparin. Pharmazie.
67:622–627. 2012.PubMed/NCBI
|
|
13
|
Bullock J, Chowdhury S, Severdia A, et al:
Comparison of results of various methods used to determine the
extent of modification of methoxy polyethylene glycol 5000-modified
bovine cupri-zinc superoxide dismutase. Anal Biochem. 254:254–262.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han DW, Lee MH, Kim HH, et al:
Epigallocatechin-3-gallate regulates cell growth, cell cycle and
phosphorylated nuclear factor-κB in human dermal fibroblasts. Acta
Pharmacol Sin. 32:637–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen DQ, Wang X, Chen L, et al: Novel
liver-specific cholic acid-cytarabine conjugates with potent
antitumor activities: Synthesis and biological characterization.
Acta Pharmacol Sin. 32:664–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan X, Wang Y, Zhang M, et al: Effects of
endostatin-vascular endothelial growth inhibitor chimeric
recombinant adenoviruses on antiangiogenesis. World J
Gastroenterol. 10:1409–1414. 2004.PubMed/NCBI
|
|
17
|
Igarashi T, Miyake K, Masuda I, et al:
Adeno-associated vector (type 8)-mediated expression of soluble
Flt-1 efficiently inhibits neovascularization in a murine choroidal
neovascularization model. Hum Gene Ther. 21:631–637. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshida M and Tabata Y: Tumor targeting of
protein through poly (ethylene glycol) conjugation with metal
coordination. J Nanosci Nanotechnol. 10:877–885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng LH, Shen W, Yong W, et al: Effects of
AMD3100 subconjunctival injection on alkali burn induced corneal
neovascularization in mice. Int J Ophthalmol. 4:44–48.
2011.PubMed/NCBI
|
|
20
|
Sun Y, Wang J, Liu Y, et al: Results of
phase III trial of rh-endostatin (YH-16) in advanced nonsmall cell
lung cancer (NSCLC) patients. J Clin Oncol. 23
(Suppl):71382005.
|
|
21
|
Folkman J: Antiangiogenesis in cancer
therapy - endostatin and its mechanisms of action. Exp Cell Res.
312:594–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dosio F, Arpicco S, Brusa P, et al: Poly
(ethylene glycol)-human serum albumin-paclitaxel conjugates:
Preparation, characterization and pharmacokinetics. J Control
Release. 76:107–117. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu B, Xu HM, Zhao L, et al: Site-specific
modification of anti-angiogenesis peptide HM-3 by polyethylene
glycol molecular weight of 20 kDa. J Biochem. 148:341–347. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Norrby K: Low-molecular-weight heparins
and angiogenesis. APMIS. 114:79–102. 2006. View Article : Google Scholar : PubMed/NCBI
|